Abstract
Background. Streptococcus pneumoniae causes serious diseases such as pneumonia and meningitis. Its major pathogenic factor is the cholesterol-dependent cytolysin pneumolysin, which produces lytic pores at high concentrations. At low concentrations, it has other effects, including induction of apoptosis. Many cellular effects of pneumolysin appear to be calcium dependent.
Methods. Live imaging of primary mouse astroglia exposed to sublytic amounts of pneumolysin at various concentrations of extracellular calcium was used to measure changes in cellular permeability (as judged by lactate dehydrogenase release and propidium iodide chromatin staining). Individual pore properties were analyzed by conductance across artificial lipid bilayer. Tissue toxicity was studied in continuously oxygenated acute brain slices.
Results. The reduction of extracellular calcium increased the lytic capacity of the toxin due to increased membrane binding. Reduction of calcium did not influence the conductance properties of individual toxin pores. In acute cortical brain slices, the reduction of extracellular calcium from 2 to 1 mM conferred lytic activity to pathophysiologically relevant nonlytic concentrations of pneumolysin.
Conclusions. Reduction of extracellular calcium strongly enhanced the lytic capacity of pneumolysin due to increased membrane binding. Thus, extracellular calcium concentration should be considered as a factor of primary importance for the course of pneumococcal meningitis.
Streptococcus pneumoniae (pneumococcus) is a common pathogen that causes life-threatening diseases in humans, including pneumonia and sepsis. This microorganism causes the most common form of bacterial meningitis and is associated with high lethality and disability. Disease rates are particularly high in young children, elderly people, and immunosuppressed patients. Only 30% of infected patients overcome the disease, and 30% of these survivors are affected by long-term sequelae, including mental retardation, learning impairment, and focal neurological deficits (eg, hearing loss) [1].
A major virulence factor of S. pneumoniae is the cholesterol-dependent cytolysin, pneumolysin (PLY), which is capable of producing lytic pores when its concentration is high and apoptosis without acute lysis at lower concentrations [2]. We have demonstrated that sublytic concentrations of PLY can modify the cellular cytoskeleton, leading to increased microtubule stabilization and actin remodeling [3, 4]. Evidence exists that the presence of PLY aggravates the course of pneumococcal pneumonia and meningitis [5–7]. In S. pneumoniae meningitis, the concentration of PLY in the cerebrospinal fluid (CSF) of patients never exceeds 0.2 μg/mL [8]. However, histological analysis of hippocampal slice cultures treated with PLY at concentrations as high as 0.5 μg/mL indicates a lack of acute lytic damage and only delayed damage in a small fraction of dentate gyrus neurons, consistent with the lack of extensive cell death in animal models of bacterial meningitis [7, 9]. Thus, the acute lysis one notices in cell culture seems to be strongly ameliorated in tissues where equivalent concentrations of PLY produce clear functional but less obvious lytic effects.
PLY consists of 4 domains arranged in an asymmetric manner. The pore formation model describes PLY monomers binding to membrane cholesterol with their C-terminal domain 4 via a tryptophan (Trp)-rich motif to form a prepore (an annular cluster of 30–50 monomers). PLY penetrates through the membrane following the unfolding of the molecule by inserting domain 3 into the lipid bilayer [10]. Thus, a barrel-structured pore with a size of ∼260 Å is formed, causing cell lysis. Experiments with artificial membranes demonstrate the existence of not only large, presumably lytic, pores, but also smaller pores with predominant cation selectivity [11, 12]. Divalent cations, such as calcium (Ca), seem to play a gating role in this smaller pore population [11]. Furthermore, Ca influx through pneumolysin pores has been demonstrated to aggravate inflammatory responses and enhance delayed cell apoptosis [2, 9, 13]. In cochlear cells, however, high concentrations of Ca (10 mM) and zinc diminished toxin binding to the membrane [14]. There is little information about the changes in brain Ca concentrations in the interstitial space during the course of various neurological diseases. In epilepsy, however, following chronic neuronal depolarization, extracellular Ca decreases [15]. Because seizures often accompany the course of meningitis [1], modulation of extracellular Ca concentrations should be considered.
