Abstract
We sought to describe change in cardiorespiratory (CR) fitness over 2 years in those with early–stage Alzheimer’s disease (AD) and nondemented aging and assess the relationship of CR fitness with cognitive decline, brain atrophy and dementia progression. Individuals with early-stage AD (n=37) and without dementia (n=53) attended clinical evaluations, cognitive and exercise tests, and MRI at baseline and 2 years later. CR fitness was lower in those with AD over the study period. Lower baseline CR fitness was associated with progression of dementia severity in AD. Declining CR fitness over 2 years was associated with brain atrophy in AD, especially in the parahippocampus. In nondemented participants, there was a trend for lower baseline fitness to be related to cognitive decline. Both lower baseline CR fitness and declining CR fitness over 2 years were associated with regional brain atrophy. We conclude that CR fitness is chronically reduced in those with AD. Further in those with AD, CR fitness is associated with progression of dementia severity and brain atrophy in AD, suggesting a link between progression of dementia severity and cardiorespiratory health.
Keywords: dementia, DARTEL, maximal exercise test
INTRODUCTION
The benefits of physical activity for brain health are receiving increased attention.(Kramer et al., 2005) In animals, exercise increases neuronal survival and resistance to brain insults,(Carro et al., 2001; Stummer et al., 1994) promotes vascularization,(Black et al., 1990; Isaacs et al., 1992) stimulates neurogenesis,(van Praag et al., 1999) and mobilizes gene expression profiles predicted to benefit brain plasticity.(Cotman and Berchtold, 2002) Additionally, running increases brain-derived neurotrophic factor levels in the hippocampus and dentate gyrus and influences long-term potentiation.(Neeper et al., 1995; van Praag et al., 1999) In humans, several randomized controlled trials have examined the cognitive effects of increasing activity in healthy, older adults and found a beneficial impact on cognitive performance.(Dustman et al., 1984; Hassmen and Koivula, 1997; Hill et al., 1993; Kramer et al., 1999; Williams and Lord, 1997) Several longitudinal studies report a positive relationship between self-reported physical activity and cognitive function.(Laurin et al., 2001; Pignatti et al., 2002; Weuve et al., 2004; Yaffe et al., 2001)
There is an increasing interest in assessing the therapeutic role of exercise and physical activity in individuals with Alzheimer’s disease (AD). Recently, greater physical activity and exercise in adults without dementia was associated with lower levels of AD biomarkers such as Pittsburgh compound B binding.(Liang et al., 2010) Additionally, a recent report showed that increased physical activity in those with dementia was associated with lower mortality risk.(Scarmeas et al., 2010) Epidemiological studies suggest regular physical activity may prevent cognitive decline and dementia, and in midlife is associated with a reduced risk of developing mild cognitive impairment and AD.(Friedland et al., 2001; Geda et al., 2010) One such study found that dancing, an aerobic activity, was associated with lower risk for developing dementia.(Verghese et al., 2003) Others have demonstrated in randomized controlled trials that aerobic fitness training improves cognitive performance in mild cognitive impairment (Scherder et al., 2005; Baker et al., 2010)
Limitations to previous studies include a reliance on reported activity levels and a lack of standard objective measures of physical activity. Physical activity and exercise influence cardiorespiratory (CR) fitness, an objective measure of an individual’s peak level of oxygen consumption during a graded exercise test. CR fitness is associated with lower rates of cognitive decline in nondemented older adults (Colcombe and Kramer, 2003) but there is a paucity of data on individuals with AD regarding the relationship of CR fitness with dementia progression and structural brain change.(Rolland et al., 2008) We previously reported cross-sectional data suggesting that CR fitness relates to whole brain (Burns et al., 2008) and medial temporal lobe volume (Honea et al., 2009) in individuals with AD. Additionally, we reported that CR fitness levels were lower in those with AD compared to nondemented peers. (Burns et al., 2008) We now extend these findings by reporting the results of a 2-year observational study of individuals with early-stage AD and nondemented controls. We hypothesized that individuals with AD would have greater CR fitness decline compared to non-demented control subjects and that CR fitness would be associated with progression of dementia severity and brain atrophy.
