Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections are frequently associated with strains harboring genes encoding Panton-Valentine leukocidin (PVL). The role of PVL in the success of the epidemic CA-MRSA strain USA300 remains unknown. Here we developed a skin and soft tissue infection model in rabbits to test the hypothesis that PVL contributes to USA300 pathogenesis and compare it with well-established virulence determinants: alpha-hemolysin (Hla), phenol-soluble modulin-alpha peptides (PSMα), and accessory gene regulator (Agr). The data indicate that Hla, PSMα, and Agr contribute to the pathogenesis of USA300 skin infections in rabbits, whereas a role for PVL could not be detected.
The vast majority of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections in the United States affect skin and soft tissue [1, 2]. These infections are caused primarily by isolates classified as pulsed-field gel electrophoresis type USA300 (USA300) [1, 2]. Notably, USA300 often causes disease in otherwise healthy individuals, and accordingly, the pathogen has enhanced virulence in animal infection models and capacity to circumvent killing by human neutrophils [3, 4]. Although significant progress has been made over the past several years, our understanding of the molecular basis of USA300 transmission and virulence is incomplete.
A cytolytic toxin known as Panton-Valentine leukocidin (PVL) is present in many strains that cause CA-MRSA infections, including those caused by USA300. As such, PVL has been thought of as a major contributor to (or the underlying determinant of) the severity of USA300 infections. However, rodent models of USA300 skin and soft tissue infection have largely failed to demonstrate a significant role for PVL in pathogenesis [5, 6]. One possible explanation for these results is that rodent neutrophils are less sensitive to PVL-mediated cytolysis compared with those from humans [7, 8]. By comparison, rabbit neutrophils are highly sensitive to PVL-mediated cytolysis—even more so than those from humans [9].
Alpha-hemolysin (Hla) and phenol-soluble modulin-alpha peptides (PSMα) are cytolytic toxins reported previously to contribute to the severity of USA300 skin infection in mouse models [10, 11]. PSMα cause comparable lysis of neutrophils from mice, rabbits, monkeys, and humans [8], whereas Hla has little or no capacity to cause lysis of neutrophils from these species [8, 12]. However, erythrocytes from mice and rabbits are far more sensitive to Hla-mediated hemolysis compared with those from humans [13].
The role of PVL, Hla, and PSMα in USA300 skin disease has not been tested in a rabbit infection model. Moreover, the relative contribution of these virulence molecules in the pathogenesis of USA300 skin infections is not known. To these ends, we developed a rabbit skin infection model in which to evaluate the relative contribution of PVL, Hla, PSMα, and the accessory gene regulator (Agr) to USA300 virulence.
METHODS
USA300 wild-type and isogenic deletion strains (LAC, LACΔpvl, LACΔpsmα, LACΔagr, and LACΔhla) were described previously [6, 10, 14]. Bacteria were cultured overnight from frozen stocks in trypticase soy broth (TSB) at 37°C with shaking (250 rpm), diluted 1:200 in fresh TSB the following morning, and then cultured to early stationary phase of growth (optical density [OD]600 = 2.0). To prepare inocula, bacteria were washed in Dulbecco’s phosphate-buffered saline (DPBS) and resuspended in DPBS at 5 × 109 colony-forming units (CFUs)/mL. Bacterial preparations were used immediately for inoculation of rabbits as described below.
Female New Zealand White rabbits (strain Cr1c:KBL, Western Oregon Rabbit Company, Philomath, OR), 5–7 weeks old, were housed singly in cages (Suburban Surgical Co., Inc., Wheeling, IL) and received food and water ad libitum. All studies conformed to the guidelines set forth by the National Institutes of Health (NIH) and were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, NIH. The day before inoculation, animals were sedated with acepromazine and hair was removed by shaving a designated area on each flank and treatment with Nair (Church & Dwight Co., Inc., Princeton, NJ), which prevented immediate regrowth of hair. The following day, rabbits were sedated with acepromazine and inoculated by subcutaneous (s.c.) injection into the left and right flank with 5 × 108 S. aureus in 0.1 mL DPBS. The optimal inoculum of USA300 was determined empirically in preliminary experiments. Inoculation with 5 × 108 USA300 caused reproducible abscesses that were easy to evaluate based upon size. Five rabbits were inoculated by s.c. injection in the right and left flanks with each strain (5 rabbits per strain and 2 abscesses per animal). One additional rabbit received sterile DPBS by s.c. injection in the right and left flanks. Abscess dimensions were measured daily for 14 days with a caliper. Length (L) and width (W) values were used to calculate abscess volume (V = 4/3π[L/2]2 × [W/2]) and area (A = π[L × W]/2) [6, 15].