In this study, we analyzed the role of extracellular Ca concentration on the acute lytic toxicity of PLY, demonstrating that decreased Ca concentration strongly increases the lytic capacity of toxins, both in cell culture and tissue modeling systems.
METHODS
Pneumolysin Preparation
Wild-type PLY and N-terminally green fluorescent protein (GFP)–tagged PLY (GFP-PLY) were expressed in Escherichia coli BL-21 cells (Stratagene, Cambridge, UK) and purified by metal affinity chromatography as described previously [16]. The purified PLY was tested for the presence of contaminating gram-negative LPS using the colorimetric limulus amebocyte lysate (LAL) assay (KQCL-BioWhittaker, Lonza, Basel, Switzerland). All purified proteins had <0.6 endotoxin unit (EU)/μg protein.
Cultures, Vital Staining, and Live Imaging
Primary astrocytes were prepared from the brains of newborn C57BL/6 mice in a mixed culture as previously reported [17], grown in Dulbecco’s modified Eagle’s medium (DMEM; GibcoBRL, Invitrogen GmbH, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (FBS; PAN Biotech GmbH, Aidenbach, Germany) and 1% penicillin/streptomycin (GibcoBRL) in 75-cm2 poly-l-ornithine–coated (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) cell culture flasks (Sarstedt AG & Co., Nuembrecht, Germany). At days 10–14 after seeding, the astrocytes were trypsinized and seeded into chamber slides coated with poly-l-ornithine.
Acute brain slices were prepared from PD 10–14-day-old C57BL/6 mice by decapitation and vibratome sectioning (Vibroslice NVSL, World Precision Instruments, Berlin, Germany) in artificial cerebrospinal fluid continuously oxygenized with carbogen gas (95% O2, 5% CO2) at 4°C. The slices were allowed to adapt in carbogenated Eagle’s basal medium (BME; GibcoBRL) with 1% penicillin/streptavidin and 1% glucose at 37°C for 1 hour before being challenged with PLY in 5% CO2-buffered medium (pH = 7.3) containing 119 mM NaCl, 26 mM NaHCO3, 1 mM NaH2PO4, 5 mM KCl, 2 mM CaCl2, and 5 mM glucose. In these acute slices, cell lysis never exceeded 7% within 12 hours.
For live imaging experiments, the cells were incubated in imaging buffer containing 135 mM NaCl, 2 mM CaCl2, 2.5 mM MgCl2, 4 mM KCl, and 5 mM Hepes (all from Carl Roth, Karlsruhe, Germany), at a pH of 7.3 at 37°C, with propidium iodide in the medium to stain permeabilized cells and Hoechst 33342 to stain the nuclei of all cells (all stains were diluted 1:1000 from 1 mg/mL stocks, Invitrogen). All variations of Ca concentration are indicated in the text. Osmolarity was adjusted using NaCl concentration changes. Both NaCl-adjusted and nonadjusted buffers were tested, and no difference was observed. The cells were visualized on an Olympus Cell M imaging system at 37°C, using ×10 and ×20 dry objectives (Olympus Deutschland GmbH, Hamburg, Germany). In all experiments, cells and tissues were treated with PLY in serum-free medium.
Lactate Dehydrogenase Test
The lactate dehydrogenase (LDH) detection kit (Roche Diagnostics GmbH, Mannheim, Germany) was used according to the manufacturer’s instructions to assess toxicity and cell lysis.
