Abstract
Malaria-specific antibody responses in children often appear to be short-lived but the mechanisms underlying this phenomenon are not well understood. In this study, we investigated the relationship between the B-cell activating factor (BAFF) and its receptors expressed on B cells with antibody responses during and after acute malaria in children. Our results demonstrate that BAFF plasma levels increased during acute malarial disease and reflected disease severity. The expression profiles for BAFF receptors on B cells agreed with rapid activation and differentiation of a proportion of B cells to plasma cells. However, BAFF receptor (BAFF-R) expression was reduced on all peripheral blood B cells during acute infection, but those children with the highest level of BAFF-R expression on B cells maintained schizont-specific immunoglobin G (IgG) over a period of 4 months, indicating that dysregulation of BAFF-R expression on B cells may contribute to short-lived antibody responses to malarial antigens in children. In summary, this study suggests a potential role for BAFF during malaria disease, both as a marker for disease severity and in shaping the differentiation pattern of antigen-specific B cells.
Children living in malaria-endemic areas in sub–Saharan Africa are repeatedly infected with the parasite Plasmodium falciparum and bear the main burden of clinical and often life-threatening severe malarial disease. Clinical immunity to the blood stage of infection is acquired over time, and eventually protects from clinical symptoms but not from infection per se. There is no doubt that antibodies play a critical role in protection from clinical malaria, since the transfer of gamma globulins prepared from immune serum of adults into children with acute malaria resulted in resolution of clinical disease [1, 2]. Many P. falciparum blood-stage antigens are either polymorphic and/or undergo clonal antigenic variation [3], and protection from clinical disease has been associated with the ability to respond to wide variety of polymorphic or variant antigens [4–6]. However, several studies reported that antibody responses to some malarial antigens are short-lived and can be detected only in the presence of parasites, suggesting a defect in the development and or maintenance of long-lived plasma cells [7–9].
Upon activation, B cells can differentiate into short-lived plasma cells [10] or undergo germinal center maturation, emerging as long-lived plasma cells (LLPCs) or long-lived memory B cells (MBCs). Upon reactivation, the latter rapidly differentiate into antibody secreting plasma cells (ASCs) [11]. Only a few studies have analyzed B-cell responses to malarial antigens in exposed children and adults showing that malaria-specific MBCs or antibodies are detectable in a proportion of infected individuals [12–14]. In addition, some studies reported altered distribution of B-cell subsets in children and adults living in malaria-endemic areas, such as an increase in the percentage of transitional B cells, CD38+IgD− MBCs [15], and atypical MBCs [13], suggesting some dysfunction of the B-cell compartment during acute and chronic P. falciparum malaria infection.
The “B-cell activating factor belonging to the tumor necrosis family” (BAFF) is a cytokine critical for the survival and differentiation of B cells throughout their developmental stages. It is expressed as a transmembrane protein on the surface of neutrophils, monocyte/macrophages, and dendritic cells [16, 17], but can be cleaved from the membrane by furin convertase [17, 18]. Both the membrane-bound [16] and soluble forms are active [18] and constitutively expressed in steady state. In humans, BAFF is essential for T-cell–dependent and –independent class switch recombination [19] and supports the survival of plasmablasts, plasma cells [20], and MBCs [21]. It has been suggested that the accumulation of plasma cells after an acute infection is a result of the effect of BAFF on both plasma-cell differentiation and survival.
Three receptors for BAFF are differentially expressed during B-cell differentiation [22]. The BAFF receptor (BAFF-R) is the sole receptor mediating homeostatic B-cell survival. Its expression increases upon B-cell activation, but is replaced by B-cell maturation antigen (BCMA) with differentiation into LLPCs. The transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) is constitutively expressed on a proportion of MBCs and can be induced on activated B cells. TACI can act as a negative regulator of B-cell homeostasis by blocking terminal B-cell differentiation, yet may be important for plasma cell differentiation because TACI-deficient humans suffer from hypergammaglobulinemia of all subclasses [22].
The expression of BAFF is increased in the presence of type I and II interferons (IFNs) and interleukin 10 (IL-10), in autoimmune conditions [23–25], and infectious diseases. Elevated levels of BAFF have been observed in human immunodeficiency virus (HIV)–infected individuals [26] and correlated with disease progression [27] and levels of auto-antibodies [26]. In addition, reduced BAFF-receptor expression on B cells has been associated with reduced B-cell survival in HIV viremic patients [28]. By contrast, BAFF plasma levels have been positively associated with B-cell proliferation in hepatitis C virus infections [29]. A recent study reported that hemozoin and soluble P. falciparum antigen induce secretion of BAFF by monocytes as well as upregulation of BAFF-R on, proliferation, and plasma-blast differentiation of B cells in vitro [30].
