Abstract
(See the editorial commentary by Gershon, on pages 815–6.)
Analysis of saliva samples from individuals aged ≥60 years who had a history of zoster (group 1), zoster and postherpetic neuralgia (PHN; group 2), or no history of zoster (group 3) revealed varicella zoster virus (VZV) DNA in saliva samples from 11 of 17 individuals in group 1, 10 of 15 individuals in group 2, and 2 of 17 individuals in group 3. The frequency of VZV DNA detection was significantly higher (P = .001) in saliva of subjects with a history of zoster, with or without PHN (21 [67%] of 32 subjects in groups 1 and 2), than in saliva of age-matched subjects with no zoster history (2 [12%] of 17 subjects in group 3). Thus, persistence of VZV DNA in saliva is the outcome of zoster, independent of PHN. Because VZV infection can produce neurological and ocular disease without zoster rash, future studies are needed to establish whether VZV DNA can be detected in the saliva of such patients.
Varicella zoster virus (VZV) is a neurotropic alphaherpesvirus. Primary infection usually causes varicella (chicken pox), after which virus become latent in ganglionic neurons along the entire neuraxis [1, 2]. Reactivation decades later produces zoster (shingles) frequently followed by chronic pain (postherpetic neuralgia [PHN]), mostly in individuals >60 years old. The cause and pathogenesis of PHN are unknown. Two non–mutually exclusive theories are that (1) the excitability of ganglionic or even spinal cord neurons is altered, and (2) there is persistent productive virus infection in ganglia. Although virological analyses of ganglia demonstrating productive VZV infection from patients with PHN have not been conducted, evidence supporting the second theory comes from the detection of VZV DNA in peripheral blood mononuclear cells (PBMCs) of elderly patients with PHN for years after zoster [3], but not in PBMCs collected >38 days after zoster in patients who did not develop PHN [4]; these observations might reflect the acquisition of virus by mononuclear cells, particularly antigen-presenting cells, while trafficking through productively infected ganglia [5]. Further evidence of persistent productive infection in PHN has been provided by an open-label study reporting clinical improvement after intravenous acyclovir in 8 (53%) of 15 patients with PHN [6].
Active VZV infection can also be confirmed on the basis of the detection of VZV DNA in human saliva. VZV DNA was first detected in saliva of patients with Ramsay Hunt syndrome (facial paralysis and zoster oticus), including patients with facial paralysis who did not have rash [7]. VZV DNA was later found in saliva of stressed healthy astronauts [8] as well as in all of 54 patients with acute herpes zoster studied over a 3-week period [9]. To test whether VZV DNA might remain in the saliva of zoster patients with PHN for a longer time than in zoster patients who did not develop PHN, we analyzed saliva samples from individuals aged ≥60 years who had (1) a history of zoster but not PHN, (2) zoster and PHN, and (3) no history of zoster.
METHODS
After informed consent was given and with approval of the University of Texas Health Science Center’s institutional review board, 49 immunocompetent subjects ≥60 years of age were enrolled. Group 1 consisted of 17 subjects (5 men and 12 women; mean age, 69.5 years [SD, 5.2 years; range, 62–77 years]) with a history of zoster; group 2 consisted of 15 subjects (8 men and 7 women; mean age, 75.1 years [SD, 7.4 years; range, 65–91 years]) with PHN; and group 3 (control group) consisted of 17 subjects (10 men and 7 women; mean age, 70.6 years [SD, 8.2 years; range, 60–91 years]) with no history of zoster (Table 1).
Table 1.