Planar Lipid Bilayer Experiments
The planar lipid bilayer (PLB) experiments were carried out as previously described [18]. Membranes were formed from a 1% (w/v) solution of oxidized cholesterol in n-decane. This artificial lipid was used instead of diphytanoylphosphatidylcholine (PC) because it facilitates the insertion of porin and PLY pores into the lipid bilayer very easily [19]. The toxin (0.5 μg/mL) was added to the aqueous phase after the membrane had turned black. The membrane current was measured with a pair of Ag/AgCl electrodes with salt bridges switched in series by a voltage source and a highly sensitive current amplifier (Keithley 427, Keithley Electronics, Garland, TX) in a buffer containing 100 mM KCl, 10 mM Hepes, and various concentrations of CaCl2 (Carl Roth). The temperature was maintained at 20°C throughout.
Evaluation and Statistics
Image processing and image analysis were performed using Image J software (version 1.43 for Windows, National Institute of Health, Bethesda, MD). Statistical analysis was performed on GraphPad Prism 4.02 for Windows (GraphPad Software Inc., La Jolla, CA). Statistical tests included a Mann–Whitney U test (comparing 2 groups, changing 1 parameter at a time) or 1-way analysis of variance (ANOVA) with Bonferroni posttest (comparing 3 or more groups, changing 1 parameter at a time).
RESULTS AND DISCUSSION
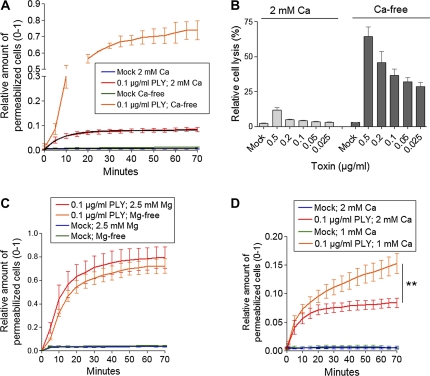
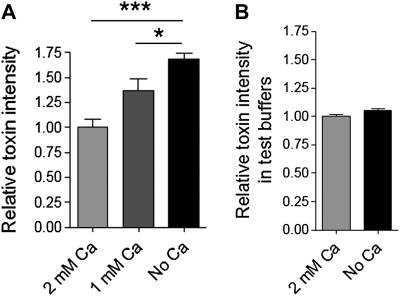
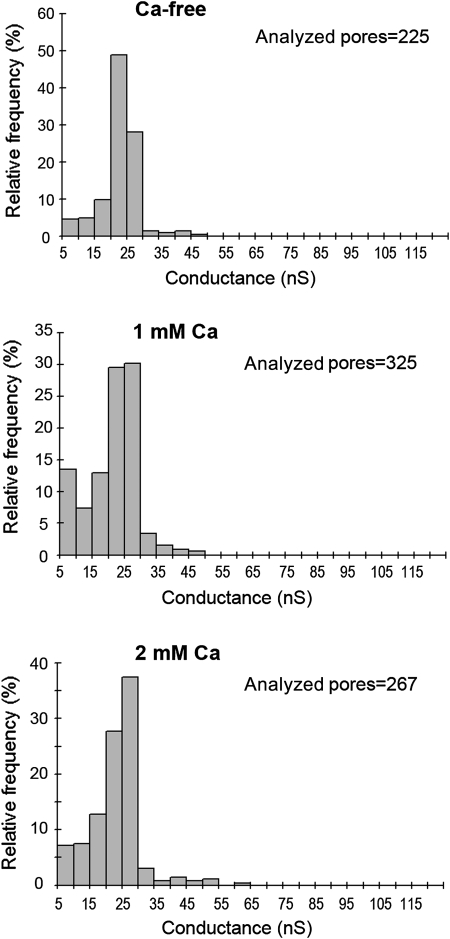
Recently, we demonstrated that sublytic concentrations of PLY can permeabilize a certain small population of cells in culture. Permeabilization occurs exponentially and remains constant within the first 20–30 minutes [20]. Here, we challenged primary mouse glial cells with 0.1 μg/mL PLY in physiological calcium concentrations (2 mM). Permeabilization increased exponentially, up to ∼7%, in the presence of toxin compared with ∼0.5% for the mock-treated control (Figure 1A). The permeabilization was evaluated by propidium iodide (PI) chromatin staining of the cells [4, 20] and by LDH release. When Ca was eliminated from the extracellular buffer, the toxicity of 0.1 μg/mL PLY dramatically increased (Figure 1A and B). Furthermore, Ca depletion conferred lytic capacity to nonlytic concentrations of PLY (Figure 1B). As the medium also contained Mg, we depleted Mg to test whether the lack of another divalent cation could have complementary effects. Lack of Mg, in contrast to Ca depletion, did not influence permeabilization kinetics (Figure 1C). Thus, Mg was neither antagonistic nor synergistic with Ca, implying a selective effect of Ca on the kinetics of PLY-induced permeabilization. The complete lack of Ca had profound effects on the lytic capacity of PLY. In pathophysiological conditions, however, complete