Abstract
Transplantation of mesenchymal stem cells (MSCs) has emerged as a potential treatment for ischemic heart repair. Previous studies have suggested that Wnt11 plays a critical role in cardiac specification and morphogenesis. In this study, we examined whether transduction of Wnt11 directly increases MSC differentiation into cardiac phenotypes. MSCs harvested from rat bone marrow were transduced with both Wnt11 and green fluorescent protein (GFP) (MSCWnt11) using the murine stem cell virus (pMSCV) retroviral expression system; control cells were only GFP-transfected (MSCNull). Compared with control cells, MSCWnt11 was shown to have higher expression of Wnt11 by immunofluorescence, real-time polymerase chain reaction, and western blotting. MSCWnt11 shows a higher expression of cardiac-specific genes, including GATA-4, brain natriuretic peptide (BNP), islet-1, and α-actinin, after being cultured with cardiomyocytes (CMs) isolated from ventricles of neonatal (1–3 day) SD rats. Some MSCWnt11 were positive for α-actinin when MSCs were cocultured with native CMs for 7 days. Electron microscopy further confirmed the appearance of sarcomeres in MSCWnt11. Connexin 43 was found between GFP-positive MSCs and neonatal rat CMs labeled with red fluorescent probe PKH26. The transdifferentiation rate was significantly higher in MSCWnt11 than in MSCNull, as assessed by flow cytometery. Functional studies indicated that the differentiation of MSCWnt11 was diminished by knockdown of GATA-4 with GATA-4-siRNA. Transduction of Wnt11 into MSCs increases their differentiation into CMs by upregulating GATA-4.
Introduction
Myocardial infarction is a leading cause of heart failure and death due in large part to the heart's limited capacity to regenerate itself in response to injury. Over the past decade, cell-based therapy has made significant breakthroughs regarding formation of new cardiomyocytes (CMs) and protection of existing, native CMs [1–3]. Mesenchymal stem cells (MSCs) are self-renewing multipotent cells. The novel information regarding the differentiation of tissue-specific MSCs to CMs has been provided [4,5]. However, growing evidence suggests a relatively low rate of stem cell differentiation [6,7]. There is a great need to develop strategies to increase their differentiation into cardiac phenotypes.
Wnt signaling plays a critical role in cardiac specification and morphogenesis [8,9] and is a primary mediator of stem cell growth and differentiation [10,11]. Wnt proteins form a family of 20 high-conserved secreted lipid-modified signaling molecules, which activate canonical (eg, Wnt1, Wnt2a, Wnt3a, and Wnt8a) or noncanonical (eg, Wnt4, Wnt5a, and Wnt11) pathways. Canonical Wnt proteins bind to Frizzled receptors, which then complex with the coreceptor LDL receptor-related protein. The signal is transduced by β-catenin, which enters the nucleus to modulate the expression of target genes [12]. Overactivation of canonical Wnt signaling suppresses cardiac differentiation in mouse embryonic stem cells [13,14] and heart formation in Xenopus and chick embryos [15,16]. In contrast, noncanonical signaling is independent of LDL receptor-related protein/β-catenin. Instead, it encompasses the Wnt/Ca2+ and Wnt/planar cell polarity pathways [17]. Activation of the noncanonical Wnt signaling pathway promotes cardiogenesis during Xenopus embryonic development [8]. Wnt11 signaling serves as a critical cell adhesion cue for the organization of CMs in the developing ventricular wall [18]. It has been reported that unfractionated bone marrow mononuclear cells (BMMNCs) cultured in Wnt11-rich medium demonstrated cardiomyogenic differentiation [10]. Supplementation with Wnt11-conditioned medium significantly enhanced the differentiation of cardiac progenitor cells (CPC) to CMs [11]. All of these suggest that Wnt11 might mediate myogenic differentiation of stem cells. We transduced Wnt11 directly into MSCs and investigated their transdifferentiation. Our results indicate that overexpression of Wnt11 increases the potential of MSC transdifferentiation into myocardial phenotypes.
Materials and Methods
All protocols were approved by the University of Cincinnati Animal Care and Use Committee and conform to the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Transfection of MSCs with Wnt11 plasmid
MSCs were obtained from femurs and tibias of male SD rats, as previously described [19]. Cells were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Fluorescence activated cell sorting (FACS) demonstrated that cultured MSCs expressed high levels of c-kit (>85%) and sca-1 (>95%).