Virologic Analysis of Saliva Obtained From Immunocompetent Individuals ≥60 Years Old With a History of Zoster, Postherpetic Neuralgia, or No History of Zoster
| Subject number | Age, years | Sex | Months from zoster to saliva analysis | No. of VZV DNA–positive samples per 7 samples | Mean VZV DNA copy number per milliliter of saliva |
| Group 1 (zoster) | |||||
| 1 | 72 | F | 144 | 3 | 62 |
| 2 | 62 | F | 30 | 3 | 1038 |
| 3 | 77 | M | 12 | 7 | 137 |
| 4 | 69 | F | 60 | 6 | 1072 |
| 5 | 70 | F | 12 | 3 | 19 |
| 6 | 78 | F | 33 | 0 | 0 |
| 7 | 72 | F | 15 | 0 | 0 |
| 8 | 74 | F | 9 | 2 | 782 |
| 9 | 63 | F | 11 | 2 | 766 |
| 10 | 77 | M | 60 | 0 | 0 |
| 11 | 64 | M | 11 | 0 | 0 |
| 12 | 70 | F | 10 | 0 | 0 |
| 13 | 67 | F | 11 | 1 | 766 |
| 14 | 69 | M | 15 | 0 | 0 |
| 15 | 65 | F | 24 | 1 | 758 |
| 16 | 62 | M | 10 | 1 | 749 |
| 17 | 70 | F | 24 | 1 | 746 |
| Mean (SD) | 69.5 (5.2) | … | 29 (34) | 2 (2) | 627a (375a) |
| Group 2 (PHN) | |||||
| 18 | 77 | M | 42 | 2 | 45 |
| 19 | 75 | M | 6 | 5 | 84 |
| 20 | 91 | F | 36 | 4 | 200 |
| 21 | 80 | M | 3 | 2 | 245 |
| 22 | 85 | F | 12 | 4 | 708 |
| 23 | 69 | M | 48 | 6 | 1274 |
| 24 | 72 | F | 36 | 1 | +b |
| 25 | 84 | F | 6 | 4 | 612 |
| 26 | 70 | M | 31 | 0 | 0 |
| 27 | 77 | M | 9 | 0 | 0 |
| 28 | 68 | M | 20 | 1 | 11 |
| 29 | 76 | M | 28 | 1 | +b |
| 30 | 65 | F | 51 | 0 | 0 |
| 31 | 69 | F | 15 | 0 | 0 |
| 32 | 69 | F | 15 | 0 | 0 |
| Mean (SD) | 75.1 (7.4) | … | 24 (16) | 2 (2) | 397a (438a) |
| Group 3 (no history of zoster) | |||||
| 33 | 72 | M | NA | 0 | 0 |
| 34 | 71 | F | NA | 0 | 0 |
| 35 | 84 | M | NA | 0 | 0 |
| 36 | 65 | M | NA | 0 | 0 |
| 37 | 71 | F | NA | 0 | 0 |
| 38 | 71 | M | NA | 0 | 0 |
| 39 | 78 | F | NA | 0 | 0 |
| 40 | 63 | F | NA | 0 | 0 |
| 41 | 71 | M | NA | 0 | 0 |
| 42 | 61 | M | NA | 0 | 0 |
| 43 | 91 | M | NA | 0 | 0 |
| 44 | 69 | F | NA | 0 | 0 |
| 45 | 73 | M | NA | 0 | 0 |
| 46 | 69 | F | NA | 0 | 0 |
| 47 | 60 | M | NA | 2 | 782 |
| 48 | 60 | M | NA | 0 | 0 |
| 49 | 71 | F | NA | 1 | 784 |
| Mean (SD) | 70.6 (8.2) | … | NA | 0.2 (0.5) | 783a (1a) |
NOTE. F, female; M, male; NA, not applicable; PHN, postherpetic neuralgia; VZV, varicella zoster virus.
Calculated from samples containing VZV DNA.
Sample contained VZV DNA but not enough to quantitate.
Seven saliva samples were obtained from each subject over a 2-week period. Samples were collected, DNA was extracted, and VZV DNA was quantified by real-time polymerase chain reaction (PCR) as described elsewhere [9]. The VZV DNA copy number was determined by comparing the cycle threshold (Ct) value of the test samples to Ct values obtained by PCR in dilutions of VZV DNA extracted from virus nucleocapsids [10]. A sample was considered to be positive if at least 2 of the 3 quantitative PCR replicates were positive. The limit of quantitative PCR detection was 10 copies of VZV DNA per milliliter of saliva; samples containing <10 copies/mL of saliva are indicated by plus signs in Table 1.
The Fisher exact 2-tailed test was used to test differences in frequencies between sample groups with significance set at P < .05. Linear regression analysis was used to determine significance (R2 > 0.95) between days positive for VZV in saliva and months after zoster. The Mann–Whitney U test was used to determine the statistical difference in VZV DNA copy number between group 1 (zoster) and group 2 (PHN).
