Abstract
Background. Although pregnant women are at increased risk of severe illness following influenza infection, there is relatively little information on the immunogenicity of influenza vaccines administered during pregnancy.
Methods. We conducted a clinical trial that enrolled 120 pregnant women in which participants were randomly assigned to receive an inactivated 2009 H1N1 influenza vaccine containing either 25 μg or 49 μg of hemagglutinin (HA) in a 2-dose series with a 21-day period between administration of the first and second doses.
Results. Following the first vaccination, HA inhibition (HAI) titers of ≥1:40 were detected in 93% (95% confidence interval [CI], 82%–98%) of subjects who received the 25-μg dose and 97% (95% CI, 88%–100%) of subjects receiving the 49-μg dose. In cord blood samples, HAI titers of ≥1:40 were found in 87% (95% CI, 73%–96%) of samples from the 25-μg dose group and in 89% (95% CI, 76%–96%) from the 49-μg dose group. Microneutralization titers tended to be higher than HAI titers, but the patterns of response were similar.
Conclusions. In pregnant women, 1 dose of an inactivated 2009 H1N1 influenza vaccine containing 25 μg of HA elicited an antibody response typically associated with protection against influenza infection. Efficient transplacental transfer of antibody was also documented.
Pregnant women are at increased risk of severe illness following infection with any influenza virus [1–3], but the risk is particularly great following infection due to the pandemic 2009 H1N1 influenza virus [4–19]. Reports from the 2009 pandemic indicate that pregnant women experienced an increased risk of hospitalization, were more likely to be admitted to the intensive care unit, and experienced increased mortality associated with 2009 H1N1 influenza infection, compared with nonpregnant women of similar age. Infection with the pandemic virus was also associated with neonatal complications, such as premature birth and adverse neonatal outcomes [11, 12, 20]. Pregnant women, who are routinely recommended to receive seasonal influenza vaccines, were targeted for priority receipt of monovalent 2009 influenza A (H1N1) vaccine during the 2009 H1N1 influenza pandemic [21, 22]. In addition, the possibility of continued or resurgent pandemic viral transmission led to the inclusion of the 2009 H1N1 influenza strain in the 2010–2011 trivalent seasonal influenza vaccine [23, 24].
Several clinical trials have shown that a single dose of an inactivated, unadjuvanted 2009 H1N1 influenza vaccine is adequately immunogenic in nonpregnant adults [25–29] but, to our knowledge, there are no reported studies of these vaccines in pregnant women. Pregnancy is associated with immunologic changes, including the development of tolerance to foreign antigens and a decrease in total circulating immunoglobulin levels, which could decrease the immune response to vaccines [30–33]. Thus, immune responses to 2009 influenza A (H1N1) vaccines in nonpregnant adults may not predict responses in pregnant women.
To evaluate the immunogenicity of an inactivated pandemic H1N1 influenza vaccine in pregnant women, we conducted a prospective clinical trial in which pregnant women were randomized to receive 2 doses of 2009 H1N1 influenza vaccine containing either 25 μg or 49 μg of hemagglutinin (HA). To evaluate the immune responses to the vaccinations, blood samples were obtained prior to vaccination and at 21 days after each vaccination. To evaluate transplacental transfer of antibodies, maternal and cord blood samples were obtained at the time of delivery.
METHODS
Vaccine
The study vaccine was a monovalent, unadjuvanted, inactivated, subvirion, preservative-free preparation of the New York Medical College X-179A reassortant of the A/California/07/2009 H1N1 and PR8 strains recommended for use in pandemic vaccine development by the World Health Organization [34]. Seed virus was propagated in embryonated chicken eggs, inactivated, and split in accordance with the process used by the manufacturer (Sanofi Pasteur) to produce licensed seasonal influenza vaccine.
The study was designed to evaluate vaccine doses of 15 μg and 30 μg of HA. Prior to release of the clinical lots for the trial, potency testing performed using a high-performance liquid chromatography (HPLC) assay estimated an HA concentration of 30 μg per mL; based on that information, injected volumes of 0.5 mL or 1.0 mL were administered to study participants randomized to the 15-μg or 30-μg dose groups, respectively. The HPLC assay was used instead of the standard single radial immunodiffusion (SRID) potency assay, because calibration reagents needed for the SRID assay were unavailable at the time the clinical lots were formulated. Subsequent retesting of the clinical lots with the standard SRID assay showed the actual HA content of the study vaccine to be 25 μg and 49 μg for volumes of 0.5 mL and 1.0 mL, respectively.
Study Design
Pregnant women 18–39 years of age who were in their second or third trimester (14–34 weeks gestation) were screened for eligibility and provided written informed consent (for eligibility criteria, see the Supplementary Appendix). At enrollment, participants were asked about prior receipt of the 2008–2009 and the 2009–2010 seasonal influenza vaccines. Women who had received the inactivated 2009–2010 seasonal influenza vaccine were eligible for enrollment if at least 2 weeks had elapsed since administration of that vaccine.