depletion is rarely observed. Therefore, we analyzed the lytic capacity of PLY in reduced Ca conditions (1 mM). Again, the lytic capacity of PLY was enhanced compared with 2 mM Ca but much less than in Ca-free buffer (Figure 1D). To clarify the exact mechanism of the increased lytic capacity at low Ca conditions, we analyzed the binding capacity of the toxin utilizing a recombinant GFP-PLY protein [16]. The GFP intensity of the cells was measured 10 minutes after challenge with GFP-PLY. Lowering the Ca concentration from 2 to 1 mM and 1 to 0 mM Ca proportionally increased the binding capacity of PLY (Figure 2A). The fluorescence increase was not due to an increase in GFP fluorescence when Ca was absent (Figure 2B). Although increased binding could obviously explain our results, we also studied the role of Ca on the size and conductance of the membrane pores produced by PLY using artificial lipid bilayers (Figure 3). Analysis of single-channel conductance in the presence of 1 or 2 mM Ca or in Ca-free conditions demonstrated identical populations of ion channels, with peak conductance in the range of 20–25 nS (Figure 3). Thus, the change in Ca concentrations did not affect the size or properties of individual PLY channels when conductance was used as a marker.
Figure 1.
A, Increased permeabilization (PI staining) with 0.1 μg/mL PLY in Ca-free buffer. B, Increased LDH release by equivalent PLY amounts in Ca-rich and Ca-free buffer. C, Depletion of Mg from the Ca-free buffer does not alter the toxicity of 0.1 μg/mL PLY, implying that the effects are Ca specific. D, Reduction of extracellular Ca from 2 to 1 mM enhanced the lytic capacity of 0.1 μg/mL PLY. ** P < .01, Mann–Whitney U test (see Methods). All values in the figures represent mean ± SEM, n = 5; PI, propidium iodide; Mg, magnesium.
Figure 2.
A, Increase of GFP fluorescence intensity of the cells following exposure to 0.5 μg/mL GFP-PLY (10 minutes after challenge) as the concentration of the extracellular Ca falls. *P < .05, ***P < .001, one-way ANOVA with Bonferroni posttest (see Methods). B, Ca depletion in the imaging buffer does not substantially affect the GFP fluorescence of PLY-GFP in the cuvette. All values represent mean ± SEM, n = 5.
Figure 3.
Effect of calcium on pore conductance in planar lipid bilayers. The panels show histograms (frequency distributions) of the single-channel conductance of PLY pores measured in Ca-free solution or in the presence of 1 or 2 mM CaCl2. The membranes were formed from 1% oxidized cholesterol dissolved in n-decane. The aqueous phase contained 100 mM KCl, 10 mM Hepes, and 0.5 μg/mL PLY. The applied voltage was 20 mV; T = 20°C. The histograms suggest virtually unchanged populations of PLY pores with a maximum conductance peak between 20 and 25 nS.