MSCs were transduced with recombinant Wnt11 using the pMSCV retroviral expression system. IRES-EGFP (Clontech) was first cloned into pMSCV at XhoI and EcoRI sites. Wnt11 was then excised from pcDNA-Wnt11 [20] with HindIII and XhoI restriction enzymes and cloned into pMSCV-EGFP. GP2-293 cells (Clontech) were transduced with pMSCV-EGFP-Wnt11 using Fugene 6 transfection reagent. After 24 h, supernatant was filtered and incubated with MSCs for 12 h in the presence of 10 μg/mL polybrene (Sigma). Stable, transduced clones were obtained by puromycin selection (Sigma; 3 μg/mL for 5 days) and verified by immunostaining, microarray, and western blot. Negative control MSCs were transduced with pMSCV-IRES-EGFP (MSCNull).
Cardiomyocyte culture and coculture with MSCs
CMs were isolated from ventricles of 1–3-day-old SD rats using a commercially available neonatal CM isolation kit (Worthington Biochemical Co.).
To investigate transdifferentiation, MSCs were plated with CMs at a ratio of 1:40 in either dual-chamber or mixed cocultures, respectively. In the dual-chamber culture system, CMs were seeded in the upper layer and MSCs were seeded in the lower layer. To quantify the transdifferentiation rate, mixed coculture cells were stained with anti-α-sarcomeric actinin antibody (clone EA-53; Sigma) conjugated with allophycocyanin. At least 20,000 cells were analyzed on a BD FACS-Calibur cell sorter (BD Biosciences).
To identify cell fusion, neonatal CMs were labeled with a red fluorochrome membrane dye, PKH26 (Sigma), and were cocultured with GFP-positive MSCs for 7 days at a ratio of 20:1 (CM:MSC). The double-positive cells were counted using FACS analysis and examined under microscope.
Real-time quantitative polymerase chain reaction
Total RNA from cells was isolated using Trizol reagent (Invitrogen), followed by DNAse treatment and purification with RNeasy mini kit (Qiagen). Complimentary DNA was synthesized in a 20 μL reaction mixture using SuperScript™ III First-Strand Synthesis for reverse transcription (RT)-polymerase chain reaction (PCR) (Invitrogen). An aliquot of cDNA was amplified using Taq DNA polymerase (2.5 U; Invitrogen) in the presence of 1 μM sense and antisense primers (Table 1). Quantitative real-time PCR was carried out on the iQ5 real-time system with iQ SYBR Supermix (Bio-Rad). Expression of each target mRNA relative to GAPDH was calculated under experimental and control conditions based on threshold cycle (CT) as r=2–Δ(ΔCT), where ΔCT=CT target – CT GAPDH and Δ(ΔCT)=ΔCT experimental – ΔCT control.
Table 1.
Sequence and Product Size for Each Primer
| Primers | Sense | Antisense |
|---|---|---|
| Wnt11 (186 bp) | 5′-CAG GAT CCC AAG CCA ATA AA | 5′-GAC AGG TAG CGG GTC TTG AG |
| GATA-4 (255 bp) | 5′-CTG TCA TCT CAC TAT GGG CA | 5′-CCA AGT CCG AGC AGG AAT TT |
| BNP (350 bp) | 5′-TTC AGC CTC GGA CTT GGA AAC | 5′-CCT TGT GGA ATC AGA AGC AGG |
| Islet-1 (441 bp) | 5′-CAG CTG CAC ACC TTG CGG AC | 5′-GTG TAT CTG GGA GCT GCG AG |
| α-Actinin (101 bp) | 5′-TCA TCC TCC TGG GCC ATG T | 5′-TAT CAC GCG GCG AAC CA |
| GAPDH (374 bp) | 5′-ATG GGA GCT GGT CAT CAA C | 5′-CCA CAG TCT TCT GAG TGG CA |
Electroimmunoblotting
Cultured cells were homogenized in lysis buffer, and protein content was determined by the Bradford method. Denatured proteins (25 and 50 μg) were separated using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane (Bio-Rad), and immunoblotted overnight at 4°C with a primary antibody: anti-Wnt11 (Abcam), anti-GATA-4 (Abcam), anti-BNP (Abcam), anti-islet-1 (Santa Cruz), or anti-β-actin (Cell Signaling). The membranes were then incubated for 1 h with HRP-conjugated secondary antibody at room temperature, washed, and developed with the ECL plus kit (GE Healthcare). The blots were analyzed by densitometry with NIH image software (AlphaEase FC, version 6.0.0).