In this paper, we have investigated whether BAFF and expression of BAFF-R on B cells could be part of the inflammatory response associated with malaria and thus may offer some explanation for the altered B-cell populations and apparent short-lived nature of some antibody responses. We report here that BAFF plasma levels increased during acute disease and reflected disease severity. The expression profiles for BAFF-R on B cells indicated rapid activation and differentiation of a proportion of B cells to plasma blasts following infection. However BAFF-R expression was reduced on all peripheral blood B cells but directly correlated with schizont-specific immunoglobin G (IgG) after the acute malaria episode.
PATIENTS, MATERIALS, AND METHODS
Study Population and Blood Collection
The study was carried out at the Kilifi District Hospital (KDH) situated 60 km north of Mombasa. Malariometric parameters for this area have been described elsewhere [31, 32]. Children under the age of 10 years presenting at the Kilifi District Hospital (KDH) with a primary diagnosis of malaria were recruited between 2006 and 2008 after their guardian provided informed written consent. Children were classified into those with mild disease (fever >37.5°C and a blood film positive for P. falciparum parasites), those with nonsevere malaria (admitted to KDH but no signs of severe life-threatening disease), and those with severe malaria (admitted to KDH and suffering from at least 1 severe syndrome: impaired consciousness [Blantyre Coma Score <5], severe respiratory distress or severe anemia [hemoglobin <5 g/L] [33]). At presentation, 3 mL blood was drawn and children invited to donate additional 5 mL blood samples 4 and 16 weeks after admission. During follow-up visits, children were clinically examined and treated if necessary. A 5 mL venous blood sample was obtained from healthy children <10 years of age during the cross-sectional survey of children under active surveillance for malaria in May 2008.
All blood samples were centrifuged, plasma removed, and stored at −80°C for later cytokine detection. Peripheral blood mononuclear cells (PBMCs) were obtained after Ficoll-Paque gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway). PBMCs were washed, counted, and stored in liquid nitrogen until use.
The study was approved by the Kenyan Medical Research Institute, Ethical Review Committee under the SSC protocol number 1131, and the Oxford Tropical Research Ethics Committee, protocol number 30-06.
Flow Cytometry
PBMCs were thawed and viable cells counted using trypan blue exclusion. The expression of BAFF-R on B cells was determined by staining 1 × 105 viable PBMCs with CD19-ECD, CD27-PC5 (Beckman Coulter), CD38-PECY7 (eBiosciences), and either CD237-FITC (BAFF-R/BR3), CD267-PE (BD Biosciences), or polyclonal antihuman BCMA-PE (R&D Systems). Cells were incubated with antibody cocktails for 30 minutes in the dark, washed, and acquired on a CyAN ADP analyzer (Beckman Coulter). Data were analyzed using FlowJo Africa (kindly donated by FlowJo Treestar). The absolute number of B cells was calculated using differential whole blood counts.
Enzyme-Linked Immunosorbent Assay
IL-10, interferon γ (IFNγ), and BAFF levels in plasma were detected using OptEIA Human IL-10 enzyme-linked immunosorbent assay (ELISA), OptEIA Human IFNγ ELISA set (BD Biosciences), and Quantikine Human BAFF Immunoassay kit (R&D systems), respectively, according to the manufacturers’ instructions. Schizont-specific antibodies were measured as described previously [34]. For the determination of total immunoglobin M (IgM) and IgG in plasma, Nunc Maxisorb plates were coated with 10 ng/mL polyclonal rabbit antihuman IgM or IgG (Sigma-Aldrich) and detected with POD-conjugated donkey antihuman IgM5Fcμ and IgG(Fab)2 fragment, respectively (Jackson Immuno Research). Standard curves were generated using purified human IgM and IgG (both Sigma-Aldrich).
Statistical Analysis
The Kruskal-Wallis test was used to compare distribution of parameters among different disease categories. Continuous variables were compared using the Mann-Whitney test and Wilcoxon rank sum test for unpaired and paired samples, respectively. Correlations among different parameters were analyzed using the Spearman rank correlation coefficient and a prediction logistic model to test if the changes in BAFF plasma concentration could predict if disease was employed. Statistical analysis was performed using Stata version 11.0, and all tests were 2-tailed, with P < .05 considered statistically significant.