METHODS
Sample
Participants were enrolled in the University of Kansas Brain Aging Project for baseline and follow-up evaluations (mean follow-up time 2.1 [SD 0.2] years). Data used in these analyses were from nondemented individuals (Clinical Dementia Rating [CDR] 0, n=53) and individuals with early-stage AD (CDR 0.5 and 1, n=37) aged 60 years and older. Study exclusions at baseline included neurologic disease other than AD with the potential to impair cognition (i.e., Parkinson disease), current or past history of diabetes mellitus (defined as a clinical diagnosis, use of an ant-diabetic agent, or 2-hour post-load serum glucose > 199), recent history of cardiovascular disease (e.g. diagnosis of congestive heart failure, acute coronary artery event or angina in the 2 years previous to the baseline evaluation), clinically significant depressive symptoms, use of investigational medications, significant visual or auditory impairment, systemic illness that may have impaired completion of the study, current or past history of alcoholism, and MRI exclusions (e.g. pacemakers). Baseline measures of these individuals have been reported previously as part of a larger cohort.(Burns et al., 2008; Honea et al., 2009) Informed consent was obtained from all participants or their legal representative as appropriate before enrollment into the study.
Clinical assessment
The clinical assessment included a semi-structured interview with the participant and a collateral source knowledgeable about the participant. Medications, past medical history, education, demographic information and family history were collected from the collateral source. Dementia status of the participant was based on clinical evaluation.(Morris et al., 2001) Diagnostic criteria for AD require the gradual onset and progression of impairment in memory and at least one other cognitive and functional domain.(McKhann et al., 1984) The CDR (Morris, 1993) assesses function in multiple domains and was used to assess dementia severity. The ratings in each of the six domains can be summed (“CDR Sum of Boxes”) to expand the CDR scale. The range of Sum of Boxes extends from 0 (no impairment) to 18 (maximum impairment). A Global CDR score is derived from individual ratings in each domain such that CDR 0 indicates no dementia, CDR 0.5 indicates very mild, CDR 1 indicates mild, CDR 2 indicates moderate, and CDR 3 indicates severe dementia. Nondemented status was defined as having a Global CDR 0 at both timepoints. Individuals with AD met criteria for very mild or mild dementia and had persistent impairment at follow up (Global CDR 0.5 or greater). These methods have a diagnostic accuracy for AD of 93% and have been shown to be accurate in discriminating those with mild cognitive impairment who have early stage AD.(Berg et al., 1998; Morris et al., 2001)
Cognitive Assessment
A trained psychometrician administered a psychometric battery including standard measures of memory, language, working memory, executive function, and visuospatial ability as described previously.(Burns et al., 2008) All cognitive performance scores were standardized (Z-score) to a larger set (n=82) of nondemented subjects (positive scores represent better performance). The mean of each participant’s Z-scores was calculated to create an index of Global Cognition, a composite measure of performance on the battery. The Mini-Mental State Examination (MMSE) (Folstein et al., 1975) was also administered to facilitate comparison across the literature.
Cardiorespiratory Fitness Assessment
CR fitness was assessed as peak oxygen consumption (VO2 peak; ml*kg−1*min−1) during a symptom-limited, graded treadmill test using a modified Bruce protocol (Burns et al., 2008; Hollenberg et al., 1998) and metabolic cart (Parvomedics, Sandy, UT) to measure the concentration of oxygen and carbon dioxide in expired air as described previously.(Burns et al., 2008) Oxygen consumption was averaged over 15-second intervals. VO2 peak was considered the highest observed value during the exercise test. Individuals included in these analyses terminated the test at voluntary exhaustion and met criteria for peak exercise (respiratory exchange ratio of 1.0 or greater during the test).(Gibbons et al., 1997)
Neuroimaging
Baseline and follow-up whole brain structural MRI data were obtained using a Siemens 3.0 Tesla Allegra MRI Scanner. High-resolution T1 weighted anatomical images were acquired (magnetization-prepared rapid gradient echo [MPRAGE]; 1×1×1mm3 voxels, repetition time [TR]=2500, echo time [TE]=4.38ms, inversion time [TI]=1,100ms, field of view=256×256, flip angle=8 degrees) and processed for voxel-based morphometric (VBM) analysis. Every scan was checked for image artifacts and gross anatomical abnormalities; eighteen data sets (9 from each group) were excluded.