Eligible subjects were randomly assigned with equal probability to receive either the lower dose (0.5 mL) or the higher dose (1.0 mL) of study vaccine. The second dose of the same vaccine was given 21 days after the first. Vaccinations were given intramuscularly in the deltoid by a member of the study team who was not involved in the subsequent assessment of adverse events, and the contents of the syringe were shielded, to the extent possible, from the subject’s view.
Following each vaccination, subjects were provided with a memory aid to record the presence and severity of local signs and symptoms (pain, tenderness, redness, and swelling), systemic symptoms (feverishness, malaise, myalgia, headache, and nausea), and oral temperature on the evening of vaccination and for the next 7 days. Participants were instructed to grade reported symptoms as mild if they did not interfere with daily activities, moderate if they resulted in some interference with daily activities, and severe if they prevented subjects from engaging in daily activities. Pain that did not interfere with normal activities but required the use of pain medications was defined as moderate. Unsolicited adverse events were collected through 21 days after the second vaccination and serious adverse events (SAEs), which included pregnancy outcomes, complications of labor and delivery, and neonatal outcomes, were collected through the last follow-up visit at 201 days after enrollment.
Blood samples were obtained before the first vaccination (baseline), before the second vaccination (21 days after the first vaccination), and at 21 days after the second vaccination. Another blood sample was obtained from the participant during the delivery hospitalization but prior to delivery, and a cord blood sample was obtained at the time of delivery.
Laboratory Assays
Microneutralization (MN) and hemagglutination inhibition (HAI) assays were performed at the Southern Research Institute laboratory using the A/California/07/2009 (H1N1) influenza virus and according to established procedures [35, 36]. The HAI assay is the most commonly used assay to assess the immune response to influenza vaccines and was used in part to have the ability to compare results with trials of influenza vaccines in healthy young adults. The microneutralization assay was used to assess the function of the antibody generated in response to vaccination. The HAI assays were performed using turkey erythrocytes with removal of nonspecific inhibitors of agglutination using receptor-destroying enzyme. The serum samples were tested at an initial dilution of 1:10, and laboratory personnel were blinded to sample identity.
Statistical Analysis
The 2 coprimary immunologic end points were defined at day 21 following the first vaccination and included the proportion of subjects who had an HAI titer of ≥1:40 and the proportion of subjects who met the definition of seroconversion by HAI (either a ≥4-fold increase in titer from baseline or a postvaccination titer ≥1:40 if the baseline titer was <1:10). The endpoints were also evaluated for titers determined by the MN assay. For analyses of the results of both assays, titers below the limit of detection were assigned a value of 5. Exact (Clopper-Pearson) confidence intervals (CIs) are reported for the endpoints of postvaccination titer ≥1:40 and the proportion of subjects who met the definition of seroconversion. Comparisons of proportions between groups were performed using Fisher’s exact test.
Geometric mean titers (GMTs) were calculated by transforming data to log scale for all computations and comparisons and transforming these results back to the original scale. Comparisons between groups were performed with the use of the t test. Unadjusted P values are reported if <.05, which indicated statistical significance. P values >.05 are not reported.
The sample size of 60 subjects per study group was selected to provide information on the dose-related immune response in a timely fashion. This sample size was not based on formal power calculations because the study was not designed to test a specific null hypothesis. However, the study was designed to generate descriptive data supportive of the hypothesis that the H1N1 inactivated influenza vaccine would be well tolerated and would elicit adequate immune responses among pregnant women. The sample size was also based on logistical considerations, such as the ability to complete recruitment in a timely fashion and a review of the precision of resulting estimates based on a range of likely outcomes. Power computations performed a priori determined that the sample size of 60 subjects per group yielded 80% power (with alpha of 0.05) to detect a difference in proportions, such as the seroconversion rate or proportion of subjects with a titer of ≥1:40, in the range of 15%–25%.
The study was approved by the institutional review boards of record of each of the participating study sites. The vaccine manufacturer provided the study product but had no role in the conduct of the study, analysis of the data, or preparation of this report.
RESULTS
Participants were enrolled from 9 September 2009 through 16 October 2009. During this period, each of the 5 states in which subjects were enrolled reported ≥3 weeks of widespread influenza activity [37]. A total of 121 subjects were enrolled; of these, 120 received the first vaccination, and 103 received the second vaccination. The characteristics of the 120 subjects given a first vaccination are shown in Table 1. The subjects’ ages, demographic characteristics, mean gestational age at enrollment, and the proportion of subjects in the second or third trimester were not significantly different between the 2 dose groups.
Table 1.