A second set of skin infection experiments was performed as described above to determine CFUs in rabbit abscesses and for histopathological analysis of abscesses. For the determination of CFUs, 2 rabbits per strain were inoculated as described above (4 abscesses per strain in total). Additionally, these rabbits received an inoculation with DPBS by s.c. injection on the right hip. For histopathological analysis, 1 additional rabbit was inoculated as above with the LAC wild-type strain and in the right hip with DPBS. Animals were euthanized on days 2, 6, and 14 post infection, and abscesses and surrounding tissue (and tissue injected with DPBS) were removed. For determination of CFUs, tissue samples were weighed, homogenized in DPBS, diluted in saline, and plated on trypticase soy agar. CFUs were enumerated the following day. No bacteria were recovered from tissues at the site of DPBS injection.
For histopathological analysis, abscesses were fixed in 10% neutral-buffered formalin for at least 48 hours. Fixed tissues and slides were prepared and stained with hematoxylin-eosin as described previously [11]. Tissue samples were evaluated by a veterinary pathologist (D. J. G.). Images of tissue sections were captured using an Olympus model BX-51 microscope (Olympus, Center Valley, PA), and brightness and contrast of images was adjusted in Adobe Photoshop CS4 (Adobe Systems Incorporated, San Jose, CA).
RESULTS AND DISCUSSION
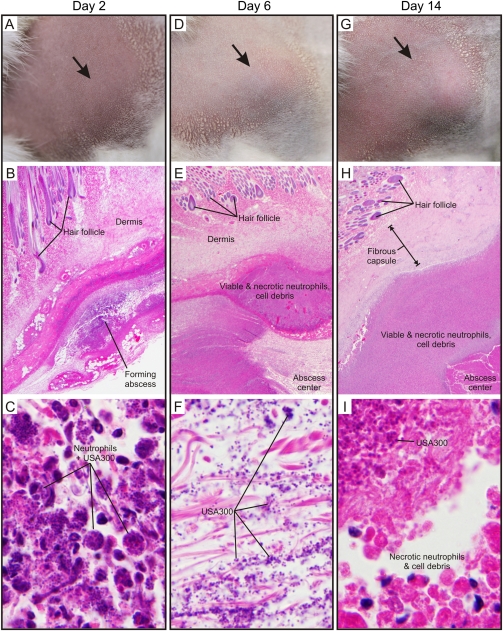
As a first step toward developing a rabbit model of skin and soft tissue infection, we infected New Zealand White rabbits with S. aureus strain LAC (5 × 108 CFUs) by s.c. inoculation and monitored the progression of abscess development by gross morphology and histopathology over a 14-day period (Figure 1). Unexpectedly, inoculation with less than 5 × 108 CFUs (eg, 1 × 108 CFUs) did not result in reproducible lesions (data not shown). The observation that a high inoculum of USA300 is needed to elicit rabbit abscesses is surprising to us, as rabbits and their leukocytes or erythrocytes are known to be highly sensitive to the effects of many S. aureus secreted toxins. S. aureus lesions in this animal model manifested initially as diffuse, raised abscesses that were detectable by day 2 post infection (Figure 1A), reached maximum size (area and volume) circa day 6 (Figure 1D), and retracted in size during the latter stages of infection (eg, day 14, Figure 1G). Histopathology revealed acute inflammation on day 2 (Figure 1B), which was characterized by an influx of neutrophils, many of which contained ingested bacteria (Figure 1C). Initial inflammatory lesions transitioned within 6 days to form abscesses that were characterized by necrosis at the center of the lesions surrounded by a dense zone of necrotic neutrophils (pus) (Figure 1E). USA300 was readily detected at the center of these abscesses (Figure 1F). Abscesses on day 14 also harbored a central area with necrotic neutrophils, cell debris, and bacteria (Figure 1, H and I). However, in contrast to day-6 lesions, these abscesses had features of chronic infection and/or resolution, such as a thick fibrous capsule surrounding the necrotic center of the lesion and mineralized and regenerating skeletal muscle at the periphery (Figure 1H and data not shown). The pathology of S. aureus lesions in the experimental rabbit model of skin and soft tissue infection largely recapitulates that of previous murine USA300 skin infection models [5, 6, 10, 11]. Moreover, several defining features of late-stage rabbit skin abscesses are similar to those reported for human abscesses, such as a central area of necrotic neutrophils (pus) surrounded by a fibrous capsule.