We examined regional brain atrophy over the 2-year study period using SPM8 algorithms (Wellcome Department of Cognitive Neurology, London, UK) running under MATLAB 7.2 (The MathWorks, Natick, MA, USA) on Linux. VBM was used to compare changes over time in regional tissue volume. In this technique, a deformation field characterizes the high dimensional warp required to approximate baseline and follow-up T1 images within subject. First, the follow-up MRI of a subject was rigidly registered onto the subject’s baseline image to remove position differences. Then we used a high dimensional deformation field to warp the follow-up T1 image to the baseline T1 image.(Ashburner et al., 2000) The result of this deformation was a field map describing changes that occurred between baseline and follow-up. The local volume change was measured in each voxel by computing the determinant of the Jacobian matrix of the deformation field. The baseline gray matter segment and Jacobian determinants (containing information about the follow-up image) were multiplied, voxel-by-voxel, to form a product image, or gray matter volume change image (GM Change).
The baseline and follow-up images were segmented using the unified segmentation (“New Segment”) model in SPM8.(Ashburner and Friston, 2005) To compare regional volume change between groups it was necessary to spatially normalize the baseline segmentation images to a common stereotactic space to ensure that the same voxel in different subjects sampled an approximately corresponding neuroanatomic structure. To do this we used DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra), which is a suite of tools for achieving more accurate inter-subject registration of brain images, (Ashburner, 2007) increasing localization as well as sensitivity for VBM studies.
All subjects’ gray matter volume segmentations were imported into DARTEL and used to create a customized template. This is a nonlinear image registration procedure, which involves iteratively matching all the selected images to a template generated from their own mean. This DARTEL template, as well as the estimated spatial normalization parameters, or flow fields from the template creation, were normalized to Montreal Neurological Insitute (MNI) standard space. The flow fields from the template creation, as well as parameters from normalization to MNI space were then applied to the GM Change images. To preserve the original tissue volumes, the normalized GM Change images were modulated and smoothed with an 8mm isotropic Gaussian kernel to accommodate inexact spatial normalization. The normalized, modulated and smoothed GM Change maps for each individual were used in the statistical analysis outlined in the next section.
Statistical Analysis
Group differences in demographic measures (age, gender, race and education) were tested with Student’s t or Chi square tests as appropriate. To test for differences between groups and over the study period, Group (AD, nondemented) and Time (baseline, follow-up) effects were examined using repeated measures ANOVA across the study for CR fitness (VO2 peak), cognition (Global Cognition score) and dementia severity (CDR Sum of Boxes) corrected for age and gender.
To assess the relationship of baseline CR fitness (VO2 peak) with cognitive change (change in Global Cognition score) and progression of dementia severity (change in CDR Sum of Boxes; AD group only) we used a multi-step, hierarchical linear regression with CR fitness as the predictor variable, separately for AD and nondemented groups. We adjusted all analyses for age and gender. In AD analyses we also controlled for baseline CDR Sum of Boxes (F=3.94; P<0.05 to retain age, gender, and CDR SB). Using the same multi-level hierarchical approach, we then explored the relationship of change in VO2 peak (follow-up – baseline) with CDR Sum of Boxes change and Global Cognition change as response variables.
To assess the relationship of CR fitness with brain atrophy, we used a full-factorial multiple regression model in SPM8 within each group (nondemented and AD separately). Baseline and change in VO2 peak served as the variables of interest, and age and gender (and baseline CDR Sum of Boxes in the AD group only) served as covariates of no interest, similar to the clinical model. The relationship between CR fitness and GM atrophy was considered significant at p≤0.05 corrected for multiple comparisons (family wise error, [FWE] and cluster size k>100), with trends presented at p<0.001, uncorrected.