RESULTS
BAFF Plasma Levels Increase With Malaria Disease Severity
We determined the plasma concentration of BAFF in 117 children with acute malaria and 54 healthy children. Children with severe malaria tended to be younger and had higher parasite load than children with nonsevere or mild malaria, but differences were not statistically significant. They also had significantly higher white blood cell (WBC) counts than healthy children, and all children suffering from acute malaria showed reduced red blood cell (RBC) counts and hemoglobin concentration (Table 1).
Table 1.
Baseline Parameters of Healthy Controls and Study Population at Presentation With Malaria
| Parameters | Healthy childrenan = 54 | Mild malariaan = 47 | Nonsevere admissionsan = 23 | Severe admissionsan = 47 |
| Age (months) | 58.6 (22.5–87.9) | 43.1 (27.8–67.9) | 47.0 (33.5–55.9) | 38.2 (25.9–50.3) |
| Parasites (103/μL) | … | 30.4 (27.5–145.5) | 200.0 (116.0–439.4) | 204.1 (113.6–331.5) |
| WBC count (103/μL) | 6.8 (5.7–9.8) | 7 (4.6–9.5) | 9.1 (5.6–12.9) | 9.8 (7.7–11.9)b |
| RBC count 106/μL | 4.5 (4.1–4.9) | 3.5 (2.5–4.3)b | 3.9 (3.5–4.3)b | 3.4 (2.5–3.9)b |
| Hb conc. (mg/dL) | 10.6 (9.9–11.1) | 7.6 (6.3–10)b | 9.2 (7.2–10.4)b | 7.3 (5.8–9.4)b |
| IL-10 (pg/mL) | 0.67 (0–14.1) | 511.0 (156–1298)b | 746.3 (269–1867)b | 908.9 (268–1412)b |
| IFNγ (pg/mL) | 0 (0–3.8) | 8.1 (0–27.4)b | 8.4 (0–110)b | 10.7 (0–51.9)b |
NOTE. Hb, hemoglobin; IFNγ, interferon γ; IL-10, interleukin 10; RBC, red blood cells; WBC: white blood cells.
Values are presented as median (25th and 75th percentile).
P < .05 Mann-Whitney U tests.
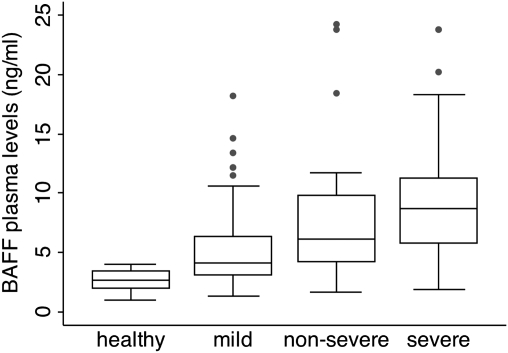
The median plasma concentration of BAFF during acute malaria was 5.8 ng/mL (interquartile range [IQR] 3.9–9.5), significantly higher than the 2.6 ng/mL (IQR 2.0–3.5) observed in healthy controls (Mann-Whitney test, P = .0001). Interestingly, there was a significant progressive increase in BAFF concentration with disease severity (Kruskal-Wallis test, P = .0001) (Figure 1). Indeed, children whose BAFF plasma levels were above the median BAFF plasma level were at a higher risk of being admitted to hospital either with nonsevere or severe malaria (odds ratio [OR] = 5.44, P < .0001, χ2 test).
Figure 1.
The plasma concentration of BAFF increases with increasing disease severity in children suffering from acute malaria. Box-and-whisker plot of the median BAFF plasma concentration in healthy children and those suffering from either mild, nonsevere, or severe, life-threatening malaria. Boxes show the 25th and 75th percentile, and whiskers present the 5th and 95th percentile. Circles denote outliers.
To determine whether the increased plasma concentration of BAFF was associated with increased levels of IL-10 and IFNγ, we analyzed their concentration in plasma of all children (Table 1). As expected, both cytokines were elevated in children suffering from acute malaria compared with healthy children (Mann-Whitney test, P = .0001, for both cytokines) but did not show the same progressive increase with disease severity as observed with BAFF (Kruskal-Wallis, P > .05, for each case). BAFF plasma levels directly correlated with IFNγ (Spearman ρ = 0.36, P = .0001) and IL-10 plasma levels (ρ = 0.44, P = .013). Parasitemia correlated with the plasma concentration of IL-10 (ρ = 0.331, P = .002) but not IFNγ (ρ = 0.042, P = .704) or BAFF (ρ = 0.008, P = .938).