Immunocytochemistry
Immunocytochemistry was performed as previously described [21]. Cells were fixed in 4% paraformaldehyde and incubated with mouse monoclonal anti-sarcomeric α-actinin (Sigma) or rabbit polyclonal anti-Wnt11 and connexin 43 (Abcam). Nuclei were stained with 4′,6-diamino-2-phenylindole. Confocal images were obtained with a Zeiss LSM 510 Confocal/Two-Photon Microscope equipped with Zeiss AIM, version 4.2.
Transmission electron microscopy
Cultured cells were rinsed and immersed in 2.5% buffered glutaraldehyde for 4 h, rinsed in 0.1 mol/L sodium cacodylate buffer (pH 7.3), and postfixed for 1 h in 1% buffered osmium tetroxide. The cells were embedded in Epon resin and cut into 60-nm-thick sections with a Sorvall MTB2 ultramicrotome. The sections were stained with uranyl acetate and lead citrate and examined with a Hitachi H-600 electron microscope at 75 kV.
Functional studies
Expression of GATA-4 in MSCWnt11 was downregulated using double-stranded SMARTpool siRNA designed to target GATA-4. Oligonucleotides were synthesized by Ambion, which are as follows: GATA-4 siRNA sequence: sense, 5′-CGG AAG CCC AAG AAU CUG A-3′, and anti-sense, 5′-UCA GAU UCU UGG GCU UCC G-3′; and nonsilencing, negative-control (scrambled) siRNA sequence: sense, 5′-UUC UCC GAA CGU GUC ACG U-3′, and antisense, 5′-ACG UGA CAC GUU CGG AGA A-3′. Transfection was performed at about 70% confluence in 6-well plates using Lipofectamine RNAiMAX Reagent (Invitrogen) following the manufacturer's instructions.
Statistical analysis
Quantitative data were expressed as mean±standard error of the mean. One-way analysis of variance (SigmaStat 3.1; Systat Software) with the Holm-Sidak method and/or Bonferroni correction was used to determine the significance of differences. Differences were considered significant if the P value was <0.05.
Results
Transduction of MSCs with Wnt11
We transduced Wnt11 into MSCs to study the effect of Wnt11 on MSC transdifferentiation into cardiac phenotypes. Retroviral-mediated transduction of Wnt11 was confirmed by immunostaining, real-time quantitative PCR, and western blotting. Although both MSCWnt11 and MSCNull were GFP immunopositive, only MSCWnt11 stained intensely for Wnt11 (Fig. 1A). In addition, MSCWnt11 exhibited higher levels of Wnt11 mRNA (Fig. 1B) and protein expression (Fig. 1C). These data demonstrate successful transduction and expression of retroviral vector encoding a Wnt11/GFP bicistronic construct.
FIG. 1.
Wnt11 expression in transduced MSCs. (A) Immunostaining of MSCs with Wnt11; nuclei were counterstained with DAPI. (B) Quantitative real-time PCR of Wnt11 expression. (C) Western blot of Wnt11 and corresponding semiquantitative data. ‡P<0.05, versus MSCNull. MSC, mesenchymal stem cell; DAPI, 4′,6-diamino-2-phenylindole; PCR, polymerase chain reaction.