Figure 1.
Pathology of rabbit skin abscesses caused by USA300 (LAC). USA300 abscesses 2 days (A–C), 6 days (D–F), or 14 days (G–I) post infection as indicated. A, D, and G, gross skin pathology. Note that hair was removed prior to inoculation. B, E, and H, representative histopathological sections of a typical rabbit skin abscess at 2, 6, and 14 days post infection as indicated. Original magnification is ×20. C, F, and I, increased magnification of an area of each abscess center at 2, 6, and 14 days post infection. Original magnification is ×1000.
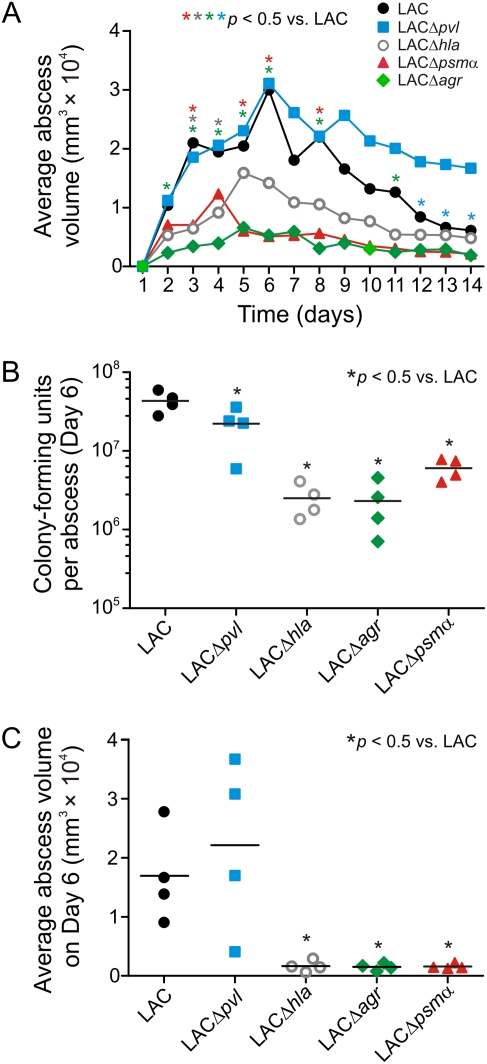
Inasmuch as the molecular basis for CA-MRSA virulence is incompletely defined, we compared the ability of LAC (USA300) wild-type, LACΔpvl, LACΔhla, LACΔpsmα, and LACΔagr strains to cause abscesses in the rabbit skin and soft tissue infection model (Figure 2A). Abscesses caused by LACΔhla, LACΔpsmα, and LACΔagr had significantly less volume and area (not shown) than those caused by the LAC wild-type strain (P < .05) (Figure 2A), indicating that Hla, PSMα, and Agr contribute to S. aureus abscess formation. In stark contrast, rabbit abscesses caused by LACΔpvl were similar in size to those caused by the LAC wild-type strain for up to 11 days post infection (Figure 2A, days 1–11). Unexpectedly, LACΔpvl abscesses were significantly larger than those caused by the LAC wild-type strain on days 12–14, suggesting that the presence of PVL promotes late resolution of infection in this model (Figure 2A, days 12–14). These data are consistent with previous observations in the mouse skin infection model, in which skin lesions caused by Δpvl strains were sometimes slightly larger than those caused by the wild-type strain [5, 16]. Collectively, the findings indicate that Hla, PSMα, and Agr are more important than PVL for staphylococcal abscess formation in the rabbit skin infection model. Moreover, these data support and extend previous observations that PVL does not contribute to pathogenesis of staphylococcal skin abscess formation in murine CA-MRSA infection models [5, 6] or clinical outcome in human complicated skin infections [17].
Figure 2.
Contribution of selected USA300 molecules to the formation of rabbit abscesses. A, Average abscess volume for rabbits infected with USA300 (LAC) wild-type or isogenic gene deletion strains as indicated. Results are the mean of 10 abscesses from 5 animals (2 per rabbit). B, USA300 CFUs recovered from rabbit abscesses on day 6 post infection. Each symbol represents data from 1 abscess and the line indicates the mean. C, average abscess volume for rabbits used in B. Data are from rabbit abscess experiments distinct from those shown in A.