Characteristics of Study Subjects at Enrollment
| Dose group | ||
| Characteristic | 25 μg (n = 60) | 49 μg (n = 60) |
| Age, mean years (range) | 31.7 (20, 39) | 31.2 (18, 39) |
| Trimester of gestation, % | ||
| Second (14–26 weeks) | 57 | 72 |
| Third (27–34 weeks) | 43 | 28 |
| Gestational age at enrollment, mean weeks (± standard deviation) | 24.4 ± 6.2 | 22.6 ± 6.0 |
| Race, % | ||
| White | 85 | 80 |
| Black | 3 | 7 |
| Asian | 7 | 10 |
| Other | 5 | 3 |
| Non-Hispanic ethnicity, % | 88 | 93 |
| Received 2008–2009 influenza vaccine,1 % | 68 | 58 |
| Received 2009–2010 influenza vaccine,2 % | 28 | 27 |
NOTE. 1The 2008–2009 influenza vaccine contained A/Brisbane/10/2007 (H3N2)-like, A/Brisbane/59/2007 (H1N1)-like, and B/Florida/4/2006-like strains.
The 2009–2010 influenza vaccine contained A/Brisbane/10/2007 (H3N2)-like, A/Brisbane/59/2007 (H1N1)-like, and B/Brisbane/60/2008-like strains.
Safety Analyses
Both vaccine dose levels were generally well tolerated (Table 2). Local injection site symptoms of pain and tenderness were more common in the 49-μg dose group following both the first and second vaccinations; however, the differences between the dose groups were not statistically significant, and nearly all of the reactions were mild in severity. Within each dose group, there was no significant change in the frequency of reported local reactions between the first and second vaccinations. The frequency of occurrence of systemic symptoms did not vary between the 2 dose groups or between the first and second vaccinations within each dose group.
Table 2.
Solicited Local and Systemic Adverse Effects During the Week After Vaccination
| Vaccinated subjects, % |
||||
| Effect | First vaccination, 25 μg (n = 60) | First vaccination, 49 μg (n = 60) | Second vaccination, 25 μg (n = 49) | Second vaccination, 49 μg (n = 54) |
| Local reactions1 | ||||
| Pain at injection site | ||||
| None | 75 | 65 | 80 | 63 |
| Mild | 23 | 35 | 20 | 35 |
| Moderate | 2 | 0 | 0 | 2 |
| Tenderness | ||||
| None | 57 | 38 | 47 | 31 |
| Mild | 40 | 60 | 53 | 69 |
| Moderate | 3 | 2 | 0 | 0 |
| Erythema | ||||
| None | 92 | 87 | 96 | 94 |
| <20 mm | 8 | 10 | 4 | 4 |
| ≥20 and <50 mm | 0 | 3 | 0 | 2 |
| Swelling/induration | ||||
| None | 93 | 98 | 98 | 100 |
| <20 mm | 5 | 2 | 0 | 0 |
| ≥20 and <50 mm | 2 | 0 | 2 | 0 |
| Systemic reactions | ||||
| Fever2 | ||||
| None | 92 | 93 | 96 | 93 |
| Mild | 7 | 5 | 2 | 7 |
| Moderate | 2 | 2 | 2 | 0 |
| Malaise2 | ||||
| None | 68 | 60 | 84 | 74 |
| Mild | 23 | 27 | 4 | 15 |
| Moderate | 8 | 13 | 12 | 11 |
| Myalgia | ||||
| None | 80 | 87 | 94 | 89 |
| Mild | 12 | 10 | 4 | 7 |
| Moderate | 7 | 3 | 2 | 4 |
| Severe | 2 | 0 | 0 | 0 |
| Nausea2 | ||||
| None | 83 | 80 | 96 | 93 |
| Mild | 12 | 15 | 0 | 7 |
| Moderate | 5 | 5 | 4 | 0 |
| Headache | ||||
| None | 72 | 70 | 78 | 80 |
| Mild | 23 | 27 | 16 | 20 |
| Moderate | 5 | 3 | 4 | 0 |
| Severe | 0 | 0 | 2 | 0 |
| Oral temperature2 | ||||
| <37.8°C | 100 | 98 | 98 | 100 |
| ≥37.8°C and <38°C | 0 | 2 | 0 | 0 |
| ≥38°C and <39°C | 0 | 0 | 2 | 0 |
NOTE. 1No severe local reactions were reported.
No severe reactions were reported.
Eighteen SAEs were reported for 15 women, and 24 SAEs were reported for 20 infants; all were considered to be unrelated to the vaccine, and the frequency of events was generally balanced across study groups, with 9 of the 15 maternal SAEs and 13 of the 20 infant SAEs reported in the 25-μg dose group. The 15 maternal SAEs included 6 reports of postpartum hemorrhage, 2 reports of preterm contractions, 2 reports of severe pre-eclampsia, and 1 report each for the outcomes of abdominal myomectomy, exacerbation of asthma, gestational hypertension at term, fetal loss at 20 weeks gestation, nonelective Cesarean section, premature delivery, retained placenta, and vaginal bleeding. The 24 infant SAEs included 5 reports of premature birth, 4 reports of sacral dimple, 3 reports of atrial septal defect, and 1 report each of congenital heart disease, Erb’s palsy, fetal demise at 36 weeks gestational age, hyperbilirubinemia, possible Hirschsprung’s disease, postaxial polydactyly, pulmonic stenosis, respiratory distress, simple complete syndactyly, tetralogy of Fallot, thickened nuchal fold, and fetal distress resulting in an emergency Cesarean section.