The lack of effect of Ca on ion conductance was surprising because other studies have indicated the gating sensitivity of cation-selective PLY pores at higher-than-physiological divalent ion concentrations (1–5 mM Zn2+ and 10–20 mM Ca). This suggests that pore characteristics may be modulated by divalent cations [11]. High Ca may also inhibit toxin binding, although at concentrations of about 10 mM [21]. The molecular mechanisms leading to increased toxin binding in our system remain unclear. One possibility is a direct effect on domain 4 of PLY (the cholesterol-binding domain), leading to improved cholesterol interaction. Alternatively, changes in Ca levels could alter the biophysical properties of the membrane. Reduction of extracellular Ca is known to increase the membrane fluidity of cortical neurons [22], and the biggest change of about 40% is observed when reducing Ca under 1 mM. We observed the largest increase in the lytic activity of PLY during the transition from 1 mM to Ca-free conditions (Figures 1A and 1D). In our artificial lipid bilayer system, we were able to accelerate pore formation by the addition of a small amount of oxidized cholesterol to the lipids in the bilayer [23] (see Methods). This is a well-known approach, but the exact molecular steps involved in improving the pore formation remain unclear. It is known, however, that the oxidized cholesterol dramatically improves both the elastic properties of artificial membranes [23] and membrane fluidity [24]. Thus, it is possible that the enhanced binding of PLY to membranes at low Ca conditions involves a similar change in membrane fluidity.
There is ample evidence that cell damage by PLY is Ca-dependent [9, 13, 21], which does not contradict our findings. Still, most of these studies investigate inflammatory markers or analyze delayed lytic cell death effects rather than delayed cell damage, as evidenced by events such as apoptosis. It is not surprising that increased intracellular Ca has a detrimental effect on cell survival because it is known to be cytotoxic in various models. Our findings, however, support the concept that apart from the beneficial effect of blocking Ca influx, maintenance of normal extracellular calcium should also be considered essential.
Depletion of extracellular Ca has been shown to inhibit endocytosis in frog motoneuronal terminals [25]. Thus, one could speculate that the lack of extracellular Ca would affect membrane component turnover and, thus, membrane adaptation. Our binding experiments, however, demonstrated a dramatic increase in membrane binding in Ca-free conditions, which cannot be explained by inhibition of endocytosis. Ca is known to affect membrane fluidic properties [26]. Furthermore, Ca binding can affect the conformation of multiple cellular molecules, many of which may demonstrate Ca-binding properties. To analyze the innate Ca-binding properties of PLY, we exposed it to ethylene glycol tetraacetic acid (EGTA) before challenging cells in Ca-free buffer to deplete the toxin of any previously bound Ca before exposure to Ca-free buffer. These experiments showed a completely identical toxicity curve in Ca-free buffer independent of preincubation of PLY with a Ca chelator, thus excluding any interfering Ca-binding properties of PLY (data not shown).
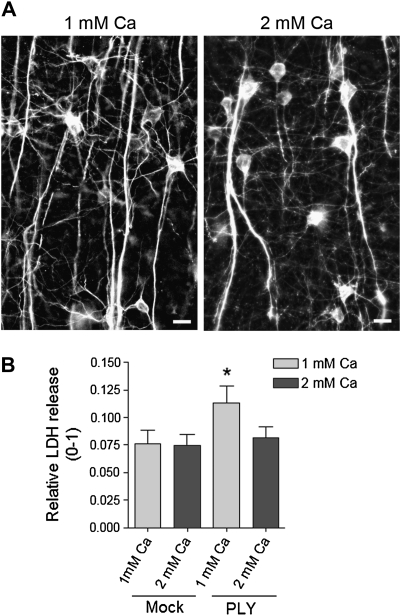
Next, we studied the acute lytic capacity of 0.2 μg/mL PLY (the mean concentration observed in the cerebrospinal fluid of patients with pneumococcal meningitis [8]) in an acute mouse brain slice model of continuous tissue oxygenation with carbogen (95% O2 and 5% CO2). This system yielded minimal cell lysis (never exceeding 8%), which we concluded to be associated with acute tissue damage induced by slicing, as it occurred immediately during the first minutes after tissue preparation and remained unchanged for at least 12 hours (data not shown). To verify the intact morphology of neurons in these conditions, we also routinely studied their microtubule-associated protein 2 (MAP2) immunostaining, which could indicate mild tissue damage even when no clear lysis increase was observed. The slices survived at least 12 hours