We also examined the relationship of change in CR fitness with medial temporal cortex atrophy in both the AD and nondemented groups. The medial temporal region of interest was selected a priori as it is affected early in AD, (Braak and Braak, 1991) is considered a valid biomarker of AD neuropathology,(Jack et al., 2002) and may be related to CR fitness.(Honea et al., 2009; Tong et al., 2001) A mask of the region was created by combining bilateral hippocampus and parahippocampus, derived from the Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu).(Maldjian et al., 2003) To correct for multiple comparisons in this restricted region of interest, small volume correction was employed (SVC) and results were considered significant at p≤0.05 FWE. Voxels are reported with reference to the MNI standard space and anatomic labels are reported with reference to the computerized Talairach Daemon (Lancaster et al., 1997) within the Pickatlas.(Maldjian et al., 2003)
RESULTS
Demographics and Cognition
AD and nondemented groups were not significantly different in age, gender, or race (p>0.2, Table 1). All nondemented participants identified as white, with one reporting Hispanic ethnicity. Thirty-five participants with AD identified as white, 1 as African-American and 1 as Native American. Individuals with AD had approximately 1 year less of formal education (p=0.04). At baseline evaluation 34 participants with AD had Global CDR of 0.5 and 3 had a Global CDR of 1. At follow-up, 23 participants with AD had a Global CDR of 0.5, 11 had a Global CDR of 1, and 3 individuals had a Global CDR of 2. Dementia severity (CDR Sum of Boxes) progressed over the 2-year study period by an average of 1.8 points. As expected, cognitive performance was lower in AD (Global Cognition, Group main effect F=117.9, p<0.001) and significantly declined over 2 years compared to the nondemented group (Group × Time interaction, F=21.2, p<0.001). The results of the MMSE score analysis paralleled the results of the Global Cognition analysis (Group × Time interaction, F=16.9, p<0.001).
Table 1.
Demographics and baseline and change at follow-up measures. Baseline group differences and change at the 2-year follow-up are presented along with the results of a repeated measures ANOVA of Group and Time.
| Nondemented (n=53) | Alzheimer’s Disease (n=37) | Sig. | |||||
|---|---|---|---|---|---|---|---|
| Age at Enrollment | 73.2 (6.7) | 73.8 (5.8) | 0.69 | -- | -- | ||
| Gender (%Female) | 54.7% | 54.1% | 0.95 | -- | -- | ||
| Education (yrs) | 16.6 (2.7) | 15.4 (3.2) | 0.04 | -- | -- | ||
| Baseline | Δ at Follow-up | Baseline | Δ at Follow-up | Group Effect | Time Effect | Group × Time Interaction | |
| Baseline MMSE | 29.5 (0.8) | −0.3 (1.1) | 26.7 (2.6) | −3.1 (5.2) | <0.001 | 0.102 | <0.001 |
| CDR Sum of Boxes | 0 (0) | 0 (0.1) | 2.7 (1.1) | 1.8 (2.7) | <0.001 | 0.042 | <0.001 |
| Global Cognition | 0.2 (0.5) | 0 (0.4) | −1.3 (0.9) | −0.6 (0.7) | <0.001 | 0.142 | <0.001 |
| Peak Oxygen Consumption, VO2 peak (ml*kg−1*min−1) | 23 (6.2) | −1.8 (2) | 20.6 (3.6) | −2.4 (2.1) | 0.003 | 0.831 | 0.214 |
Relationship of CR Fitness with Dementia
We then assessed between group differences in CR fitness (VO2 peak) over the course of the 2-year study period. CR fitness was lower in the AD group compared to the nondemented group (Group main effect, F=9.2, p=0.003, Table 1). There was no Time × Group interaction (p=0.2).