Expression of BAFF Receptors on B-cell Subsets Is Altered During Malaria Disease
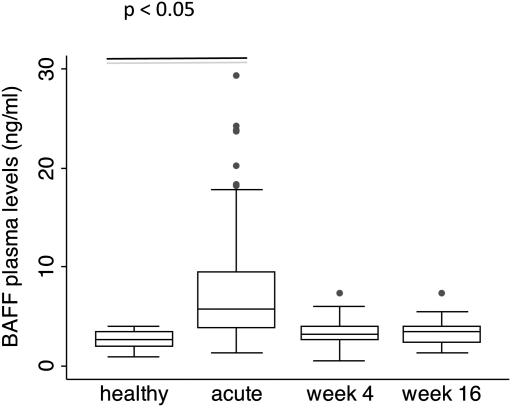
We determined the expression levels of BAFF-R, TACI, and BCMA on cryopreserved PBMCs from a subset of 30 children who returned for their follow-up visit 4 and 16 weeks after an acute malaria episode, and 9 healthy children. The clinical parameters and cytokine concentrations in plasma in this subset of children was comparable to those of the wider group described above. The percentage of B cells and the absolute number of B cells in blood were significantly increased in children suffering from acute malaria and during follow up compared with healthy children (Table 2). During follow up, the plasma concentration of BAFF in children who suffered from acute malaria returned to concentrations comparable to those observed in healthy children, with no significant differences between either of the follow-up visits and healthy children (Figure 2).
Table 2.
Baseline Characteristics of the Subset of Children Who Returned for Follow Up
| Parameters | Healthy childrenan = 9 | Acutean = 30 | Week 4an = 30 | Week 16an = 30 |
| Age (months) | 84.7 (52.9–100.3) | 51.8 (41.1–65.9) | 52.8 (42.8–66.9) | 55.8 (45.8–69.9) |
| WBC count (103/μL) | 6.8 (5.7–9.8) | 8.05 (5.1–10.6) | 8.1 (6.7–9.4) | 8.6 (7.4–9.8) |
| RBC count (106/μL) | 4.7 (4.2–5.4) | 3.5 (2.5–4.1)b | 4.4 (4.1–4.9) | 4.7 (4.4–5.2) |
| Hb conc. (mg/dL) | 11.0 (10–11.2) | 7.8 (6.0–9.2)b | 10.3 (9.8–11.3) | 10.5 (9.8–11.9) |
| B cells (% PBMC) | 8.9 (7.7–12.8) | 19 (13.2–26.5)b | 17.7 (12.8–21.9)b | 18 (13.5–22)b |
| B cells (103/μL) | 0.28 (0.21–0.35) | 1.8 (0.7–2.8)b | 1.2 (0.95–2.0)b | 1.3 (0.9–2.0)b |
| BAFF (ng/mL) | 3.5 (3.1–3.9) | 5 (4.1–9.1)b | 3.3 (2.6–4.2) | 3.5 (2.3–4.0) |
| IL-10 (pg/mL) | 0 | 721 (491–1368)b | … | … |
| IFNγ (pg/mL) | 0 | 4.7 (0–14.9)b | … | … |
NOTE. BAFF, B-cell activating factor; Hb, hemoglobin; IFNγ, interferon γ; IL-10, interleukin 10; PBMC, peripheral blood mononuclear cell; RBC, red blood cell; WBC, white blood cell.
Values are presented as median (25th and 75th percentile).
P < .05 Mann-Whitney U tests.
Figure 2.
The plasma concentration of BAFF is increased only during acute disease. Median BAFF plasma concentration determined in healthy children and children suffering from acute malaria (acute) and 4 weeks (week 4) and 16 weeks (week 16) after the acute episode. Boxes show the 25th and 75th percentile, and whiskers present the 5th and 95th percentile. Circles denote outliers.
