Abstract
Our purpose was to project and compare clinical and quality-adjusted life year (QALY) outcomes of adjuvant radiotherapy (ART) vs. salvage radiotherapy (SRT) after radical prostatectomy for men with locally advanced prostate cancer.
We constructed a Markov model to simulate the randomized studies of observation vs. ART, assuming 75% of observation patients would receive SRT at prostate specific antigen (PSA) recurrence. Transition probabilities and utility inputs were drawn from randomized trials of ART and cohort studies of SRT. We projected 10-year PSA recurrence-free survival, metastasis-free survival and overall survival.
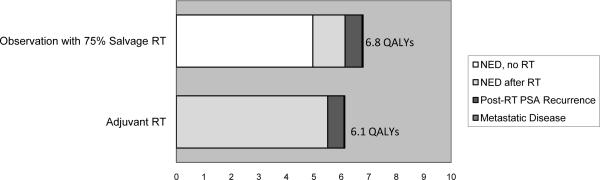
We found that observation with selective SRT yielded slightly worse outcomes than ART for post-RT PSA recurrence-free survival (47% and 52%), metastasis-free survival (69% and 70%) and overall survival (72% and 73%). Findings were robust to sensitivity analyses. After adjusting for the disutility of RT, observation plus SRT yielded better QALYs at 10 years than ART (6.80 and 6.13 QALYs).
Thus, observation plus SRT may be optimal for men likely to comply with surveillance who wish to minimize treatment side effects. These findings reflect outcomes for the average patient given the current level of evidence and are meant to help inform current decision-making as we await future clinical studies of comparative effectiveness.
Keywords: prostate cancer, radiotherapy, decision analysis
Approximately one-third of men undergoing radical prostatectomy have locally advanced prostate cancer (PC), characterized by positive surgical margins (PSM), extracapsular extension (ECE) and/or seminal vesicle invasion (SVI).1 Given a negative post-operative prostate specific antigen (PSA), the 5-year PSA recurrence risk in such men varies from 2% in a 65-year old man with preoperative PSA of 4 ng/mL, Gleason grade 3+3 tumor with only one pathologic findings of locally advanced disease to 70% in a 65-year old man with preoperative PSA of 10 ng/mL, Gleason grade 4+4 tumor with PSM, ECE and SVI.2 In such men, post-operative pelvic radiotherapy (RT) significantly improves disease control3–7 but has side effects.8
For men with locally advanced PC, RT can be administered as either adjuvant RT (ART, given post-operatively to prevent recurrence) or salvage RT (SRT) given after a period of initial observation, if/when local recurrence is suspected. Three randomized trials of men with locally advanced PC demonstrated a 5-year PSA recurrence probability following prostatectomy of about 50% for men assigned to observation and 25% after ART.3,6,7 One trial also showed a statistically significant difference in metastasis (HR 0.71, 95% CI 0.54–0.94) and overall survival (HR 0.72, 95% CI 0.55–0.96).9 However, results for metastasis and death were possibly biased: only 32% of men with PSA recurrence on observation received SRT and of those only 55% received it early (i.e., at the first signs of PSA recurrence) when SRT is most effective. Moreover, the design biased against observation -- in this group the endpoint was pre-SRT PSA recurrence, not post-SRT PSA recurrence. There are no randomized trials of SRT, but several cohort studies4,5,10–17 suggest improved metastasis-free and overall survival.17
Given the current state of knowledge, the ideal comparative effectiveness trial would randomize men with locally advanced PC at prostatectomy to either (1) ART or (2) observation plus early SRT. Such a trial is underway, but will take over a decade to recruit enough patients (>8,000) and evaluate clinically important endpoints.18 Some argue that for these reasons, the ART vs. SRT question is unlikely to ever be settled by a clinical trial.19 To aid current patient-provider discussions, we used decision analysis to synthesize the current evidence and project outcomes over a 10-year time horizon: post-RT PSA recurrence-free survival, metastasis-free survival, overall survival, and quality-adjusted life years (QALYs).