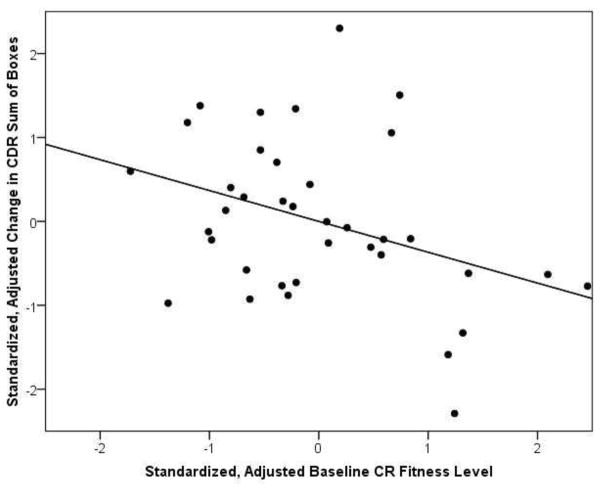
We next assessed the relationship of baseline CR fitness to cognitive change and progression of dementia severity. In the AD group, lower baseline levels of CR fitness were associated with greater progression of dementia severity over the study (increase in CDR Sum of Boxes, β=−0.42, p=0.03, Table 2), controlling for age, gender and baseline CDR Sum of Boxes. Figure 1 depicts the relationship of baseline VO2 peak and change in CDR Sum of Boxes. Baseline fitness was not associated with decline in Global Cognition in the AD group. We also examined the relationship of change in CR fitness over the study period with cognitive change and progression of dementia severity, again controlling for age, gender and baseline CDR Sum of Boxes. CR fitness change was not associated with progression of dementia severity or change in Global Cognition in the AD group.
Table 2.
Standardized Coefficients (β) Predicting Progression of Dementia Severity and Change in Global Cognition After Controlling for Age, Sex and Baseline Dementia Severity.
| Dementia Progression | Change in Global Cognition | |
|---|---|---|
| Baseline VO2 peak | −0.42* | 0.27 |
| Change in VO2 peak | −0.15 | 0.02 |
| Baseline VO2 peak | -- | 0.36^ |
| Change in VO2 peak | -- | 0.19 |
p<0.05,
p=0.06
Figure 1.
Scatter plot of baseline CR fitness (VO2peak) plotted against progression of dementia severity (Change in CDR Sum of Boxes), full model adjusted r2=0.356, p=0.001. Higher CR fitness level (positive values along the x-axis) are associated with less progression in dementia severity over the course of the study (negative values along the y-axis), β = −0.42, p=0.03. Variables are adjusted for age, gender and baseline CDR Sum of Boxes.
In the nondemented group there was a trend for baseline CR fitness to be associated with change Global Cognition (β=0.36, p=0.06), with lower CR fitness associated with a decline in Global Cognition after controlling for age and sex. Two-year change in CR fitness was not associated with decline in Global Cognition.
CR Fitness and Regional Brain Atrophy
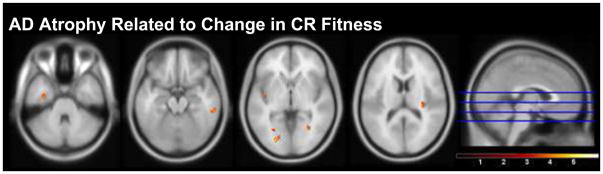
We then assessed the relationship of baseline and 2-year change in CR fitness with atrophy patterns in AD. Though baseline CR fitness was not significantly related to regional gray matter atrophy in AD, we observed several trends (p<0.001, uncorrected, k>100) where decline in CR fitness over the 2-year study period was associated with atrophy in the left parahippocampus, bilateral insular lobes, right lingual gyrus, right putamen and right inferior temporal gyrus (Table 3A, Figure 2). When our analysis was restricted to our a priori region of interest, the medial temporal lobe, there was a significant relationship of change in CR fitness and atrophy in left parahippocampus (SVC, p<0.05 FWE), with 2-year decline in CR fitness associated with greater atrophy, controlling for age, gender, and baseline CDR Sum of Boxes).
Table 3.