without any sign of neurite damage in a buffer with 1 or 2 mM Ca (Figure 4A). Next, we challenged these slices for 4 hours with 0.2 μg/mL PLY and analyzed the amount of acute lysis by assaying the release of LDH. In such conditions, LDH did not increase following toxin exposure, confirming the sublytic nature of the toxin at these concentrations (Figure 4B). As soon as the Ca concentration in the perfusion buffer was reduced from 2 to 1 mM, PLY revealed its lytic capacity, inducing ∼4% higher LDH release above the control levels (Figure 4B). With this data, we proved that toxin concentrations that normally are sublytic in brain tissue can be lytic when extracellular levels of Ca are decreased. Still, our slice system has several characteristics that could ameliorate the effects of PLY such that they are less drastic than those seen in clinical cases of severe pneumococcal meningitis. Specifically, we exposed cells and tissues to PLY in a single sublytic dose. However, during clinical progression of meningitis, it is continuously released for more than 4 hours; other bacterial factors, continuously released in the course of bacterial lysis, such as bacterial CpG-DNA and peroxide, could further compromise the defense capacity of brain tissue [2, 5, 17]. The extracellular concentration of Ca in the human brain, as measured in the CSF, is one-fourth of that in the serum (CSF ∼0.6 mM vs ∼2.4 mM in the plasma [27, 28]). Thus, the true damaging potential of PLY in diseased brains should be higher than that observed in classical experimental cell culture and slice culture conditions. However, the concentration of Ca in the CSF of newborn rats is similar to their plasma levels (∼2 mM) [28], demonstrating an important difference from humans. Thus, researchers have to carefully interpret the experimental evidence of the role of PLY in rodents, humans, and cell culture conditions, considering that, by default, the human brain is likely more vulnerable to the lytic effects of PLY due to a lower Ca content.
Figure 4.
A, Verification of the MAP2 immunostaining of pyramidal neurons in the cortex of mice 8 hours after preparation of acute slices in ACSF with both 1 and 2 mM Ca shows completely intact neurite morphologies. Scale bars: 20 μm. B, LDH release from brain slices, incubated for 4 hours with or without 0.2 μg/mL PLY in the presence of 1 and 2 mM extracellular Ca. * P < .05 vs all, Mann–Whitney U test (see Methods). Values represent mean ± SEM, n = 6 experiments (5 slices/condition/experiment). ACSF, artificial cerebrospinal fluid.
The level of Ca in the CSF of patients with brain infections has not been studied in detail. A mild reduction of Ca was observed in a small study group of patients with various brain infections (including tuberculosis and viral infections). The patients in the group that were comatose had the largest changes in Ca [29]. Much more information is available concerning the level of serum Ca in systemic infections. Hypocalcemia is common in patients with streptococcus group A bacteremia and sepsis, and this correlates with an aggravated course of the disease [30, 31]. It is interesting to note that the group A streptococci also produce a cholesterol-dependent cytolysin (streptolysin O) [32]. Furthermore, reduction of serum Ca also occurs in septic conditions of nonstreptococcus origin [33]. Our work suggests that attention should be paid to maintaining physiological Ca levels in cases where S. pneumoniae is suspected or present.
In conclusion, we provide evidence for the dependence of the lytic capacity of PLY on the extracellular concentration of Ca. Reduction of Ca concentration could have profound effects on PLY neurotoxicity enhancement. Reduction of Ca concentration can transform sublytic pathophysiological concentrations into lytic concentrations, worsening the course of the disease. Unfortunately, there is no systematic evidence for the dynamic changes of Ca concentration in the brain during the clinical course of pneumococcal meningitis. Further work is needed to verify whether these findings might have prognostic and therapeutic importance throughout the course of pneumococcal meningitis.