RESULTS
VZV DNA was found in the saliva samples from 11 of 17 individuals with a history of zoster but not PHN (group 1), 10 of 15 subjects with PHN (group 2), and 2 of 17 subjects with no zoster history (group 3) (Table 2). Overall, VZV DNA was significantly more prevalent in saliva of subjects with a history of zoster with or without PHN (21 [67%] of 32 subjects) than in age-matched subjects with no zoster history (2 [12%] of 17 subjects; P = .001 [Fisher exact test]). Thirty (25%) of 119 total saliva samples obtained were positive for VZV DNA in group 1, 30 (28%) of 105 samples were positive in group 2, and 3 (2%) of 119 samples were positive in group 3 (Table 2), but the prevalence of positive saliva samples in subjects with a history of zoster with or without PHN (60 positive samples of the total 224) did not differ significantly from that in the subjects with no zoster history (P = .461).
Table 2.
Prevalence of Varicella Zoster Virus DNA in Saliva of Immunocompetent Individuals ≥60 Years Old With a History of Zoster, Postherpetic Neuralgia, or No History of Zoster
| Group |
|||
| Measure | Zoster | Postherpetic neuralgia | No history of zoster |
| No. (%) of individuals with saliva positive for VZV DNA | 11/17 (65) | 10/15 (67) | 2/17 (12) |
| No. (%) of saliva samples positive for VZV DNA | 30/119 (25) | 30/105 (28) | 3/119 (2) |
NOTE. The prevalence of varicella zoster virus (VZV) DNA in saliva of subjects with zoster or postherpetic neuralgia compared with age-matched control subjects with no history of zoster was highly significant (P = .001; Fisher exact test).
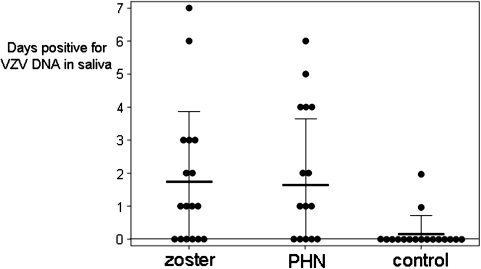
The mean (± SD) time from zoster to saliva collection in group 1 was 29 ± 34 months (range, 9–144 months), and that in group 2 was 24 ± 16 months (range, 3–51 months) (Table 1). Of the 7 saliva samples collected from each individual in the 3 groups, a mean (± SD) of 2 ± 2 samples (range, 0–7 samples) were positive for VZV DNA in group 1, 2 ± 2 samples (range, 0–6 samples) were positive in group 2, and 0.2 ± 0.5 samples (range, 0–2 samples) were positive in group 3. Comparison among the groups with respect to the mean number of days positive for VZV DNA in the saliva (Figure 1) revealed no significant difference between groups 1 and 2 (P = .75); however, the mean number of days positive for VZV DNA in saliva differed significantly between group 1 (subjects with zoster) and group 3 (controls; P = .005), and between group 2 (subjects with PHN) and group 3 (controls; P = .001).
Figure 1.
Days positive for varicella zoster virus (VZV) DNA in saliva of individuals with zoster, postherpetic neuralgia (PHN), and no history of zoster (control). The mean number of days testing positive for VZV DNA in saliva did not differ significantly between individuals with zoster and those with PHN (P = .75), but it did differ significantly between individuals with zoster and control individuals (P = .005) and between individuals with PHN and control individuals (P = .001). Thick horizontal lines indicate mean values, and thin horizontal lines indicate standard deviations of each group.