Regional atrophy associated with decline in VO2 peak in AD (A) and with baseline (B) and decline (C) in VO2 peak nondemented participants.
| A) Decline in VO2 peak, AD group | ||||||
|---|---|---|---|---|---|---|
| k | T | Z | x | y | z | |
| Left insula | 191 | 4.35 | 3.65 | −39 | −10 | 0 |
| Left fusiform/parahippocampal gyrus** | 235 | 4.72 | 3.88 | −35 | −13 | −30 |
| Left lingual gyrus | 176 | 4.34 | 3.65 | −28 | −61 | −4 |
| Left middle occipital cortex | 153 | 5.32 | 4.22 | −25 | −82 | −2 |
| Right caudate | 109 | 3.82 | 3.31 | 23 | 4 | 20 |
| Right insula | 327 | 4.24 | 3.58 | 30 | −24 | 15 |
| Right occipital cortex | 101 | 4.50 | 3.75 | 33 | −64 | −2 |
| Right inferior temporal gyrus | 476 | 4.55 | 3.78 | 60 | −32 | −21 |
| B) Baseline VO2 peak, nondemented group | ||||||
| Left inferior temporal cortex | 332 | 3.90 | 3.58 | −69 | −25 | −20 |
| Left middle occipital | 345 | 3.67 | 3.39 | −49 | −84 | 10 |
| Left superior occipital cortex | 480 | 4.14 | 3.76 | −34 | −86 | 23 |
| Right uncus | 160 | 4.31 | 3.89 | 20 | 14 | −33 |
| Right middle temporal pole | with above | 3.63 | 3.36 | 21 | 12 | −43 |
| Right middle occipital | 309 | 3.75 | 3.46 | 49 | −81 | −19 |
| C) Decline in VO2 peak, nondemented group | ||||||
| Left inferior frontal gyrus | 227 | 4.71 | 4.18 | −48 | 20 | 6 |
| Left middle frontal | 128 | 3.88 | 3.56 | −25 | 47 | −13 |
| Left putamen | 319 | 3.91 | 3.59 | −20 | 15 | −7 |
| Right caudate | 138 | 3.83 | 3.52 | 14 | 27 | 5 |
Regions are listed left to right. X, Y and Z coordinates coincide with the MNI brain atlas. A minimum cluster size (k= 100) was set as a threshold for uncorrected analyses.
Region is significant with Small Volume Analysis in the Medial Temporal Lobe with Family-wise Error correction (p<0.05)
Figure 2.
Statistical parametric maps showing regions of brain atrophy associated with decline in CR fitness in the AD group over 2 years. Anatomic locations are in Table 3A. Color bar represents T-statistics, with voxels presented at p<0.001 uncorrected, cluster size (k) > 100. Slices are presented inferior to superior at the location identified by the blue lines on the sagittal image on the right. The cluster in the right parahippocampal gyrus is significant (FWE p<0.05) under small volume corrected analysis of the medial temporal lobe.
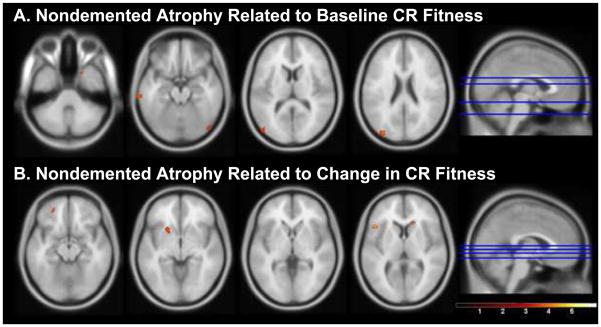
In the nondemented group, there was a trend for lower baseline CR fitness to be associated with regional atrophy in bilateral occipital and temporal cortices and right uncus (p<0.001, uncorrected, k>100, Table 3B, Figure 3A). There was also a trend for an association between decline in CR fitness and atrophy in the left frontal cortex and putamen and right caudate nucleus (Table 3C, Figure 3B). Neither baseline CR fitness nor change in CR fitness was associated with significant atrophy in the medial temporal region of interest in the nondemented group.
Figure 3.