Immunogenicity Analyses
At baseline, most participants were seronegative for the 2009 H1N1 influenza virus (Table 3). Following the first vaccination, an HAI antibody titer of ≥1:40 was detected in 93% (95% CI, 82%–98%) of subjects who received the 25-μg vaccine and 97% (95% CI, 88%–100%) of subjects who received the 49-μg vaccine, with GMTs of 384.2 (95% CI, 259.6–568.6) and 460.7 (95% CI, 325.2–652.7) in the 25-μg and 49-μg dose groups, respectively. These differences were not statistically significant. Microneutralization antibody titers were higher than HAI titers, with GMTs of 444.1 (95% CI, 309.7–636.7) and 595.7 (95% CI, 443.5–800.2) in the 25-μg and 49-μg dose groups, respectively, but as with the HAI titers, there were no significant differences between the dose groups for any of immunogenicity endpoints (proportion with titer ≥1:40, proportion meeting the definition of seroconversion, or postvaccination GMT) following the first vaccination. There were no significant increases in GMT, either by HAI or MN assays, following the second vaccination.
Table 3.
Serum Hemagglutination Inhibition and Microneutralization Assay Responses Before and After Each Dose of the 2009 H1N1 Influenza Vaccine
| Hemagglutination inhibition assay |
Microneutralization assay |
|||
| Immunogenicity end point | 25-μg vaccine dose | 49-μg vaccine dose | 25-μg vaccine dose | 49-μg vaccine dose |
| Titer ≥1:40, % (95% CI) | ||||
| Baseline | 7 (2–18) | 7 (2–17) | 13 (5–24) | 16 (7–27) |
| 21 Days after dose 1 | 93 (82–98) | 97 (88–100) | 96 (87–100) | 97 (88–100) |
| 21 Days after dose 2 | 95 (82–99) | 92 (81–98) | 100 (91–100) | 100 (93–100) |
| Delivery | ||||
| Maternal | 85 (71–94) | 62 (46–75)1 | 95 (83–99) | 87 (74–95) |
| Cord blood | 87 (73–96) | 89 (76–96) | 92 (79–98) | 91 (79–98) |
| Seroconversion, % (95% CI) | ||||
| 21 Days after dose 1 | 89 (78–96) | 97 (88–100) | 93 (82–98) | 97 (88–100) |
| 21 Days after dose 2 | 95 (82–99) | 92 (81–98) | 97 (86–100) | 100 (93–100) |
| Geometric mean titer (95% CI) | ||||
| Baseline | 6.8 (5.3–8.7) | 6.3 (5.1–7.8) | 9.5 (7.3–12.4) | 9.6 (7.5–12.3) |
| 21 Days after dose 1 | 384.2 (259.6–568.6) | 460.7 (325.2–652.7) | 444.1 (309.7–636.7) | 595.7 (443.5–800.2) |
| 21 Days after dose 2 | 360.3 (225.0–577.0) | 347.2 (233.3–516.6) | 509.5 (349.6–742.6) | 543.7 (413.1–715.5) |
| Delivery | ||||
| Cord blood | 230.3 (152.1–348.7) | 150.3 (100.9–223.9)2 | 503.5 (329.6–769.1) | 299.3 (197.7–453.2) |
| Maternal | 132.1 (83.0–210.3) | 50.9 (30.5–84.9)1 | 333.8 (216.5–514.6) | 191.0 (121.3–300.6) |
| Geometric mean ratio of cord blood:maternal delivery titer (range) | 1.81 (1.48–2.21) | 2.96 (2.16–4.06) | 1.52 (1.24–1.86) | 1.60 (1.30–1.98) |
NOTE. The number of specimens tested by the hemagglutination inhibition assay for the 25-μg dose group included 55 specimens obtained at baseline and after dose 1, 38 specimens obtained after dose 2, 41 maternal delivery specimens, and 39 cord blood specimens. For the 49-μg dose group, the number of specimens included 58 specimens obtained at baseline and after dose 1, 51 specimens obtained after dose 2, 47 maternal delivery specimens, and 46 cord blood specimens. The number of specimens tested by the microneutralization assay for the 25-μg dose group included 55 specimens obtained at baseline and after dose one, 38 specimens obtained after dose 2, 41 maternal delivery specimens, and 39 cord blood specimens. For the 49-μg dose group, the number of specimens included 58 specimens obtained at baseline and after dose 1, 51 specimens obtained after dose 2, 47 maternal delivery specimens, and 45 cord blood specimens. CI, confidence interval.
P ≤ .02 for comparison of 25-μg dose group with 49-μg dose group.
P = .002 for comparison of cord blood geometric mean titer and maternal delivery geometric mean titer.