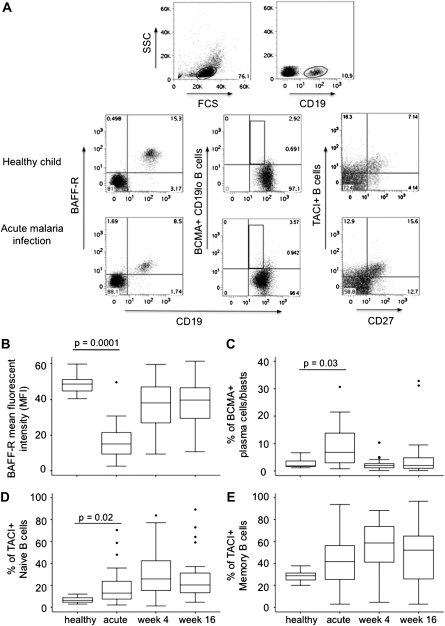
The BAFF-R expression on mature B cells was significantly decreased on all B cells from children suffering from malaria compared with healthy controls (Mann-Whitney test, P = .0001) (Figure 3B), but during follow up, BAFF-R expression levels on B cells had returned to a level comparable to those of healthy children. We observed a significant increase in the proportion of BCMA-positive plasma cells during acute disease compared with healthy children (Mann-Whitney test, P = .003) which returned to levels similar to those in healthy children by weeks 4 and 16 of follow up (Figure 3C). The percentage of naive B cells expressing TACI significantly increased during acute disease compared with healthy children (Mann-Whitney test, P = .02; Figure 3D). Although not significant, a similar trend was observed for the proportion of TACI-positive memory B cells (Mann-Whitney U test, P = .07; Figure 3E). Interestingly, the proportion of TACI-positive B cells continued to increase for both naive and memory B cells over the follow-up period, and were maintained at a significantly higher level than that observed in healthy children (Mann-Whitney test, P < .03 for both naive and memory B cells). We sought to determine if observed changes in expression of BAFF-R were associated with the plasma concentration of BAFF. At acute disease, the increase in BAFF plasma levels significantly correlated with a reduction of BAFF-R expression on B cells (ρ = −0.52, P = .003). Interestingly, BAFF-R expression on B cells was also inversely correlated with the plasma concentration of IFNγ (ρ = –0.56, P = .002) but not with IL-10. It is likely that reduced BAFF-R expression indicated the rapid activation and differentiation of some B cells into long-lived plasma cells. Indeed, we observed a significant inverse correlation between BAFF-R expression and the percentage of BCMA+ B cells (ρ = −0.57, P = .002) but not with the percentage of TACI+ memory B cells during acute disease.
Figure 3.
Expression of BAFF receptors on B cells in children with acute malaria and during follow up. CD19-positive B cells were identified in the lymphocyte gate. A, Representative dot plot of BAFF-R-positive CD19-positive B cells, BCMA-positive CD19-low, and TACI-positive naive (CD27-) or memory B cells (CD27+) of 1 healthy child and 1 child suffering from acute malaria. Box plot of MFI of BAFF-R B, the proportion of BCMA-positive CD19-low B cells C, and TACI-positive naive D, and memory E B cells in healthy children and children with malaria during acute disease and week 4 and week 16 follow up. Boxes show the 25th and 75th percentile, and whiskers present the 5th and 95th percentile. Circles denote outliers.
Expression of BAFF-R Is Correlated With the Maintenance of Schizont-Specific Antibodies
Protection against malarial disease is associated with the induction and maintenance of antibody responses to a wide variety of malarial antigens. To determine whether the plasma concentration of BAFF or BAFF-R expression on B cells was associated with the induction or maintenance of antibodies, we measured total and schizont-specific IgM and IgG antibody concentrations in plasma. The plasma concentration of total IgM but not IgG was significantly lower in children with acute malaria at acute disease and at both follow-up time points compared with healthy controls. As expected, schizont-specific IgM responses were significantly higher at acute disease and dropped to levels similar to those observed in healthy children by week 4 of follow up (Table 3). The concentration of schizont-specific IgG was similar to that in healthy children during acute malaria and at week 4 of follow up, but decreased by week 16 of follow up.
Table 3.
Total and Schizont-Specific IgM and IgG Schizont in Plasma
| Healthy childrenan = 8 | Acutean = 30 | Week 4an = 30 | Week 16an = 30 | |
| IgM (ng/mL) | 253 (182–278) | 158 (94–257)b | 144 (41–216)b | 168 (57–201)b |
| IgG (ng/mL) | 1051 (861–1061) | 966 (905–1090) | 980 (870–1031) | 932 (881–995) |
| IgM schizont (OD) | 0.06 (0.05–0.13) | 0.16 (0.09–0.34)b | 0.08 (0.06–0.17) | 0.05 (0.03–0.11) |
| IgG schizont (OD) | 0.21 (0.06–0.37) | 0.17 (0.10–0.38) | 0.21 (0.11–0.34) | 0.10 (0.04–0.25) |
NOTE. IgG, immunoglobin G; IgM, immunoglobulin M; OD, optical density.
Values are presented as median (25th and 75th percentile).
P < .05 Mann-Whitney U tests.