For A–C, *P < .05 vs LAC wild-type using a 1-way analysis of variation (ANOVA) and Dunnett test to correct for multiple comparisons. Asterisks in A are matched by color to the appropriate mutant strain. CFUs, colony-forming units.
We performed a second set of experiments to evaluate the number of S. aureus CFUs per rabbit abscess on day 6 following s.c. inoculation with LAC wild-type and isogenic mutant strains (Figure 2B). The number of USA300 CFUs per abscess was significantly less for animals infected with isogenic deletion strains compared with that of wild-type infected rabbits (P < .05) (Figure 2B); the corresponding abscess volumes were consistent with the previous experiment (compare day 6 in Figure 2, A and C). Although the number of CFUs recovered from LACΔpvl abscesses was reduced slightly (<2-fold) compared with that from LAC wild-type abscesses, the magnitude of the decrease in CFUs was far greater for LACΔhla (17.3-fold), LACΔagr (18.8-fold), and LACΔpsmα (7.2-fold) abscesses (Figure 2B).
We reported previously that PVL failed to contribute to abscess formation or disease severity in mouse skin infection models [5, 6]. One possible explanation for these results is the reduced susceptibility of rodent neutrophils to the effects of PVL in vitro compared with human neutrophils [7, 8]. On the other hand, rabbit neutrophils have significantly increased in vitro susceptibility to PVL-mediated cytolysis relative to cells from humans [9]. Therefore, if susceptibility of neutrophils to PVL in vitro is a reflection of its capacity to promote disease in vivo, the rabbit skin infection model should be optimal for revealing any contribution of PVL to disease. Nevertheless, the experiments described above failed to detect a contribution of PVL to disease establishment or severity (as estimated by abscess volume) in the rabbit skin infection model (Figure 2A). These observations suggest that in vitro activity or capacity of S. aureus cytolytic toxins does not necessarily correlate with a virulence phenotype in vivo—perhaps due to the high redundancy of cytolytic toxins encoded by most S. aureus strains.
The relative role of individual USA300 virulence molecules in the pathogenesis of staphylococcal skin infection has remained incompletely determined. Here we demonstrated that Hla and PSMα contribute prominently to the skin disease in the rabbit, whereas PVL does not. These findings provide strong support to the idea that Hla and PSMα (but not PVL) are key CA-MRSA virulence determinants. Identification of molecules that contribute to the severity of S. aureus infections is an important step in our efforts to develop new therapeutics or vaccines directed to prevent or moderate disease. Thus, we anticipate future studies will be directed to evaluate Hla and PSMα as potential vaccine antigens in the rabbit model of S. aureus skin infection.
Funding
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). J. B. W. and O. S. acknowledge membership in and support from the Region V Great Lakes Regional Center of Excellence (RCE) (NIH award 2-U54-AI-057153).
Acknowledgments
The authors thank National Institute of Allergy and Infectious Diseases (NIAID) colleagues Ralph Larson, Don Dale, and Adam Kennedy for technical assistance, and Austin Athman, Gary Hettrick, and Anita Mora for photography.
References
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Voyich JM, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–19. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Cheung GY, Hu J, et al. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202:1866–76. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 7.Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- 8.Löffler B, Hussain M, Grudmeier M, et al. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci USA. 2010;107:5587–92. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Braughton KR, Kretschmer D, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy AD, Bubeck WJ, Gardner DJ, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–8. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valeva A, Walev I, Pinkernell M, et al. Transmembrane β-barrel of staphylococcal α-toxin forms in sensitive but not resistant cells. Proc Natl Acad Sci USA. 1997;94:11607–11. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernheimer AW. Staphylococcal alpha toxin. Ann NY Acad Sci. 1965;128:112–23. doi: 10.1111/j.1749-6632.1965.tb11633.x. [DOI] [PubMed] [Google Scholar]
- 14.Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 15.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with gram-positive cocci. Infect Immun. 1992;60:2636–40. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci USA. 2010;107:2241–6. doi: 10.1073/pnas.0910344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae IG, Tonthat GT, Stryjewski ME, et al. Presence of genes encoding the Panton-Valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J Clin Microbiol. 2009;47:3952–7. doi: 10.1128/JCM.01643-09. [DOI] [PMC free article] [PubMed] [Google Scholar]