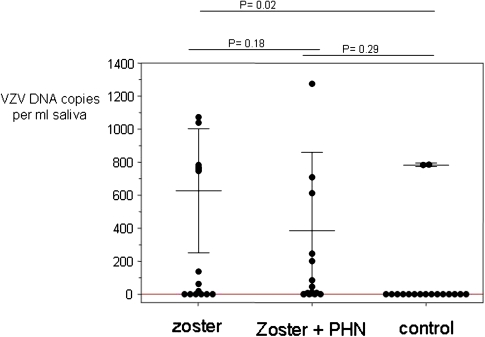
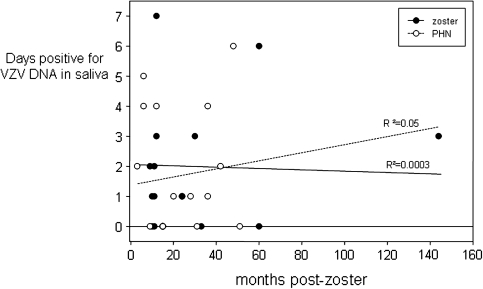
The mean (± SD) number of VZV DNA copies per milliliter of saliva in samples positive for VZV DNA was 627 ± 375 copies/mL (range, 19–1,072 copies/mL) in group 1, 397 ± 438 copies/mL (range 11–1,274 copies/mL) in group 2, and 783 ± 1 copies/mL (range, 783–784 copies/mL) in group 3 (Table 1 and Figure 2). No statistically significant difference in VZV DNA copy number was found between group 1 (subjects with zoster) and group 2 (subjects with zoster and PHN; P = .14). Furthermore, there was no significant correlation between the time interval from zoster to saliva collection and the number of days testing positive for VZV DNA in either group 1 or group 2 (R2 = 0.05 and 0.0003, respectively) (Figure 3).
Figure 2.
Varicella zoster virus (VZV) DNA copies per milliliter of saliva in individuals with zoster, postherpetic neuralgia (PHN), and no history of zoster (control). The mean (± SD) VZV DNA copy number per milliliter of saliva for all positive samples was 627 ± 375 copies/mL in individuals with a history of zoster, 397 ± 438 copies/mL in individuals with PHN, and 783 ± 1 copies/mL in individuals with no history of zoster.
Figure 3.
Days positive for varicella zoster virus (VZV) DNA in 7 days of saliva collection with respect to months after zoster in subjects with zoster or postherpetic neuralgia (PHN). No significant correlation between the number of days with VZV DNA in saliva and time after zoster was found in subjects with zoster (group 1; R2 = 0.05) or among subjects with PHN (group 2; R2 = 0.0003).
DISCUSSION
In this study, we examined saliva for the presence of VZV DNA in immunocompetent individuals aged ≥60 years and grouped according to zoster status. Remarkably, we detected VZV DNA in saliva samples from subjects with a history of zoster up to 12 years after illness, regardless of whether they had PHN, whereas only 2 (12%) of 17 age-matched subjects with no zoster history showed VZV DNA in their samples. Note that neither the number of positive saliva samples nor the copy number of VZV DNA in saliva differed significantly between the subjects with zoster alone (group 1) and those with zoster and PHN (group 2), indicating that zoster predicts the presence of VZV DNA in saliva, not the chronic pain afterward. The detection of VZV DNA in the saliva of 2 subjects aged ≥60 years with no history of zoster is most likely the outcome of VZV reactivation without rash. This is not surprising given the magnitude of virologically confirmed VZV-induced neurological and ocular disease such as cerebellar ataxia, meningoencephalitis, VZV vasculopathy, myelitis, zoster sine herpete, and retinitis without any history of zoster rash (reviewed in [11]).
An earlier study reported the detection of VZV DNA in the saliva of 54 zoster patients examined, although the age range of those zoster patients was 21–82 years and their saliva was studied for only 3 weeks after zoster [9]. The detection of VZV DNA [8] and of infectious VZV [12] in the saliva of healthy stressed astronauts points to the need to compare the duration of detectable VZV DNA in saliva of zoster patients <60 years old with that in zoster patients ≥60 years old. Furthermore, VZV DNA has also been detected in saliva of individuals ≥60 years old for 1 month after immunization with Zostavax (Zoster Vaccine Live) [13], suggesting that human saliva should be examined for VZV DNA and infectious virus for longer times after immunization.