At delivery, 85% (95% CI, 71%–94%) of women who had received the 25-μg vaccine had an HAI antibody titer of ≥1:40, but this level was detected in only 62% (95% CI, 46%–75%) of women who had received the 49-μg dose (P = .02). The difference in proportion with titer ≥1:40 at delivery was less pronounced for MN titers. Similarly, the GMTs in the maternal delivery samples were significantly higher in the 25-μg dose group compared with the 49-μg dose group by HAI (132.1 vs 50.9; P = .01), but not by MN (333.8 vs 191.0; P = .14).
Cord blood HAI and MN GMTs were also higher in the 25-μg dose group compared with the 49-μg dose group, but these differences were not statistically significant. Cord blood HAI GMTs were higher than maternal delivery sample GMTs in both dose groups, and this difference was statistically significant for the 49-μg dose group (P = .002). The geometric mean ratio (GMR) of cord blood to maternal blood HAI titers was 1.81 (95% CI, 1.48–2.21) in the 25-μg group and 2.96 (95% CI, 2.16–4.06) in the 49-μg group. The GMR for MN titers was 1.52 (95% CI, 1.24–1.86) in 25-μg group and 1.60 (95% CI, 1.30–1.96) in the 49-μg group. In analyses of paired maternal delivery and cord blood samples, the cord blood titer was higher than the corresponding maternal delivery sample titer in most pairs (Figure 1).
Figure 1.
Comparison of antibody titers by hemagglutination inhibition (HAI) (A) and microneutralization (B) assays in maternal delivery and infant cord blood pairs, by dose group (circles, 25 μg; crosses, 49 μg). The diagonal lines indicate equal values for the paired maternal delivery and infant cord blood specimens. Points above the line indicate a cord blood titer higher than the maternal delivery titer within each pair.
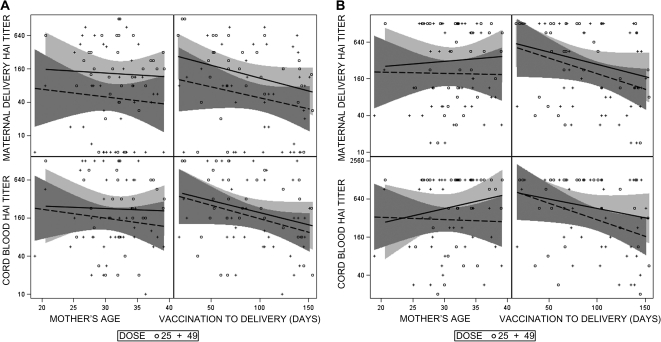
The panels in Figure 2 display the relationships between the variables of maternal age at first vaccination and interval in days between the mother’s second vaccination and delivery with the endpoints of HAI and MN antibody titers in cord blood and maternal delivery samples. There was no relationship between mother’s age and these endpoints. The downward slopes in the plots of the relationship of the interval from the second vaccination and delivery suggest trends toward lower cord blood and maternal delivery titers with vaccination earlier in pregnancy. This relationship was confirmed in analyses of covariance that adjusted for dose group. Those analyses found significant associations between the interval in days variable and the endpoints of HAI and MN log titer in cord blood and maternal delivery samples (P < .05 for all 4 analyses).
Figure 2.
Relationships between the variables of mother’s age in years at the first vaccination and interval in days between the second vaccination and delivery with the endpoints of cord blood and maternal delivery titers by hemagglutination inhibition (HAI) (A) and microneutralization (MN) (B) assays. The markers indicate the individual values for the 25-μg (circles) and 49-μg (crosses) dose groups, the lines are the estimated linear regression lines for each dose group (solid line, 25 μg; dashed line, 49 μg), and the shaded areas indicate the 95% confidence bands for each regression line.
In analyses stratified by prior receipt of the 2008–2009 and/or 2009–2010 seasonal influenza vaccines, there was no evidence of a lower response to the first or second vaccination among participants reporting prior receipt of seasonal influenza vaccine (Table 4).
Table 4.
Geometric Mean Hemagglutination Inhibition and Microneutralization Titers to the 2009 H1N1 Influenza Vaccine According to Prior Receipt of 2008–2009 and/or 2009–2010 Seasonal Influenza Vaccines
| Assay | Dose group | Received 2008–2009 and/or 2009–2010 seasonal influenza vaccine | No. of subjects | Day 0, GMT (95% CI) | No. of subjects | Day 21 after dose 1, GMT (95% CI) | No. of subjects | Day 21 after dose 2, GMT (95% CI) |
| HAI | 25 μg | No | 10 | 5.2 (4.8–5.6) | 10 | 355.1 (142.5–884.9) | 8 | 349.0 (123.8–983.4) |
| Yes | 44 | 7.2 (5.3–9.8) | 44 | 380.5 (240.8–601.4) | 29 | 347.9 (196.1–617.2) | ||
| 49 μg | No | 17 | 5.8 (4.3–7.8) | 17 | 417.1 (225.3–772.1) | 14 | 430.7 (264.0–702.5) | |
| Yes | 40 | 6.7 (5.0–8.9) | 40 | 489.3 (312.8–765.2) | 36 | 320.0 (186.2–549.9) | ||
| MN | 25 μg | No | 10 | 5.0 (…)1 | 10 | 320.0 (129.4–791.2) | 8 | 293.4 (106.6–807.8) |
| Yes | 44 | 11.0 (8.0–15.0) | 44 | 467.0 (309.1–705.6) | 29 | 574.7 (376.5–877.3) | ||
| 49 μg | No | 17 | 6.8 (4.5–10.3) | 17 | 461.9 (257.1–829.7) | 14 | 551.7 (320.8–948.7) | |
| Yes | 40 | 11.3 (8.3–15.4) | 40 | 662.6 (462.2–949.8) | 36 | 543.4 (385.3–766.4) |
NOTE. CI, confidence interval.