MATERIALS AND METHODS
Data Sources
Articles for inclusion were drawn from a systematic review of radiation after radical prostatectomy published in 200820 as well as a PubMed search with MeSH term “prostate cancer” and the subheading “radiotherapy” as well as the free text terms “prostate cancer AND salvage radiation” and “prostate cancer AND adjuvant radiation”. We used published results from three randomized trials of initial observation versus ART – the Southwestern Oncology Group (SWOG)6,9 European Organization for Research and Treatment of Cancer (EORTC)3 and the German Cancer Society (GCS)7 trials – and one cohort study of SRT.4 Participants in all studies were similar age, pre- and post-prostatectomy PSA, pathologic stage, and positive margin rate, but slightly different in Gleason score, lymph node status, and pre-RT PSA (Table 1). In the trials, PSA recurrence rate was significantly higher with observation than ART. We estimated progression probabilities for our base-case analysis from the SWOG trial, because it was the only trial with follow-up long enough to detect a difference in the important long-term outcomes of metastasis and death. However, it should be noted that the more contemporary EORTC and GCS trials with shorter follow-up have similar 5-year PSA recurrence-free survival compared with SWOG: observation vs. ART in SWOG was 45% vs. 72%, in EORTC was 53% vs. 74% and in GCS was 54% vs. 72%. Thus, using the results from the SWOG study as the primary reference in our model is the more conservative approach (i.e. favors ART). Results from cohort studies of SRT4,5,10–17 can be used to estimate progression probabilities following SRT. We used data from Stephenson and colleagues4 because it is the largest study by nearly 10-fold and reports results stratified by pre-RT PSA which was essential to our model development. Importantly, a subset of 328 men in this study was treated at PSA ≤0.5 ng/mL, which approximates the threshold for recurrence in the SWOG and EORTC studies (PSA >0.4 ng/mL).
Table 1.
Features of patient cohorts in reference articles
| SWOG (ART vs. Observation)6 | EORTC (ART vs. Observation)3 | GCS (ART vs. Observation)7 | Stephenson (SRT)4 | |
|---|---|---|---|---|
| N | 425 | 1005 | 307 | 1603 |
| Years of study enrollment | 1988–1997 | 1992–2001 | 1997–2004 | 1987–2005 |
| Age in years, mean/median (range) | 65 (44–79) | 65 (61–69) | 65 | 62 (58–67) |
| Pre-prostatectomy PSA (ng/ml), median | 10.0 | 12.3 | 9.6 | 10.5 |
| Post-prostatectomy PSA (ng/ml) | <0.2 (66%) ≥ 0.2 (34%) | median 0.2; range (0.1–0.3) | 0 | Persistently elevated in 29%. (Pre-radiotherapy PSA = 1.1) |
| Pathologic Features | ||||
| ECE or PSM = 67% | ECE = 77% | ECE = 65%; T4 = 6% | ECE = 65% | |
| SVI = 11% | SVI = 26% | SVI = 27% | SVI = 24% | |
| ECE+ SVI + PSM = 22% | PSM = 63% | PSM = 64% | PSM = 51% | |
| Positive lymph nodes | 0% | 0.2% | 2% | 3% |
| Pathologic Grade | ||||
| ■ WHO 1 or Gleason ≤6 | 51% | 13% | 37% | 26% |
| ■ WHO 2 or Gleason 7 | 36% | 63% | 52% | 52% |
| ■ WHO 3 or Gleason 8–10 | 13% | 24% | 11% | 22% |
| Radiation Dose | 60Gy | 60Gy | 60Gy | 70Gy |
| Follow-up (mean*, median**) | 10.9* years after randomization | 5.0** years after randomization | 4.5* years after randomization | 7.5** years after prostatectomy, 4.4** years after RT |
Model Development
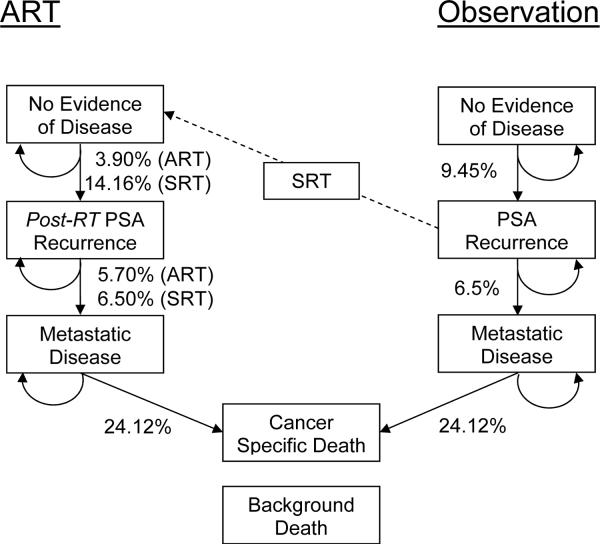
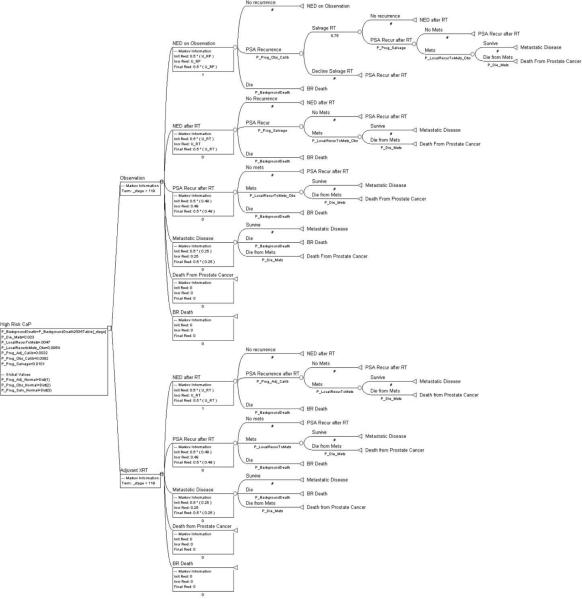