Cardiac genes expression
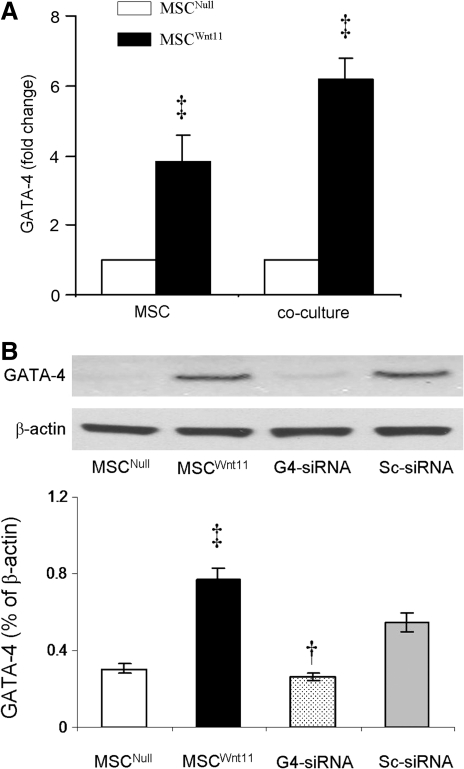
GATA-4 is a cardiac transcription factor. GATA-4 levels in MSCWnt11 were examined. GATA-4 expression was significantly upregulated in MSCWnt11 (compared with MSCNull) when MSCs were either cultured alone or cocultured with CMs in a dual-chamber system (Fig. 2A). To knock down GATA-4 activity, MSCWnt11 were transduced with GATA-4-siRNA. Quantitative real-time PCR showed that the expression of GATA-4 in GATA-4-siRNA-MSCWnt11 decreased by 54%±4%, compared with scrambled siRNA-MSCWnt11. GATA-4 protein was reduced in GATA-4-siRNA-MSCWnt11 cells compared with scrambled siRNA-MSCWnt11 (Fig. 2B).
FIG. 2.
Overexpression of Wnt11 upregulates GATA-4 in MSCs. (A) Quantitative real-time PCR of GATA-4 expression in MSCs cultured alone (MSC) and with CMs in a dual-chamber set (coculture). (B) Western blot of GATA-4 and corresponding semiquantitative data for various MSCs. ‡P<0.05, versus MSCNull; †P<0.05, versus MSCWnt11. G4-siRNA, GATA-4-siRNA-MSCWnt11; Sc-siRNA, scrambled siRNA-MSCWnt11; CM, cardiomyocytes.
Expression of the cardiac markers α-actinin, BNP, and islet-1 was significantly upregulated in MSCWnt11 either cultured alone (Fig. 3A) or cocultured with CMs in a dual-chamber system for 7 days (Fig. 3B). However, these proteins in MSCWnt11 were suppressed when GATA-4-siRNA was introduced (Fig. 3C).
FIG. 3.
Expression of cardiac markers in MSCs. (A, B) Quantitative real-time PCR of cardiac markers in MSC alone (A) or cocultured with CMs in dual-chamber dishes for 7 days (B). (C) Representative western blot of cardiac markers and semiquantitative data expressed as ratio to native CMs. ‡P<0.05, versus MSCNull; †P<0.05, versus MSCWnt11. BNP, brain natriuretic peptide.
Differentiation into cardiac phenotype
To determine whether GFP+ cells in 7-day mixed cocultures with native CMs had differentiated into myocardial phenotypes, cells were immunostained for α-actinin and visualized using confocal microscope with Z-stack reconstruction. MSCs were distinguished from CMs on basis of GFP positivity. To exclude nonspecific α-actinin staining, an isotype control was used (Fig. 4A, control). Some GFP+ MSCs were also α-actinin+, indicating that these cells were transdifferentiated CMs. These cells had GFP-positive nuclei, which excluded the possibility of GFP+ cell overlay with α-actinin+ CMs. Differentiation was also confirmed by transmission electron microscopy. The ultramicroscopic features of a differentiated MSC showed sarcomeres, but these cells contained nucleus with multiple nucleoli, as observed in the MSC nucleus (Fig. 4B). However, these differentiated CMs had weak α-actinin staining and less sarcomeres compared with native CMs.
FIG. 4.
Transdifferentiation of MSCs into cardiac phenotypes. (A) Representative micrographs showing GFP, α-actinin immunolabeling, and DAPI staining after MSCs cocultured with CMs for 7 days. White stars: differentiated CM (GFP+/α-actinin+ cell); green arrow: nontransdifferentiated MSC (GFP+/α-actinin− cell); white arrows: native CMs. Yellow arrow in “control” panel indicates apoptotic CMs with condensed nuclei and shrunk phenomenon, which is distinguished from normal CM (white arrow). (B) Electron microscopic images. Typical CM has myofibers with clear sarcomeres (S) and central nucleus with single nucleolus (red arrow). MSCs have no myofibers, and nucleus has multiple nucleoli (red arrows). Differentiated CMs from MSCs is confirmed by multinucleoli (red arrows) and appearance of sarcomeres (S). It had physical contacts with neighboring myocytes (red arrowheads). (C, D) Representative FACS data of α-actinin+/GFP+ cells (C) and quantitative assay in various groups (D). ‡P<0.05, versus MSCNull; †P<0.05, versus MSCWnt11; FACS, fluorescence activated cell sorting.