All samples had the same value (below the limit of detection of a titer of 1:10), and therefore confidence intervals could not be calculated.
There was good correlation between the results of the HAI and MN assays in postvaccination samples. The Spearman correlation coefficient for the HAI and MN titers among all participants prior to vaccination was 0.34 and increased to 0.81 for the post–dose 1 and post–dose 2 visits. The correlation between the 2 assays for the vaccine group-specific dose responses was similar.
DISCUSSION
This evaluation of the immunogenicity of a 2009 H1N1 influenza vaccine in pregnant women indicates that a single dose of an inactivated 2009 H1N1 influenza vaccine is highly immunogenic in women vaccinated during the second or third trimester of pregnancy. At 3 weeks after administration of a 25-μg dose, 93% of women had an HAI titer of ≥1:40, which is a level typically associated with protection against influenza infection. There was no further increase in HAI GMTs with the second vaccination, which suggested no apparent benefit of a second vaccination for the mother. The vaccinations were well tolerated, with reactogenicity profiles that were similar to those reported for 2009 H1N1 influenza vaccines in nonpregnant adults [25, 26, 28, 38].
We followed women through the time of delivery, to evaluate persistence of the immune response in the vaccinated women and the transplacental transfer of antibody, as determined by antibody levels in infant cord blood samples, compared with samples obtained from the mother at the time of delivery. We found that, although antibody titers in the vaccinated women decreased in the interval between vaccination and delivery, relatively high titers were maintained up to the time of delivery, and cord blood titers tended to be higher than maternal delivery titers. These findings suggest that clinical protection from vaccination during pregnancy may persist to the postpartum period and can be efficiently transferred to the infants.
The relatively high cord blood titers are consistent with clinical benefits reported in 3 recent evaluations of inactivated seasonal influenza vaccines given during pregnancy. A clinical trial of influenza vaccine given to women in Bangladesh during their third trimester of pregnancy reported that an HAI titer of ≥1:40 to the vaccine influenza A H1N1 strain was detected in >80% of maternal delivery and cord blood samples [39] and that influenza vaccination during pregnancy was associated with a reduction in the risk of febrile illness in both the women and their infants [40]. An observational cohort study of 1160 mother-infant pairs in the United States found that infants born to mothers who had received influenza vaccine during pregnancy had a 41% lower risk of laboratory-confirmed influenza infection than did infants born to mothers who had not been vaccinated [41]. Similarly, a case control study found that infants whose mothers were vaccinated during pregnancy were 91% less likely to be hospitalized with influenza infection during the first 6 months of life than were infants whose mothers had not been vaccinated [42].
We found consistent trends toward higher HAI antibody levels in cord blood samples, compared with maternal delivery samples. Other evaluations of transplacental transfer of maternal antibodies have found higher levels of antibodies to protein antigens, such as pertussis antigens, and to pneumococcal antigens, such as pneumococcal polysaccharide, in cord blood samples than in maternal delivery specimens, which is believed to be the result of active placental transport of maternal antibody [43–46]. Interestingly, this phenomenon has not been previously demonstrated in evaluations of maternal influenza vaccination. In fact, a recent evaluation of an MF59-adjuvanted 2009 H1N1 influenza vaccine given in the third trimester of pregnancy found that HAI GMTs were significantly lower in the cord blood samples than in the maternal delivery samples (141.8 vs 257.9) [47]. Similarly, in the clinical trial of seasonal influenza vaccine conducted in Bangladesh, HAI GMTs in the cord blood specimens were either lower than or comparable to those detected in the maternal delivery samples [39]. Finally, in the observational study of seasonal influenza vaccines reported by Eick et al [41], HAI GMTs to vaccine strains in the cord blood samples were generally similar to those in maternal postpartum specimens. Our findings could represent chance observations, they may be a function of the administration of 2 doses of a higher–antigen content vaccine, or they may be attributable to other factors. We also found that vaccination later in pregnancy may be associated with higher cord blood antibody levels, which could have implications for the level of clinical protection or duration of persistence of passively acquired antibody in the neonates.
There are limitations to this study that may be relevant to the interpretation of the results. First, we enrolled only women in the second and third trimesters of pregnancy and so did not evaluate antibody response to vaccines administered earlier in pregnancy. Second, we did not collect information on height and weight of the pregnant women and so could not evaluate the possible influence of body mass index on vaccine response. Last, as noted in the methods, because of issues with the potency testing of the vaccine, we evaluated doses of 25 μg and 49 μg of HA, instead of the intended doses of 15 μg and 25 μg of HA.