We developed a state-transition Markov model with one-month cycle length to evaluate clinical outcomes after prostatectomy for a hypothetical cohort of 65-year-old men with locally advanced PC managed post-operatively with (1) ART or (2) initial observation plus SRT delivered at early PSA recurrence (PSA ≤0.5 ng/mL). We assumed that men had PSA <0.4 ng/mL post-operatively and similar demographic and cancer characteristics to SWOG participants. Figure 1 provides an overview of the model (for complete model see Figure 2). Each month, ART-treated men faced a lower PSA recurrence risk than men on observation.3,6 For men on observation, we assumed that 75% would receive SRT at PSA recurrence, and varied this assumption in sensitivity analysis. Each month, men in either treatment arm could experience a post-RT PSA recurrence, either local or metastatic. The outcome of post-RT PSA recurrence was designed to be similar to an intent-to-treat analysis: for the 25% of men with PSA recurrence on observation who didn't receive SRT, their initial PSA recurrence was considered as their post-RT PSA recurrence. Men with local recurrence faced a monthly risk of metastasis. All men with a post-RT PSA recurrence, whether local or metastatic, were managed with androgen deprivation therapy. Death from PC only occurred among subjects with metastases. Men were at risk of dying from other causes, based on the US life tables.21 Analyses were performed with TreeAge Pro 2009 (TreeAge Software, Inc.; Williamstown, MA).
FIGURE 1.
Simplified flow chart representing complex Markov model. Patients are assigned to ART or initial observation. With each 1-month cycle, subjects can remain in their current state or progress vertically through the model. For ease of interpretation, annual rather than monthly progression probabilities are shown. A variable portion of those with a PSA recurrence on observation may proceed through SRT to no evidence of disease in the RT vertical axis.
Figure 2.
Complete Markov decision analytic model as constructed using TreeAge Pro 2009 software. Pictured is the version we used for our cohort simulation. Background death rates with the strategies of observation and ART are both equal to the rates in the 2004 U.S. Life Table. 75% of men with a PSA recurrence on observation actually receive indicated SRT. SRT is delivered at a PSA threshold of ≤0.5 ng/ml. Note that the rate of progression from local recurrence to metastases is higher in the observation group than the ART group but the rate of progression from metastases to death is equal in the two groups. Note that to decline indicated SRT places someone in a health state equivalent to a post-RT PSA recurrence.
Estimating Model Parameters
Progression Probabilities
We derived the annual probabilities of PSA recurrence with and without ART from the 10-year PSA recurrence-free survival probabilities reported in the SWOG trial (52% for ART, 28% for observation),6 assuming constant rates over 10 years (Table 2).22 We derived the annual probabilities of PSA recurrence following SRT from the 10-year PSA recurrence-free probability in Stephenson et al.4 For this variable, we included only the subset of participants who were treated at PSA 0.51–1.0 ng/mL, to approximate the mean PSA at which SRT was delivered in the SWOG trial (0.7 ng/mL). Metastasis was defined in the reference articles as bony or visceral metastases or extra-pelvic nodal metastases.3,9 The cancer-specific mortality rate among men with metastases was derived from the 5-year relative survival probability for stage IV patients reported by the National Cancer Institute.23
TABLE 2.