To approximate differentiation rates, cells from mixed cocultures were performed FACS after cocultured cells were stained with α-actinin. Native CMs were α-actinin positive, MSCs were GFP positive, and cardiac phenotypes, which transdifferentiated from MSCs, were positive for both α-actinin and GFP. The percentage of α-actinin+/GFP+ cells was significantly higher in MSCWnt11 compared with MSCNull, which was abolished by transducing GATA-4-siRNA (Fig. 4C, D). These results indicate that Wnt11 promotes MSC transdifferentiation into cardiac phenotypes via upregulating GATA-4.
To confirm whether fusion plays an important role in cell differentiation, GFP-positive MSCs were cocultured with PKH26-prelabeled neonatal CMs (1:20 ratio). Flow cytometry analysis data showed that about 40% of MSCs expressed PKH26 after coculture with CMs for 7 days (Fig. 5A). To confirm whether GFP+/PKH26+ cells were fused cells, the cultured cells were further examined under inverted microscope (Fig. 5B–F). A clear cell edge between 2 types of cells was seen and connexin 43 was expressed. Some MSCs were not positive for PKH26, and even they were located near the CMs. These phenomena indicate that cells positive for both GFP and PKH26 contact with CMs by gap junctions but not by fusion.
FIG. 5.
Myogenic transformation of MSCs may involve cell fusion or connection with native CM. (A) Representative FACS data of PKH26+/GFP+ cells. (B–F) Representative confocal images illustrate PKH26 (red, B), GFP (green, C), nucleus (blue, D), and connexin 43 (white, E) labeling. Seven days after MSCs were cocultured with PKH26-prelabeled neonatal CMs (1:20 ratio), some MSCs were positive for GFP and PKH26 (F; white star indicates double positivity for PKH26 and GFP cell). Connexin 43 was observed between MSCs and native CMs (yellow arrows). GFP, green fluorescent protein; FACS, fluorescence activated cell sorting.
Discussion
It has been reported that Wnt11 plays a vital role in myocardial development [8,9,22] and Wnt11-conditioned medium promotes BMMNC and CPC differentiation into cardiac phenotypes [10,11]. Wnt11-conditioned medium also increased cord blood-derived CD133+ cells differentiation into endothelial cells in vitro [23]. Overexpression of Wnt11 also increases osteoblast maturation and mineralization [24]. Bone morphogenetic protein (BMP)-treated Wnt11-overexpressing MC3T3 E1 preosteoblasts show the greatest increase in alkaline phosphatase activity, ∼2-fold higher than the BMP-treated control cells after the start of BMP induction for 6 days [24].
In this study, we demonstrate that transduction of Wnt11 into MSCs promotes their differentiation into cardiac phenotypes. Loss-of-function experiments suggest that these effects are associated with upregulation of GATA-4. Our study is novel in 3 aspects. First, we used 2 controls—the original, basal MSCs (MSCbas) and the transduction control (MSCNull)—to exclude the possibility that viral transduction may impair the ability of MSCs to differentiate into cardiac phenotypes. Second, the quantitative analysis of cardiac marker expression was performed in MSCs cultured alone and cocultured with CMs as well as analysis of cardiac marker expression at the RNA and protein level was carried out. Third, transdifferentiation experiments included ultrastructural analysis—revealing MSC-derived CMs with typical developing sarcomeres.
We quantified the expression of cardiac markers under different treatments and observed the morphological changes of MSCs using electron and confocal microscopes. Overexpression of Wnt11 not only significantly increases the expression of cardiac markers in MSCs, but also promotes MSC transdifferentiation into cardiac phenotype in coculture, which is consistent with previous reports [10,11]. The quantitative analysis of the expression of cardiac markers was performed under MSCs cultured alone and those cultured in a dual-chamber system, which prevented any possibility of CM contaminations. Compared with native CMs, differentiated cells are immature CMs with weak α-actinin staining, few sarcomeres (Fig. 4A, B), and less cardiac marker expression (Fig. 3C). Therefore, Wnt11 transduction can significantly increase the potential of MSC transdifferentiation into cardiac phenotype.