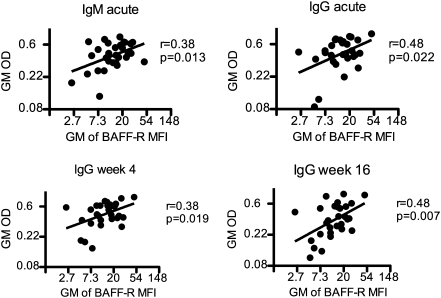
We further analyzed if the changes observed in BAFF plasma concentrations or expression of BAFF-R on B cells during acute disease correlated with total or schizont-specific antibody responses during acute disease and follow up. The plasma concentrations of total IgG and IgM were not correlated with the plasma concentration of BAFF or BAFF-R expression on B cells. By contrast, during acute disease, IgM and IgG schizont-specific responses significantly correlated with BAFF-R expression on B cells after normalization of values by log-transformation (Figure 4) and was maintained over the period of follow up, indicating that maintenance of BAFF-R expression is important for the maintenance of antigen-specific antibody responses.
Figure 4.
The expression of BAFF-R on B cells correlates with schizont-specific IgM and IgG during acute malaria and follow up. Shown are scatter plots of log-transformed OD values for schizont-specific IgM and IgG during acute malaria and week 4 and week 16 follow up. OD, optical density; GM, geometric mean.
DISCUSSION
Given the role of BAFF in differentiation, survival, and function of mature B cells, we investigated whether short-lived antibody responses to malarial antigens observed in some children may be due to dysregulation of BAFF or expression of its receptors on B cells.
Our results demonstrate that BAFF plasma levels increased during acute malaria but returned to normal levels within 4 weeks of an acute malaria episode. Noticeably, the plasma concentration of BAFF reflected disease severity more closely than either IFNγ or IL-10, independent of parasitemia. Both IL-10 and IFNγ can induce BAFF secretion by myeloid cells, and their plasma concentration correlated with the plasma concentration of BAFF. BAFF acts on dendritic cells, probably in an autocrine manner, and induces their maturation and secretion of interleukin 12 (IL-12), supporting the development of T-helper 1 (Th1) T-cell responses [35]. Thus, BAFF may play an important role in the inflammatory response and contribute to inflammation-associated immunopathology during acute malaria.
The plasma concentration of BAFF is upregulated in many autoimmune conditions [23–25] and in some infections that present with polyclonal activation of B cells, hypergammaglobulinemia, and autoimmune-like syndromes [26, 27]. Interestingly, we did not observe a correlation between the plasma concentration of BAFF and total or schizont-specific antibody concentrations during acute disease or during follow up. However, several studies reported overall higher levels of auto-antibodies in people living in malaria-endemic areas and linked this observation to repeated immune activation with frequent malaria episodes [36]. It seems possible that repeatedly elevated levels of BAFF during acute malaria episodes contribute to a rescue of auto-reactive B cells in people living in malaria-endemic areas [37–39].
BAFF is detected in plasma of all individuals and constitutively occupies BAFF-R expressed on all resting mature B cells. Upon B-cell receptor (BCR) activation in a T-cell–dependent manner, BAFF enhanced B-cell proliferation, differentiation into plasma cells, and the secretion of immunoglobulin [19, 21]. In contrast, activation of B cells in a T-cell-independent manner in the presence of BAFF attenuates differentiation to plasma cells [40]. B cells that differentiate into plasma cells downregulate expression of BAFF-R and upregulate expression of BCMA, a BAFF receptor exclusively expressed on long-lived plasma cells [21, 41]. The role of TACI is less well understood. TACI is expressed on a proportion of MBCs and also an early marker of B-cell activation [41]. Expression of TACI is upregulated upon engagement of Toll-like receptor 9 (TLR9), CD40, or the BCR on B cells, and some studies have suggested that it is indispensable for the differentiation of B cells into plasma cells and class switch recombination in response to T-independent antigens [42, 43] and to a lesser extend for T-dependent antigens [44, 45].