Although we were not able to evaluate a dose of 15 μg of HA (the standard dose per strain contained in licensed inactivated influenza vaccines), our results suggest that inactivated vaccines containing 15 μg of 2009 H1N1 influenza hemagglutinin are likely to be adequately immunogenic in pregnant women. In dose-ranging studies of seasonal influenza vaccines and 2009 H1N1 influenza vaccines involving nonpregnant adults, the proportion of subjects who achieved an HAI titer of ≥1:40 appears to be relatively insensitive to dose [25–29, 48–50]. For example, in a study of an inactivated 2009 H1N1 vaccine made by Sanofi Pasteur in a cohort of adults 18–64 years of age, a postvaccination titer of ≥1:40 was detected in 95% of subjects given a vaccine containing 11 μg of antigen, compared with 98% of subjects given a vaccine containing 24 μg of antigen [28]. Thus, based on these findings, it is highly likely that a single dose of vaccine containing 15 μg of HA given to pregnant women would induce antibody titers typically correlated with protection and that transplacental transfer of antibody would confer passive protection to the newborns.
Supplementary Data
Supplementary data are available at The Journal of Infectious Diseases online.
Funding
This work was supported by Vaccine and Treatment Evaluation Unit contracts from the National Institutes of Health (contracts HHSN272200800004C [Group Health], HHSN272200800057C [Duke], HHSN27220080000C [Vanderbilt], and HHSN272200800002C [Baylor], HHSN272200800003C [SLU]) and by contract N01-AI-30063 to Southern Research Institute.
Supplementary Material
Acknowledgments
We thank the many members of the study teams and other individuals who contributed to the conduct of this clinical trial, including Jane Dimer, Barbara Carste, Maya Dunstan, Patty Starkovich, Nancy Dorn, C. Hallie Phillips, Theresa Shea, Bill Lee, Carol Dean, Joyce Benoit, Tom Archer, Michelle Hill, Shannon Byler, Farah Hawasili, Marie Schwartz (Group Health); Robert Atmar, Hana El Sahly, W. Paul Glezen, Connie Rangel, Tracey Lanford, Nanette Bond, Maurizio Maccato, Phillip Pinell, Alan Jewell, and Annette Machado (Baylor College of Medicine); Linda Eggemeyer, Irene Graham, Edwin Anderson, and Brittney Whisenand (Saint Louis University School of Medicine); Shanda Phillips, Gayle Johnson, Faith Brendle, Teresa Sims, Michaela Toney, Wendi McDonald, Deborah Myers, Deborah Hunter, Sandra Yoder (Vanderbilt University School of Medicine); Emmanuel Walter, Bonnie Thiele, Amanda Anderson, Kristin Weaver, Kristen Lynam, Tammy Bishop, Liping Feng, Beth Patterson, Lynn Harrington (Duke University Medical Center); Lisa Slappey, Valerie Johnson, Crystal Coleman, Kristen Southworth, Tiffany Pitts, Barbara Taggart, and Logan Haller (Southern Research Institute); Mekhala Acharya, Kuo Guo, Fenhua He, Heather Hill (EMMES); Robin Mason, Fran Rubin, Mirjana Nesin, Wendy Buchanan, Linda Lambert, Shy Shorer, and Suzanne Murray (National Institute of Allergy and Infectious Diseases); and the members of the Safety Monitoring Committee (Robert Salata [Chair]) David Kimberlin, Margo Schilling, Donald Stablein, and Jeanne Sheffield), for their valuable input.