Base-Case Estimates and Ranges Used in Sensitivity Analysis
| Transition Probabilities* | Base-Case Value |
|---|---|
| Annual probability of PSA recurrence (ranges for sensitivity analysis) | 0.0945 (0.0500 – 0.2000) |
| ■ Observation2, 7 | |
| ■ ART2, 7 | 0.0390 (0.0200 – 0.0800) |
| ■ SRT given at PSA≤0.5ng/mL5 | 0.1416 (0.1151 – 0.2486) |
| Annual probability of PSA recurrence progressing to metastases7 ■ Observation ■ ART |
0.065 |
| 0.057 | |
| Annual probability of death in those with metastatic disease25 ■ Observation or ART |
0.2412 |
| Utility23* | 1 |
| ■ No PSA recurrence after radical prostatectomy ± RT | |
| ○ No complications | |
| ○ Erectile dysfunction | 0.89 |
| ○ Urinary obstruction | 0.88 |
| ○ Urinary incontinence | 0.83 |
| ○ Bowel dysfunction | 0.71 |
| ■ Radiation therapy (acute effects for 6 weeks) | 0.73 |
| ■ PSA Recurrence after RT (includes worry associated with a chance of metastasis and the disutility of androgen suppression therapy) | 0.49 |
| ■ Metastatic disease | 0.25 |
| ■ Death | 0 |
| Probability of adverse events† | Radical Prostatectomy | Radical Prostatectomy + RT |
|---|---|---|
| ■ Erectile dysfunction | 0.84–0.88 | 0.89–0.91 |
| ■ Urinary incontinence | 0.03 | 0.07 |
| ■ Urinary obstruction/irritation | 0.1 | 0.22 |
| ■ Bowel dysfunction | 0.05 | 0.14–0.32 |
Annual probabilities are presented for ease of understanding, although model inputs were monthly probabilities.
Model Calibration
We used calibration to estimate transition probabilities that could not be directly calculated from the studies.24 For instance, the 10-year risks of post-RT PSA recurrence and metastasis was known but the risk of transition from a post-RT PSA recurrence to metastasis was unknown. Assuming all metastases were preceded by a PSA recurrence, the unknown transition probability (i.e., from post-RT PSA recurrence to metastases) was derived by varying its value over a plausible in order to match the model-predicted 10-year risk of metastasis with the observed risk. For all calibrations, we allowed 32% of the PSA recurrences on observation to receive SRT at an average PSA of 0.7 ng/mL, consistent with SWOG.
Adverse Effects
We considered all possible combinations of four common adverse effects of radical prostatectomy and RT: erectile dysfunction, urinary obstruction, urinary incontinence, and bowel dysfunction. We obtained the prevalence of these effects after radical prostatectomy, with and without RT, from the SWOG trial (Table 2).6,8
To derive QALYs, we multiplied the probability of each unique health state by the health-related quality-of-life weight (utility) of that state (Table 2). Utilities were drawn from Stewart and colleagues25 who used standard gamble to elicit utilities for RT, metastatic disease, and several single and joint health states for a mixed sample of older men from the community and men recently diagnosed with PC. Similar utility values were obtained by other investigators using different methods.26 We analyzed several models of the interaction between utilities for health states with a single complication and utilities for health states with multiple complications. The best-fit model (additive model) was used to impute the joint health state utilities not directly assessed in the Stewart study (see Appendix A).
Cohort Simulation
Having calibrated the model using conditions determined by SWOG: (1) 32% with PSA recurrence on observation received SRT; (2) SRT delivered at PSA 0.51–1.0 ng/ml and (3) background death rates drawn from the 1996 U.S. life table21 (time frame of SWOG trial), we then ran the model for a modern cohort, using the most recent life table (2004)27 and allowing 75% of men with PSA recurrence on observation to receive SRT at PSA ≤0.5 ng/ml (assuming some patients will be lost to follow-up or otherwise refuse SRT). We compared ART and observation with SRT on the basis of post-RT PSA recurrence-free survival, metastasis-free survival, and overall survival at 10 years post-surgery. We also projected quality-adjusted life years over 10 years, using utilities shown in Table 1, to illustrate the tradeoff between side effects and benefits of ART. The QALY analysis was repeated for a hypothetical 55- and 75-year old man, inputting the different background death rates specific to these ages.
Sensitivity Analysis
We performed sensitivity analyses for post-RT PSA recurrence by varying the probabilities of PSA recurrence on observation, after ART, and after SRT. The first two probabilities were varied between half and twice their initial values. The third probability was varied over four probabilities of post-RT PSA recurrence in the Stephenson study when men were treated at PSA levels of ≤0.50, 0.51–1.00, 1.01–1.5, and >1.5 ng/mL.4 To account for the possibility that some men may not proceed with recommended SRT, we also varied the probability of receiving SRT between 0% and 100%.