Although no immediate explanation is apparent for the persistence of VZV DNA in saliva of subjects with a history of zoster, our data support those of earlier studies which revealed that the presence of virus is not restricted to the affected dermatome. For example, VZV DNA was found in the saliva of each of 54 patients with zoster on the face, trunk, and upper and lower extremities [9]. VZV DNA is also present in blood mononuclear cells during acute zoster [14] as well as in blood mononuclear cells of some elderly individuals with no history of zoster [15]. Overall, the detection of VZV DNA in saliva and blood indicates that after reactivation from ganglia, virus does more than travel transaxonally anterograde to skin. Furthermore, although the origin of VZV DNA in saliva is still unknown, infectious virus has been found in the saliva of patients with acute zoster [9] and in healthy astronauts [12]. The detection of VZV DNA in saliva of some elderly individuals for many years after zoster may reflect their inability to drive virus back to the latent state, just as a smoldering ganglionitis has been speculated to explain the development of postherpetic neuralgia [5]. Both phenomena could readily be explained by individual differences in host-cell--mediated immune responses to VZV.
Finally, the potential usefulness of saliva in diagnosis of patients with neurological and ocular disease should be considered. Future studies are needed to establish whether VZV DNA can be detected in the saliva of such patients. To date, definitive virological confirmation has required blood and cerebrospinal fluid examination for VZV DNA and anti-VZV immunoglobulin G and M.
Funding
This work was supported by the National Institutes of Health (grant AG032958 to D. G. and R. J. C., grant AG006127 to D. G., and grant NS067070 to M. A. N.); and the National Aeronautics and Space Administration (grant SMO-015 to D. L. P.).
Acknowledgments
We thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
References
- 1.Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol. 1992;31:444–8. doi: 10.1002/ana.410310417. [DOI] [PubMed] [Google Scholar]
- 2.Gilden DH, Gesser R, Smith J, et al. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes. 2001;23:145–7. doi: 10.1023/a:1011883919058. [DOI] [PubMed] [Google Scholar]
- 3.Mahalingam R, Wellish M, Brucklier J, Gilden DH. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–3. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- 4.Gilden DH, Devlin M, Wellish\ M, et al. Persistence of varicella-zoster virus DNA in blood MNCs of patients with varicella or zoster. Virus Genes. 1988;2:299–305. doi: 10.1007/BF00684037. [DOI] [PubMed] [Google Scholar]
- 5.Gilden DH, Cohrs RJ, Mahalingam R. VZV vasculopathy and postherpetic neuralgia: progress and perspective on antiviral therapy. Neurology. 2005;64:21–5. doi: 10.1212/01.WNL.0000148484.19070.4D. [DOI] [PubMed] [Google Scholar]
- 6.Quan D, Hammack BN, Kittelson J, Gilden DH. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2006;63:940–2. doi: 10.1001/archneur.63.7.noc60049. [DOI] [PubMed] [Google Scholar]
- 7.Furuta Y, Ohtani F, Sawa H, Fukuda S, Inuyama Y. Quantitation of varicella-zoster virus DNA in patients with Ramsay Hunt syndrome and zoster sine herpete. J Clin Microbiol. 2001;39:2856–9. doi: 10.1128/JCM.39.8.2856-2859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–9. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SK, Tyring SK, Gilden DH, et al. Varicella-zoster virus in the saliva of patients with herpes zoster. J Infect Dis. 2008;197:654–7. doi: 10.1086/527420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilden DH, Shtram Y, Friedmann A, et al. Extraction of cell-associated varicella-zoster virus DNA with X-100-NaCl. J Virol Methods. 1982;4:263–75. doi: 10.1016/0166-0934(82)90073-8. [DOI] [PubMed] [Google Scholar]
- 11.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–53. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–22. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierson DL, Mehta SK, Gilden D, et al. Varicella zoster virus DNA at inoculation sites and in saliva after Zostavax immunization. J Infect Dis. 2011;203:1542–5. doi: 10.1093/infdis/jir139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilden DH, Hayward AR, Krupp J, Hunter-Laszlo M, Huff JC, Vafai A. Varicella-zoster virus infection of human mononuclear cells. Virus Res. 1987;7:117–29. doi: 10.1016/0168-1702(87)90074-8. [DOI] [PubMed] [Google Scholar]
- 15.Devlin ME, Gilden DH, Mahalingam R, Dueland AN, Cohrs R. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–22. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]