References
- 1.Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176:463–8. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 5.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 6.Kelly H, Mercer G, Cheng A. Quantifying the risk of pandemic influenza in pregnancy and indigenous people in Australia in 2009. Euro Surveill. 2009;14 [PubMed] [Google Scholar]
- 7.Webb SA, Pettila V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–34. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 10.Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis. 2010;50:686–90. doi: 10.1086/650460. [DOI] [PubMed] [Google Scholar]
- 11.ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–26. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 13.Hanslik T, Boelle PY, Flahault A. Preliminary estimation of risk factors for admission to intensive care units and for death in patients infected with A(H1N1)2009 influenza virus, France, 2009–2010. PLoS Curr Influenza. 2010;2:RRN1150. doi: 10.1371/currents.RRN1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callaghan WM, Chu SY, Jamieson DJ. Deaths from seasonal influenza among pregnant women in the United States, 1998–2005. Obstet Gynecol. 2010;115:919–23. doi: 10.1097/AOG.0b013e3181d99d85. [DOI] [PubMed] [Google Scholar]
- 16.Oluyomi-Obi T, Avery L, Schneider C, et al. Perinatal and maternal outcomes in critically ill obstetrics patients with pandemic H1N1 influenza A. J Obstet Gynaecol Can. 2010;32:443–7. doi: 10.1016/S1701-2163(16)34497-8. [DOI] [PubMed] [Google Scholar]
- 17.Miller AC, Safi F, Hussain S, Subramanian RA, Elamin EM, Sinert R. Novel influenza A(H1N1) virus among gravid admissions. Arch Intern Med. 2010;170:868–73. doi: 10.1001/archinternmed.2010.126. [DOI] [PubMed] [Google Scholar]
- 18.Koegelenberg CF, Irusen EM, Cooper R, et al. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM. 2010;103:319–25. doi: 10.1093/qjmed/hcq022. [DOI] [PubMed] [Google Scholar]
- 19.Maravi-Poma E, Martin-Loeches I, Regidor E, et al. Severe 2009 H1N1 influenza in pregnant women in Spain. Crit Care Med. 2011;39:945–51. doi: 10.1097/CCM.0b013e318208ee12. [DOI] [PubMed] [Google Scholar]
- 20.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 Pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2010.12.033. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Strategic Advisory Group of Experts on Immunization—report of the extraordinary meeting on the influenza A (H1N1) 2009 pandemic, 7 July 2009. Wkly Epidemiol Rec. 2009;84:301–4. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–8. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Update: influenza activity—United States, August 30, 2009–March 27, 2010, and composition of the 2010–11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423–30. [PubMed] [Google Scholar]
- 24.World Health Organization. Recommended viruses for influenza vaccines for use in the 2010–2011 northern hemisphere influenza season. http://www.who.int/csr/disease/influenza/recommendations2010_11north/en/index.html. Accessed 6 December 2010. [Google Scholar]
- 25.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 26.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 27.Liang XF, Wang HQ, Wang JZ, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 28.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 29.Talaat KR, Greenberg ME, Lai MH, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J Infect Dis. 2010;202:1327–7. doi: 10.1086/656601. [DOI] [PubMed] [Google Scholar]
- 30.Weetman AP. The immunology of pregnancy. Thyroid. 1999;9:643–6. doi: 10.1089/thy.1999.9.643. [DOI] [PubMed] [Google Scholar]
- 31.Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161–70. doi: 10.1385/CRIAI:26:3:161. [DOI] [PubMed] [Google Scholar]
- 32.Gordon CL, Johnson PD, Permezel M, et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50:672–8. doi: 10.1086/650462. [DOI] [PubMed] [Google Scholar]
- 33.Escribese MM, Kraus T, Rhee E, Fernandez-Sesma A, Lopez CB, Moran TM. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood. 2008;112:4574–84. doi: 10.1182/blood-2008-04-148692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Summary of status of development and availability of A/California/7/2009 (H1N1)-like candidate vaccine viruses and potency testing reagents. http://www.who.int/csr/resources/publications/swineflu/summary_candidate_vaccine.pdf. Accessed 6 December 2010. [Google Scholar]
- 35.Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16:558–66. doi: 10.1128/CVI.00368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keitel WA, Dekker CL, Mink C, et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine. 2009;27:6642–8. doi: 10.1016/j.vaccine.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. 2009. Fluview. http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/weekly41.htm. Accessed 1 August 2011. [Google Scholar]
- 38.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03(A)-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–5. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy—antibody responses in mothers and infants. N Engl J Med. 2010;362:1644–6. doi: 10.1056/NEJMc0912599. [DOI] [PubMed] [Google Scholar]
- 40.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 41.Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;165:104–11. doi: 10.1001/archpediatrics.2010.192. [DOI] [PubMed] [Google Scholar]
- 42.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51:1355–61. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quiambao BP, Nohynek HM, Kayhty H, et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine. 2007;25:4470–7. doi: 10.1016/j.vaccine.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J Infect Dis. 2004;190:335–40. doi: 10.1086/421033. [DOI] [PubMed] [Google Scholar]
- 45.de Voer RM, van der Klis FR, Nooitgedagt JE, et al. Seroprevalence and placental transportation of maternal antibodies specific for Neisseria meningitidis serogroup C, Haemophilus influenzae type B, diphtheria, tetanus, and pertussis. Clin Infect Dis. 2009;49:58–64. doi: 10.1086/599347. [DOI] [PubMed] [Google Scholar]
- 46.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–84. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 47.Zuccotti G, Pogliani L, Pariani E, Amendola A, Zanetti A. Transplacental antibody transfer following maternal immunization with a pandemic 2009 influenza A(H1N1) MF59-adjuvanted vaccine. JAMA. 2010;304:2360–1. doi: 10.1001/jama.2010.1729. [DOI] [PubMed] [Google Scholar]
- 48.Jackson LA, Austin G, Chen RT, et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine. 2001;19:4703–9. doi: 10.1016/s0264-410x(01)00225-0. [DOI] [PubMed] [Google Scholar]
- 49.Engler RJ, Nelson MR, Klote MM, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;168:2405–14. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 50.Treanor J, Keitel W, Belshe R, et al. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine. 2002;20:1099–105. doi: 10.1016/s0264-410x(01)00440-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.