Additional one-way sensitivity analyses were done in which we varied the utility of the following health states over the range of 80–120% of their reference value: (1) NED after RT, (2) Post-RT PSA recurrence, and (3) metastatic disease. We did not vary the utility of radical prostatectomy because it was common to all patients, regardless of whether they received RT or not.
RESULTS
Model Calibration
Our model accurately predicted the 10-year PSA recurrence-free survival in both the observation (28%) and ART arms (52%) of the SWOG trial. It also accurately predicted metastasis-free survival (71%) and overall survival (74%) in the ART arm, after we adjusted the background death rate to 80% of the rate in the 1996 U.S. life table (i.e., representing a healthier trial population in terms of non-cancer mortality). Using the annual probability of progressing from PSA recurrence to metastatic disease as estimated from the ART arm, our model under-predicted the incidence of metastases among men on observation. By increasing the annual probability of progression from PSA recurrence to metastatic disease from 5.7% to 6.5% in the observation arm, we were able to achieve the same 10-year metastasis-free survival as in the SWOG trial (61%).
Even with the correct 10-year metastasis-free survival, our model under-predicted deaths in the observation arm. To achieve the same 10-year overall survival probability as in the SWOG study (66%), we needed to either increase the annual probability of progression from metastasis to death in the observation arm to almost 3-fold that of the adjuvant arm (from 24% to 67%), or increase the background death rate to equal the rate in the 1996 U.S. life table, which is 1.25-fold the rate for the adjuvant arm.
Cohort Simulation
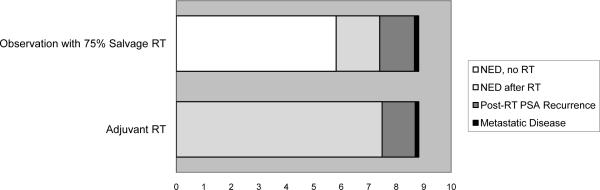
Using 2004 background death rates and assuming that 75% of men with PSA recurrence received SRT, our model demonstrated slightly lower 10-year PSA recurrence-free survival rates on observation vs. ART (47% vs. 52%; Table 3). We assumed that the probability of PSA recurrence progressing to metastases was higher in the observation patients but the annual probability of cancer death in those with metastatic disease was similar between strategies (Table 2). Under these assumptions we found that metastasis-free survival and overall survival were similar between strategies (Table 3). With observation plus SRT, 28% of men similar to those in the SWOG trial would never experience PSA recurrence over 10 years and thus avoid SRT. A distribution of the expected time spent in each health state over 10 years is shown (Figure 3a).
TABLE 3.
Ten-Year Model Outcomes with ART versus Observation plus Variable Rates of SRT
| Outcomes at 10 Years | Observation | ART | ||||
|---|---|---|---|---|---|---|
| 0% SRT | 25% SRT | 50% SRT | 75% SRT | 100% SRT | ||
| % PSA Recurrence-Free Survival | 28 | 35 | 41 | 47 | 53 | 52 |
| % Metastasis-Free Survival | 62 | 64 | 66 | 69 | 71 | 70 |
| % Overall Survival | 68 | 69 | 71 | 72 | 74 | 73 |
| % Prostate Cancer-Specific Death | 9 | 8 | 6 | 4 | 3 | 4 |
FIGURE 3a.
Distribution of average time spent in each health state over ten years in men managed with Observation vs Adjuvant RT where 75% of PSA recurrences on observation receive Salvage RT (empty area to the right represents death).
Expected QALYs
When the disutility of radiation was modeled, observation with SRT (given in 75% of PSA recurrences) was slightly preferred over ART at 10 years (6.80 vs. 6.13 QALYs, Figure 3b). When varying the age of the hypothetical patient to 55 years (7.23 vs. 6.54 QALYs) or 75 years (5.82 vs. 5.24 QALYs), observation remained preferred.
Figure 3b.
Quality-adjusted life expectancy (10 year time horizon) in men managed with Observation vs Adjuvant RT where 75% of PSA recurrences on observation receive Salvage RT.
Sensitivity Analysis
Given a man has a PSA recurrence on observation, we varied between 0–100% the probability that he would receive SRT (Table 3). The resulting PSA recurrence-free survival varied from 28–53%, metastasis-free survival from 62–71%, overall survival from 68–74%, and PC-specific deaths from 3–9%.