In our study, all peripheral B cells from healthy children expressed BAFF-R in line with previous reports [41, 46]. However, BAFF-R expression was significantly downregulated on all B cells of children during acute malaria. The reduction in BAFF-R expression was transient, and all children exhibited normal expression levels of BAFF-R 4 weeks and 4 months after the acute malaria episode. Kumsiri et al. [30] reported that BAFF-R expression is upregulated on B cells in vitro in response to hemozoin or P. falciparum–soluble antigen. These observations are not necessarily contradictory, since children with acute malaria will have been infected with asexual blood-stage parasites for several days before developing clinical symptoms, and BAFF-R expression on B cells might increase during the early phase of blood stage infection. It is likely that B-cell activation during acute infection resulted in reduced BAFF-R expression due to B-cell differentiation into plasma cells accompanied by increased BCMA expression, at least for a proportion of B cells [41]. Indeed, we observed a significant inverse correlation between BAFF-R expression and the proportion of BCMA-positive B cells, indicating that long-lived plasma cells are induced during an acute malaria episode. However, only a small proportion of peripheral blood B cells differentiated into plasma cells whereas the reduction in BAFF-R expression was observed on the entire population of B cells, suggesting either internalization or shedding of BAFF-R in the presence of high and sustained levels of BAFF [40], as has been described for patients with Sjogren’s syndrome and systemic lupus erythematosus [47]. Whether the reduction of BAFF-R expression on B cells during acute malaria is a normal homeostatic response to high concentrations of BAFF or indicative of a pathological mechanism or both requires further investigation. However, those children suffering from acute malaria who maintained the highest expression levels of BAFF-R on B cells showed significantly higher levels of antischizont IgG 4 weeks and 4 months after the acute malaria episode, suggesting that the overall reduction of BAFF-R expression on all B cells renders at least a proportion of them insensitive to the action of BAFF. In this context, it is noteworthy that TLR9 agonists such as CpG reduces the expression of BAFF-R but increases the expression of TACI on B cells [41]. In the presence of high concentrations of BAFF, TLR9 agonists attenuate the differentiation of memory B cells into antibody-secreting plasma cells [40]. Interestingly, the percentage of TACI-positive naive and memory B cells increased during acute malaria and was maintained over the entire 4 months of follow up. The proportion of TACI-positive naive or memory B cells and the plasma concentration of BAFF or reduced expression of the BAFF-R in children suffering from acute malaria did not correlate, suggesting that the induction of TACI on B cells was independently regulated. Parasite DNA complexed by polycationic carriers or hemozoin is a potent TLR9 agonist [48, 49], although the latter remains controversial. Whether parasite DNA activates B cells directly has not been investigated. At least during time of acute disease, when the concentration of BAFF is high, DNA from parasites may provide a constant trigger for TLR9-induced TACI expression on B cells and attenuate their differentiation into antibody-secreting plasma cells.
The effect of blockade of BAFF by a monoclonal anti-BAFF IgG and BAFF-R Ig-fusion protein have been tested in clinical trials for treatment of systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), and ameliorated disease activity [50]. Blocking BAFF during acute severe malarial disease may interrupt positive feedback between IFNγ and BAFF and thus reduce inflammation while facilitating the induction of long-lived plasma cells. However, a better understanding of the action of BAFF during acute malaria is required before its therapeutic use can be considered.
In summary, we show that the concentration of BAFF is increased during acute malaria and reflects disease severity. In addition, we observed dynamic regulation of the expression of the 3 BAFF receptors (BAFF-R, BCMA, and TACI) on B cells during acute malaria and follow up. It is likely that a proportion of B cells received T cell help and differentiated into BCMA-expressing plasma cells. However, the reduction of the BAFF-R on all peripheral blood B cells and the parallel increase of TACI-positive B cells indicated that the regulation of BAFF receptor expression during B-cell differentiation may be altered, rendering a proportion of B cells insensitive to BAFF. Further studies are necessary to identify whether parasite DNA could engage TLR9 expressed on activated MBCs and inhibit their differentiation to plasma cells in the presence of BAFF.
Funding
This work was supported by the European Commission (BioMalPar; contract no LSPH-CT-2004-503578). E. N. received a BioMalPar PhD studentship, and B. C. U. is a Wellcome Trust Senior Research Fellow in Biomedical Science (grant number 079082). J. L. is funded by the Medical Research Council, UK (U117584248).
Acknowledgments
This study is published with permission of the Director of the Kenya Medical Research Institute (KEMRI). We thank the children and their guardians for participation in the study, and community-based fieldworkers and hospital staff for their dedicated work with patients and study participants.