Funding
The research in Würzburg was supported by the Emmy Noether Programme of the German Science Foundation (DFG; grant IL-151.1 to A. I.); the DFG Sonderforschungsbereich 487 (A5) to R. B.; the Rudolf Virchow Center for Experimental Medicine, Würzburg; and the University of Würzburg. The research in Glasgow was supported by the Wellcome Trust; the Biotechnology and Biological Sciences Research Council (BBSRC); and European Science Foundation.
Acknowledgments
We are grateful to Alexandra Bohl for excellent technical assistance.
References
- 1.Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–8. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 2.Braun JS, Sublett JE, Freyer D, et al. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliev AI, Djannatian JR, Opazo F, et al. Rapid microtubule bundling and stabilization by the Streptococcus pneumoniae neurotoxin pneumolysin in a cholesterol-dependent, non-lytic and Src-kinase dependent manner inhibits intracellular trafficking. Mol Microbiol. 2009;71:461–77. doi: 10.1111/j.1365-2958.2008.06538.x. [DOI] [PubMed] [Google Scholar]
- 4.Iliev AI, Djannatian JR, Nau R, Mitchell TJ, Wouters FS. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc Natl Acad Sci USA. 2007;104:2897–02. doi: 10.1073/pnas.0608213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiss A, Braun JS, Jäger K, et al. Bacterial pore-forming cytolysins induce neuronal damage in a rat model of neonatal meningitis. J Infect Dis. 2011;203:393–400. doi: 10.1093/infdis/jiq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirst RA, Gosai B, Rutman A, et al. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J Infect Dis. 2008;197:744–51. doi: 10.1086/527322. [DOI] [PubMed] [Google Scholar]
- 7.Wellmer A, Zysk G, Gerber J, et al. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect Immun. 2002;70:6504–8. doi: 10.1128/IAI.70.11.6504-6508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spreer A, Kerstan H, Bottcher T, et al. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob Agents Chemother. 2003;47:2649–54. doi: 10.1128/AAC.47.8.2649-2654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stringaris AK, Geisenhainer J, Bergmann F, et al. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol Dis. 2002;11:355–68. doi: 10.1006/nbdi.2002.0561. [DOI] [PubMed] [Google Scholar]
- 10.Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121:247–56. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Korchev YE, Bashford CL, Pasternak CA. Differential sensitivity of pneumolysin-induced channels to gating by divalent cations. J Membr Biol. 1992;127:195–203. doi: 10.1007/BF00231507. [DOI] [PubMed] [Google Scholar]
- 12.Korchev YE, Bashford CL, Pederzolli C, et al. A conserved tryptophan in pneumolysin is a determinant of the characteristics of channels formed by pneumolysin in cells and planar lipid bilayers. Biochem J. 1998;329:571–7. doi: 10.1042/bj3290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fickl H, Cockeran R, Steel HC, et al. Pneumolysin-mediated activation of NFkappaB in human neutrophils is antagonized by docosahexaenoic acid. Clin Exp Immunol. 2005;140:274–81. doi: 10.1111/j.1365-2249.2005.02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco-Vidal V, Beurg M, Darrouzet V, Bebear JP, Skinner LJ, Dulon D. Zinc protection against pneumolysin toxicity on rat cochlear hair cells. Audiol Neuro-otol. 2008;13:65–70. doi: 10.1159/000108763. [DOI] [PubMed] [Google Scholar]
- 15.Somjen GG, Aitken PG, Giacchino JL, McNamara JO. Interstitial ion concentrations and paroxysmal discharges in hippocampal formation and spinal cord. Adv Neurol. 1986;44:663–80. [PubMed] [Google Scholar]