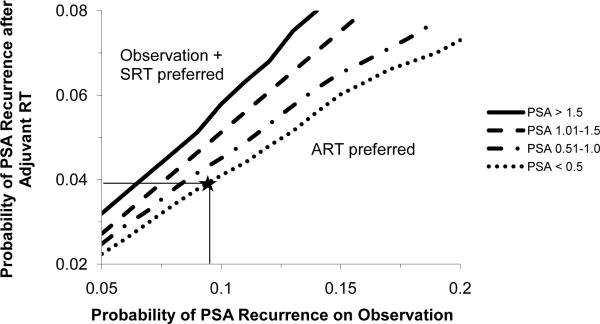
Using 3-way sensitivity analysis (Figure 4), we varied (1) the probability of PSA recurrence with observation, (2) the probability of PSA recurrence with ART, and (3) the PSA prompting SRT. The outcome of interest was cumulative time free of post-RT PSA recurrence over 10 years. As SRT is delayed until higher PSA values, ART becomes preferred over a wider range of effectiveness. Given the base-case values of probability of PSA recurrence with observation (9.5%/year) and ART (3.9%/year), the two strategies were equivalent when 75% of PSA recurrences on observation received SRT at PSA <0.5 ng/mL; however, when SRT was delayed until reaching a higher PSA, ART was preferred. These findings were robust to variations in the annual probability of PSA recurrence to the values reported in the EORTC trial: 12.1% for observation and 5.8% for ART.
FIGURE 4.
Three-way sensitivity analysis on outcome of post-RT PSA recurrence-free survival. To interpret, select a probability of recurrence after ART (base case = horizontal line at 3.9%) and on observation (base case = vertical line at 9.5%). If the corresponding point on the graph is above the line representing one's pre-determined PSA threshold at which SRT is delivered (base case = PSA < 0.5 ng/ml) then observation + SRT is preferred. If it corresponds to a point below that line then ART is preferred. In the base case analysis (represented by a “star”) ART is slightly preferred.
In one way sensitivity analyses where the utility of post-RT PSA recurrence and the utility of metastatic disease were varied and the outcome was QALYs over a 10-year time horizon, observation remained preferred over ART throughout the range of utility scores (results not shown). However, QALYs were sensitive to changes in the utility of RT. Specifically, the threshold value for the utility of NED after RT was 0.85; at values above this, ART was preferred. In this analysis, the utility of NED after radical prostatectomy was held constant at its reference value of 0.86.
DISCUSSION
Our model predicted that for men with locally advanced PC who undergo radical prostatectomy, initial observation with early SRT (PSA ≤ 0.5 ng/mL) is slightly less effective than ART for 10-year PSA recurrence-free survival, metastasis-free survival, and overall survival. However, observation with early SRT confers similar or slightly better health-related quality of life to ART; 28% of men similar to those in the SWOG study avoid RT through 10 years and many more delay RT, thus avoiding or delaying the adverse effects of RT. Our model underestimates the real disutility of RT; we assumed RT toxicity to be grade 1 or 2, whereas in the randomized trials grade 3 toxicity occurred in 4%.3,6 Furthermore, assessment of the late effects of RT was only available through 5 years.3,6 Including delayed complications would make ART less attractive.
Our model successfully projected the outcomes of PSA recurrence, metastasis, and death as reported for the SWOG randomized trial of ART versus observation. An obstacle to modeling the SWOG study results, particularly for overall survival, was that the background death rate in SWOG's observation group appeared to be higher than in the ART group. Specifically, the number needed to treat to prevent one metastasis was 12.2 men, but the number needed to treat to prevent one death was only 9.1 men.9 If the survival benefit of ART was achieved by reducing the number of metastases and thus cancer-specific deaths, then one would expect the number needed to treat to prevent one death to be more than the number needed to treat to prevent one metastasis. This raises the question of whether the observed survival benefit in the ART group was at least partially due to lower non-cancer-specific (i.e., background) death, despite randomization. We were able to account for this difference in survival in our model by either assigning a 67% annual probability of progression from metastasis to cancer death or a 1.25-fold higher background death rate in observation patients. Because a 67% annual death probability from metastatic PC is extremely high, we opted to use the higher background death rate for model calibration, then used equal background death rates for the cohort simulation. For all analyses, we applied a 24% probability of cancer death from metastases in both groups.