It has been reported that no convincing evidence of transdifferentiation of human endothelial progenitor cells into CMs was obtained in coculture system [6]. Same outcome was observed in our preliminary study when MSCs were cocultured with CMs in various ratios (from 1:5 to 1:40). The result showed that no differentiated cells could be found when the ratio of CM to MSC was <10:1. This suggests that stem cell differentiation into CMs needs a cardiac environment facilitated by enough CMs. A recent study supports this hypothesis by showing that the numbers of bone marrow stem cells expressing cardiac markers were positively correlated with the numbers of neonatal CM in the culture [25].
We did not find α-actinin+/GFP+ cell in either MSCWnt11 culture alone or those cocultured with native CMs in a dual- chamber system. It suggests that specific communication between stem cells and native myocytes is required in the transdifferentiation of stem cells into mature CMs. Some studies have suggested that the phenotypic changes of stem cells are the result of cell fusion [26,27]. It has been reported that cell fusion occurred in ∼30%–40% of cells that acquired a myogenic phenotype [25]. We investigated the possibility of cell fusion between GFP-positive MSCs and CMs prelabeled with PKH26. Approximately 40% MSCs were PKH26 positive after being cocultured with PKH26-prelabeled CMs for 7 days. Our morphological data show a clear cell edge between 2 types of cells. Further, connexin 43 staining was positive between CMs and PKH26-positive MSCs. These data indicated that gap junctions were formed between MSCs and CMs, which is similar to those among existing resident CMs. Moreover, stem cells fused with other cells, resulting in the formation of multinucleated cells. Very few multinucleated cells were observed. Therefore, the positive MSCs for both PKH26 and GFP were connected with CMs by gap junctions or other specific channels, which result in PKH26 transfer. However, the possibility of MSCs fusion with CMs cannot be excluded in this study.
Wnt11 signaling is reportedly sufficient to induce cardiomyogenic differentiation in unfractionated BMMNCs in the absence of CMs [10]. In our study, Wnt11 increased the potential of MSCs to transdifferentiate into cardiac phenotypes, both cultured alone and with CMs in a dual-chamber system. However, no MSCs were transformed into beating cells or α-actinin–positive cell without making contact with native CMs. These different outcomes may result from duration of cell culture (7 days in our study), and/or culture environment, as well as the type of stem cells.
We show in this study that upregulation of GATA-4 plays a pivotal role in the observed Wnt11-mediated cardiac differentiation. Specifically, transfection of GATA-4-siRNA into MSCWnt11 dramatically reduced transdifferentiation of MSCWnt11. It is well known that GATA-4 is a potent transcriptional activator of several cardiac muscle-specific genes and a key regulator of the CM gene program [28,29]. Inhibition of GATA-4 expression interferes with expression of cardiac muscle genes and blocks development of beating CMs [28]. Upregulation of GATA-4 in MSCWnt11 may well commit these cells to a higher cardiac transdifferentiation rate, and this effect may be mediated by Wnt11 activation of PKC or c-Jun NH2-terminal kinase signaling pathways [10]. Further study is needed to explore how Wnt11 upregulates GATA-4, resulting in MSC transdifferentiation into cardiac phenotypes.
Conclusions
Transduction of Wnt11 increases MSC differentiation into myocardial phenotypes. The underlying mechanisms may involve upregulation of GATA-4 by Wnt11.
Acknowledgments
This work was supported by National Institutes of Health grants HL083236, HL105176 (to M.X.), and HL087246 (to M.A.).
Author Disclosure Statement
None.