- 16.Douce G, Ross K, Cowan G, Ma J, Mitchell TJ. Novel mucosal vaccines generated by genetic conjugation of heterologous proteins to pneumolysin (PLY) from Streptococcus pneumoniae. Vaccine. 2010;28:3231–7. doi: 10.1016/j.vaccine.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Iliev AI, Stringaris AK, Nau R, Neumann H. Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9) Faseb J. 2004;18:412–4. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- 18.Benz R, Janko K, Boos W, Lauger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978;511:305–19. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- 19.Benz R, Ishii J, Nakae T. Determination of ion permeability through the channels made of porins from the outer membrane of Salmonella typhimurium in lipid bilayer membranes. J Membr Biol. 1980;56:19–29. doi: 10.1007/BF01869348. [DOI] [PubMed] [Google Scholar]
- 20.Förtsch C, Hupp S, Ma J, et al. Changes in astrocyte shape induced by sublytic concentrations of the cholesterol-dependent cytolysin pneumolysin still require pore-forming capacity. Toxins. 2011;3:43–62. doi: 10.3390/toxins3010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beurg M, Hafidi A, Skinner L, et al. The mechanism of pneumolysin-induced cochlear hair cell death in the rat. J Physiol. 2005;568:211–27. doi: 10.1113/jphysiol.2005.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohba S, Hiramatsu M, Edamatsu R, Mori I, Mori A. Metal ions affect neuronal membrane fluidity of rat cerebral cortex. Neurochem Res. 1994;19:237–41. doi: 10.1007/BF00971570. [DOI] [PubMed] [Google Scholar]
- 23.Benz R, Beckers F, Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979;48:181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- 24.Wood WG, Igbavboa U, Rao AM, Schroeder F, Avdulov NA. Cholesterol oxidation reduces Ca(2+)+MG (2+)-ATPase activity, interdigitation, and increases fluidity of brain synaptic plasma membranes. Brain Res. 1995;683:36–42. doi: 10.1016/0006-8993(95)00347-s. [DOI] [PubMed] [Google Scholar]
- 25.Zefirov AL, Abdrakhmanov MM, Mukhamedyarov MA, Grigoryev PN. The role of extracellular calcium in exo- and endocytosis of synaptic vesicles at the frog motor nerve terminals. Neuroscience. 2006;143:905–10. doi: 10.1016/j.neuroscience.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Orlov SN, Aksentsev SL, Kotelevtsev SV. Extracellular calcium is required for the maintenance of plasma membrane integrity in nucleated cells. Cell Calcium. 2005;38:53–7. doi: 10.1016/j.ceca.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Bereczki D, Fekete I, Loof I, et al. Cations of cisternal cerebrospinal fluid in humans and the effect of different doses of nimodipine on CSF calcium after stroke. Clin Neuropharmacol. 2000;23:318–23. doi: 10.1097/00002826-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Jones HC, Keep RF. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol. 1988;402:579–93. doi: 10.1113/jphysiol.1988.sp017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey VK, Parmeswaran M, Soman SD, Dacosta HJ. Altered sensorium in neurological infections and the homeostasis of Mg, Zn, Cu and Ca. Sci Total Environ. 1982;25:277–85. doi: 10.1016/0048-9697(82)90020-1. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Abraham R, Keller N, Vered R, Harel R, Barzilay Z, Paret G. Invasive group A streptococcal infections in a large tertiary center: epidemiology, characteristics and outcome. Infection. 2002;30:81–5. doi: 10.1007/s15010-002-1182-6. [DOI] [PubMed] [Google Scholar]
- 31.Cooper BW, Morganelli E. Group B streptococcal bacteremia in adults at Hartford Hospital 1991–1996. Conn Med. 1998;62:515–7. [PubMed] [Google Scholar]
- 32.Johnson MK, Geoffroy C, Alouf JE. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980;27:97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaloga GP, Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann Intern Med. 1987;107:36–41. doi: 10.7326/0003-4819-107-1-36. [DOI] [PubMed] [Google Scholar]