Decision models are limited by the assumptions used to build them. In general, ours is a conservative model, with assumptions constructed to bias against observation and SRT. Still, our model has limitations. We assumed that men on observation received SRT at earliest evidence of PSA recurrence (≤ 0.5 ng/mL). In reality, there will be variation in follow-up, and some men may progress beyond the point at which they would maximally benefit from SRT.4 Decisions about a patient's commitment to intense surveillance may need to be made on an individual basis. While our results were robust when assuming that 75% of the men needing SRT actually received it, when that number dropped to 50%, ART was preferred. If >75% of men with a PSA recurrence on observation can be expected to complete SRT then observation becomes an even more attractive option. Such a high SRT capture rate might be achievable in a trial setting; however, in community-based registries the frequency of SRT in men with a PSA recurrence has been as low as 11% in the early 1990s and as high as 32% in 2000–0428,29. Thus, the ability to follow an observation protocol and willingness to proceed with SRT should be integral to selecting observation vs. ART.
There are limits to the external validity of the results. First, roughly a third of the men in the SWOG and EORTC trials had a persistently elevated PSA post-prostatectomy, which could represent residual cancer. Second, contemporary men have on average lower grade, stage and PSA than men in the era of the SWOG and EORTC trials.2 Although, the more contemporary GCS trial in which all men had a negative post-operative PSA still had a probability of PSA recurrence similar to that in the SWOG and EORTC trials, the GCS men had high risk cancer (8% had T4 disease). A modern patient with only one of the features of locally advanced PC (ECE, PSA or SVI) will typically have a 10-year PSA recurrence-free survival of 70–80%, compared to the 28% used in this model. Third, men in the SWOG trial received SRT at higher PSA thresholds than those used currently. In summary, all three of these features that contrast with current practice (lower risk patients, a negative post-operative PSA and earlier SRT delivery) would only lend stronger support for initial observation in the modern era.
Other limitations bear mention. The negative impact of RT on QALYs significantly impacts the interpretation of our model. As utilities are subjective, future investigations should incorporate sensitivity analyses around the utility values in the model. Our findings should only be applied to men with disease similar to the average participant in the reference cohorts, and not men with metastatic disease, T4 disease, or PSA ≥0.4 post-prostatectomy. In subset analyses, the benefits of adjuvant and SRT were experienced by select high-risk patients;9,17 however, the reference studies lacked sufficient detail to design a model that accommodates factors predictive of individual response to therapy. We model results for a cohort of 65 year old men. When varying the background death rate down to that of a 55 year old man or up to that of a 75 year old man, QALYs remained higher with observation than ART. In sensitivity analysis we demonstrate that ART is preferred over observation for the outcome of 10-year QALYs only when the utility of RT is nearly equal to the utility of observation (0.85 vs. 0.86, respectively). In practical terms this means that ART would be preferred over observation only if radiation was essentially side effect free or if men placed little value on the additional risk of those radiation side effects. Indeed, modern radiation techniques may yield different results from those seen in this study; specifically, rectal complications are lower but urinary complications may be higher with intensity modulated radiation therapy than 3-D conformal radiotherapy.30 While our sensitivity analyses show our findings to be robust over a wide range of ART and SRT effectiveness, the optimum model would allow the clinician to input an individual's disease characteristics and arrive at a preferred treatment strategy. A combination of our model with input probabilities from nomograms such as the Kattan nomogram is intriguing area for future research.2
In our model, ART showed slightly better clinical outcomes compared with observation plus SRT in terms of post-RT PSA recurrence-free survival, metastasis-free survival, and overall survival. When the adverse effects of RT were included in the decision model, observation plus SRT became the preferred treatment. These results were highly dependent on delivering SRT early (at PSA ≤0.5ng/mL) and in ≥75% of observed patients with a PSA recurrence. This model can inform physician-patient treatment discussions as we await the results of randomized clinical studies.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Dr. Anne Marie Weber-Main for critically reviewing and editing manuscript drafts.
Footnotes
CONFLICT OF INTEREST STATEMENT The authors declare no conflict of interest.