References
- 1.Zeng L. Hu Q. Wang X. Mansoor A. Lee J. Feygin J. Zhang G. Suntharalingam P. Boozer S. Mhashilkar A. Panetta CJ. Swingen C. Deans R. From AH. Bache RJ. Verfaillie CM. Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 2.Quevedo HC. Hatzistergos KE. Oskouei BN. Feigenbaum GS. Rodriguez JE. Valdes D. Pattany PM. Zambrano JP. Hu Q. McNiece I. Heldman AW. Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajstura J. Rota M. Whang B. Cascapera S. Hosoda T. Bearzi C. Nurzynska D. Kasahara H. Zias E. Bonafe M. Nadal-Ginard B. Torella D. Nascimbene A. Quaini F. Urbanek K. Leri A. Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 4.Arminan A. Gandia C. Bartual M. Garcia-Verdugo JM. Lledo E. Mirabet V. Llop M. Barea J. Montero JA. Sepulveda P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907–918. doi: 10.1089/scd.2008.0292. [DOI] [PubMed] [Google Scholar]
- 5.Rangappa S. Entwistle JW. Wechsler AS. Kresh JY. Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype. J Thorac Cardiovasc Surg. 2003;126:124–132. doi: 10.1016/s0022-5223(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 6.Gruh I. Beilner J. Blomer U. Schmiedl A. Schmidt-Richter I. Kruse ML. Haverich A. Martin U. No evidence of transdifferentiation of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation. 2006;113:1326–1334. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 7.Koninckx R. Hensen K. Daniels A. Moreels M. Lambrichts I. Jongen H. Clijsters C. Mees U. Steels P. Hendrikx M. Rummens JL. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11:778–792. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 8.Pandur P. Lasche M. Eisenberg LM. Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 9.Hardy KM. Garriock RJ. Yatskievych TA. D'Agostino SL. Antin PB. Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty MP. Abdel-Latif A. Li Q. Hunt G. Ranjan S. Ou Q. Tang XL. Johnson RK. Bolli R. Dawn B. Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells. Circulation. 2008;117:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.741066. [DOI] [PubMed] [Google Scholar]
- 11.Koyanagi M. Haendeler J. Badorff C. Brandes RP. Hoffmann J. Pandur P. Zeiher AM. Kuhl M. Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280:16838–16842. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- 12.Moon RT. Kohn AD. De Ferrari GV. Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita JK. Takano M. Hiraoka-Kanie M. Shimazu C. Peishi Y. Yanagi K. Nakano A. Inoue E. Kita F. Nishikawa S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005;19:1534–1536. doi: 10.1096/fj.04-3540fje. [DOI] [PubMed] [Google Scholar]
- 14.Naito AT. Shiojima I. Akazawa H. Hidaka K. Morisaki T. Kikuchi A. Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marvin MJ. Di Rocco G. Gardiner A. Bush SM. Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzahor E. Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikuchi A. Yamamoto H. Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Nagy II. Railo A. Rapila R. Hast T. Sormunen R. Tavi P. Rasanen J. Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc Res. 2010;85:100–109. doi: 10.1093/cvr/cvp254. [DOI] [PubMed] [Google Scholar]
- 19.Xu M. Wani M. Dai Y-S. Wang J. Yan M. Ayub A. Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 20.Dai YS. Markham BE. p300 functions as a coactivator of transcription factor GATA-4. J Biol Chem. 2001;276:37178–37185. doi: 10.1074/jbc.M103731200. [DOI] [PubMed] [Google Scholar]
- 21.Xu M. Wang Y. Hirai K. Ayub A. Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am J Physiol Heart Circ Physiol. 2001;280:H899–H908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- 22.Garriock RJ. D'Agostino SL. Pilcher KC. Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev Biol. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Nikolova T. Wu M. Brumbarov K. Alt R. Opitz H. Boheler KR. Cross M. Wobus AM. WNT-conditioned media differentially affect the proliferation and differentiation of cord blood-derived CD133+ cells in vitro. Differentiation. 2007;75:100–111. doi: 10.1111/j.1432-0436.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 24.Friedman MS. Oyserman SM. Hankenson KD. Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J Biol Chem. 2009;284:14117–14125. doi: 10.1074/jbc.M808337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He XQ. Chen MS. Li SH. Liu SM. Zhong Y. McDonald Kinkaid HY. Lu WY. Weisel RD. Li RK. Co-culture with cardiomyocytes enhanced the myogenic conversion of mesenchymal stromal cells in a dose-dependent manner. Mol Cell Biochem. 2010;339:89–98. doi: 10.1007/s11010-009-0372-2. [DOI] [PubMed] [Google Scholar]
- 26.Metzele R. Alt C. Bai X. Yan Y. Zhang Z. Pan Z. Coleman M. Vykoukal J. Song YH. Alt E. Human adipose tissue-derived stem cells exhibit proliferation potential and spontaneous rhythmic contraction after fusion with neonatal rat cardiomyocytes. FASEB J. 2010 doi: 10.1096/fj.09-153221. [Epub ahead of print]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonde S. Pedram M. Stultz R. Zavazava N. Cell fusion of bone marrow cells and somatic cell reprogramming by embryonic stem cells. FASEB J. 2010;24:364–373. doi: 10.1096/fj.09-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grepin C. Nemer G. Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development (Cambridge, England) 1997;124:2387–2395. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- 29.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]