References
- 1.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 2.McGregor IA. The Passive transfer of human malarial immunity. Am J Trop Med Hyg. 1964;13:S237–9. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- 3.Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annu Rev Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- 4.Riley EM, Allen SJ, Wheeler JG, et al. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–37. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 5.Egan AF, Morris J, Barnish G, et al. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–9. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 6.Metzger WG, Okenu DM, Cavanagh DR, et al. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 2003;25:307–12. doi: 10.1046/j.1365-3024.2003.00636.x. [DOI] [PubMed] [Google Scholar]
- 7.Bull PC, Lowe BS, Kaleli N, et al. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J Infect Dis. 2002;185:1688–91. doi: 10.1086/340420. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh DR, Elhassan IM, Roper C, et al. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–59. [PubMed] [Google Scholar]
- 9.Fruh K, Doumbo O, Muller HM, et al. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991;59:1319–24. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLennan IC, Toellner KM, Cunningham AF, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 11.Arpin C, Banchereau J, Liu YJ. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med. 1997;186:931–40. doi: 10.1084/jem.186.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman JR, Bejon P, Ndungu FM, et al. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis. 2005;191:1623–30. doi: 10.1086/429671. [DOI] [PubMed] [Google Scholar]
- 13.Weiss GE, Crompton PD, Li S, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–82. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wipasa J, Suphavilai C, Okell LC, et al. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asito AS, Moormann AM, Kiprotich C, Ng'ang'a ZW, Ploutz-Snyder R, Rochford R. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J. 2008;7:238. doi: 10.1186/1475-2875-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardelli B, Belvedere O, Roschke V, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 17.Scapini P, Nardelli B, Nadali G, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–97. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 23.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–9. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 26.Stohl W, Cheema GS, Briggs WS, et al. B lymphocyte stimulator protein-associated increase in circulating autoantibody levels may require CD4+ T cells: lessons from HIV-infected patients. Clin Immunol. 2002;104:115–22. doi: 10.1006/clim.2002.5238. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS. 2003;17:1983–5. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 28.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–99. [PubMed] [Google Scholar]
- 29.Sene D, Limal N, Ghillani-Dalbin P, Saadoun D, Piette JC, Cacoub P. Hepatitis C virus-associated B-cell proliferation—the role of serum B lymphocyte stimulator (BLyS/BAFF) Rheumatology (Oxford) 2007;46:65–9. doi: 10.1093/rheumatology/kel177. [DOI] [PubMed] [Google Scholar]
- 30.Kumsiri R, Potup P, Chotivanich K, Petmitr S, Kalambaheti T, Maneerat Y. Blood stage Plasmodium falciparum antigens induce T cell independent immunoglobulin production via B cell activation factor of the TNF family (BAFF) pathway. Acta Trop. 2010;116:217–26. doi: 10.1016/j.actatropica.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Mbogo CN, Snow RW, Khamala CP, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am J Trop Med Hyg. 1995;52:201–6. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- 32.Okiro EA, Hay SI, Gikandi PW, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 34.Ndungu FM, Bull PC, Ross A, Lowe BS, Kabiru E, Marsh K. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 2002;24:77–82. doi: 10.1046/j.0141-9838.2001.00440.x. [DOI] [PubMed] [Google Scholar]
- 35.Chang SK, Mihalcik SA, Jelinek DF. B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol. 2008;180:7394–403. doi: 10.4049/jimmunol.180.11.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniel-Ribeiro CT, Zanini G. Autoimmunity and malaria: what are they doing together? Acta Trop. 2000;76:205–21. doi: 10.1016/s0001-706x(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 37.Abele DC, Tobie JE, Hill GJ, Contacos PG, Evans CB. Alterations in serum proteins and 19s antibody production during the course of induced malarial infections in man. Am J Trop Med Hyg. 1965;14:191–7. doi: 10.4269/ajtmh.1965.14.191. [DOI] [PubMed] [Google Scholar]
- 38.Banic DM, Viana-Martins FS, De Souza JM, Peixoto TD, Daniel-Ribeiro C. Polyclonal B-lymphocyte stimulation in human malaria and its association with ongoing parasitemia. Am J Trop Med Hyg. 1991;44:571–7. doi: 10.4269/ajtmh.1991.44.571. [DOI] [PubMed] [Google Scholar]
- 39.Donati D, Zhang LP, Chen Q, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun. 2004;72:5412–8. doi: 10.1128/IAI.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J Immunol. 2007;178:5612–22. doi: 10.4049/jimmunol.178.9.5612. [DOI] [PubMed] [Google Scholar]
- 41.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179:7276–86. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 42.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–95. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 43.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–8. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 44.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–91. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakurai D, Kanno Y, Hase H, Kojima H, Okumura K, Kobata T. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur J Immunol. 2007;37:110–8. doi: 10.1002/eji.200636623. [DOI] [PubMed] [Google Scholar]
- 46.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–8. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sellam J, Miceli-Richard C, Gottenberg JE, et al. Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjogren's syndrome and systemic lupus erythematosus. Ann Rheum Dis. 2007;66:790–7. doi: 10.1136/ard.2006.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coban C, Ishii KJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol. 2010;184:4338–48. doi: 10.4049/jimmunol.0903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009;158:155–63. doi: 10.1111/j.1365-2249.2009.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]