REFERENCES
- 1.Surveillance, Epidemiology, and End Results (SEER) Program . Limited-Use Data (1973–2006) National Cancer Institute; www.seer.cancer.gov. released April 2009. [Google Scholar]
- 2.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr., Dotan ZA, DiBlasio CJ, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23(28):7005–7012. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366(9485):572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson GP, Goldman B, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. The prognostic impact of seminal vesicle involvement found at prostatectomy and the effects of adjuvant radiation: data from Southwest Oncology Group 8794. J Urol. 2008;180(6):2453–2457. doi: 10.1016/j.juro.2008.08.037. discussion 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson IM, Jr., Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 7.Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 8.Moinpour CM, Hayden KA, Unger JM, Thompson IM, Jr., Redman MW, Canby-Hagino ED, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26(1):112–120. doi: 10.1200/JCO.2006.10.4505. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buskirk SJ, Pisansky TM, Schild SE, Macdonald OK, Wehle MJ, Kozelsky TF, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol. 2006;176(3):985–990. doi: 10.1016/j.juro.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 11.Catton C, Gospodarowicz M, Warde P, Panzarella T, Catton P, McLean M, et al. Adjuvant and salvage radiation therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother Oncol. 2001;59(1):51–60. doi: 10.1016/s0167-8140(01)00302-4. [DOI] [PubMed] [Google Scholar]
- 12.Chawla AK, Thakral HK, Zietman AL, Shipley WU. Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: analysis of efficacy and prognostic factors. Urology. 2002;59(5):726–731. doi: 10.1016/s0090-4295(02)01540-6. [DOI] [PubMed] [Google Scholar]
- 13.Katz MS, Zelefsky MJ, Venkatraman ES, Fuks Z, Hummer A, Leibel SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483–489. doi: 10.1200/JCO.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald OK, D'Amico AV, Sadetsky N, Shrieve DC, Carroll PR. Predicting PSA failure following salvage radiotherapy for a rising PSA post-prostatectomy: from the CaPSURE database. Urol Oncol. 2008;26(3):271–275. doi: 10.1016/j.urolonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Taylor N, Kelly JF, Kuban DA, Babaian RJ, Pisters LL, Pollack A. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56(3):755–763. doi: 10.1016/s0360-3016(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 16.Trabulsi EJ, Valicenti RK, Hanlon AL, Pisansky TM, Sandler HM, Kuban DA, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72(6):1298–1302. doi: 10.1016/j.urology.2008.05.057. discussion 1302-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker C, Catton CN, Sydes MR, Parulekar WR, Murphy CM, Parmar MK, et al. RADICALS - an Intergroup randomised controlled trial in prostate cancer of optimal timing of radiotherapy and duration of hormone therapy after radical prostatectomy (MRC PR10, NCIC PR13). 2007 Prostate Cancer Symposium; Orlando, Florida. [Google Scholar]
- 19.Thompson IM, Tangen CM, Klein EA. Is there a standard of care for pathologic stage T3 prostate cancer? J Clin Oncol. 2009;27(18):2898–2899. doi: 10.1200/JCO.2008.20.9460. [DOI] [PubMed] [Google Scholar]
- 20.Pasquier D, Ballereau C. Adjuvant and salvage radiotherapy after prostatectomy for prostate cancer: a literature review. Int J Radiat Oncol Biol Phys. 2008;72(4):972–979. doi: 10.1016/j.ijrobp.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Anderson R. United States Abridged Life Tables, 1996. National Vital Statistics Reports. 1998;47(13):1–20. [PubMed] [Google Scholar]
- 22.Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Making. 1994;14(1):52–58. doi: 10.1177/0272989X9401400107. [DOI] [PubMed] [Google Scholar]
- 23.SEER Stat Fact Sheets: Prostate. [Google Scholar]
- 24.Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics. 2009;27(7):533–545. doi: 10.2165/11314830-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart ST, Lenert L, Bhatnagar V, Kaplan RM. Utilities for prostate cancer health states in men aged 60 and older. Med Care. 2005;43(4):347–355. doi: 10.1097/01.mlr.0000156862.33341.45. [DOI] [PubMed] [Google Scholar]
- 26.Krahn M, Ritvo P, Irvine J, Tomlinson G, Bremner KE, Bezjak A, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care. 2003;41(1):153–164. doi: 10.1097/00005650-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56(9):1–39. [PubMed] [Google Scholar]
- 28.Mehta SS, Lubeck DP, Sadetsky N, Pasta DJ, Carroll PR. Patterns of secondary cancer treatment for biochemical failure following radical prostatectomy: data from CaPSURE. J Urol. 2004;171(1):215–219. doi: 10.1097/01.ju.0000100087.83112.23. [DOI] [PubMed] [Google Scholar]
- 29.Moreira DM, Banez LL, Presti JC, Jr., Aronson WJ, Terris MK, Kane CJ, et al. Predictors of secondary treatment following biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. BJU Int. 105(1):28–33. doi: 10.1111/j.1464-410X.2009.08684.x. [DOI] [PubMed] [Google Scholar]
- 30.Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.