Statistical parametric maps showing regions of brain atrophy associated with CR fitness in the nondemented group. Anatomic locations are in Table 3B-C. Regional atrophy is related to lower baseline (A) and decline (B) in CR fitness in the nondemented group. Color bar represents T-statistics, with voxels presented at p<0.001 uncorrected, cluster size (k) > 100. Slices are presented inferior to superior at the location identified by the blue lines on the sagittal image on the right.
DISCUSSION
Our data show that higher baseline CR fitness in individuals with AD was associated with attenuated progression of dementia severity, independent of age, gender and baseline dementia severity. Additionally, decline in CR fitness over 2 years most notably associated with greater rates of medial temporal atrophy. In nondemented older adults, lower baseline levels of CR fitness were marginally associated with cognitive decline (p=0.06) and atrophy in temporal and posterior regions, whereas declining fitness was associated with frontal and subcortical atrophy.
CR Fitness Change Over Time
Our first aim was to characterize CR fitness change in a population with early-stage AD. The AD group demonstrated consistently lower CR fitness levels than the nondemented group over the course of the study, extending our previously reported cross-sectional findings.(Burns et al., 2008) However, our data do not demonstrate exacerbated decline in CR fitness in the AD group compared to the nondemented group. These results suggest preclinical AD may impact physical activity and CR fitness levels before the clinical recognition of the disease. Early disease-related changes in physical activity may influence a cycle of decline that includes reduced CR fitness, brain and body changes and functional decline that may exacerbate the AD syndrome. Alternatively, individuals with a lifetime history of physical inactivity may be at increased risk of developing the disease and thus are over-represented in our AD cohort. This is consistent with prior reports linking low levels of physical activity, even in midlife, with mild cognitive impairment and dementia.(Geda et al., 2010; Larson et al., 2006; Podewils et al., 2005; Rovio et al., 2005)
Cardiorespiratory Fitness, Physical Activity and Dementia Progression
Our second aim was to explore the relationship of fitness with dementia progression. In the AD group we found that higher baseline CR fitness was associated with less progression of dementia severity (CDR box score) over the 2-year study period, independent of age, gender and baseline dementia severity. This is consistent with the hypothesis that higher CR fitness may be associated with slower disease progression. CR fitness was not related to decline in cognitive performance despite the relationship with dementia severity. We suspect that CR fitness more closely reflects the functional domains assessed in the CDR. It is possible that individuals with higher CR fitness have greater physical function reserve that may reduce the functional impact of disease. Future studies may wish to use more robust statistical techniques to accurately assess the relationship of CDR constructs to physical and cognitive measures.
When assessing change in CR fitness over the 2-year study period, we found that declining CR fitness was associated with brain atrophy in several regions in the AD group, including those most affected by AD neuropathology. The findings extend our prior cross-sectional report and suggest a relationship between a validated neuroimaging marker of AD neuropathological burden, atrophy in the medial temporal lobes,(Barnes et al., 2004; Jack et al., 1998; Wang et al., 2003) and maintenance of CR fitness. We found specific regional brain volume associations with CR fitness in the left parahippocampus. This is interesting as this region is recruited to a greater degree during verbal memory tasks in those with mild AD and MCI than nondemented controls.(Hamalainen et al., 2007; Peters et al., 2009) If the parahippocampal areas provide compensatory activity for task execution, CR fitness may further support sustained cognitive function in early AD, although it is important to note these data do not establish a causal relationship between physical activity and parahippocampal function.
While the observational nature of this study is unable to assess causal relationships, these data are consistent with the possibility that interventions to maintain CR fitness, such as exercise, may slow brain atrophy related to AD. This hypothesis is supported by data from animals that suggest exercise enhances neurogenesis, is anti-apoptotic and promotes angiogenesis.(Kim et al., 2010; Pereira et al., 2007) Exercise has also been reported to influence imaging markers of neurogenesis in human hippocampus.(Pereira et al., 2007)
The relationship of CR fitness and brain health is also moderately supported in our nondemented group, where greater baseline CR fitness was associated with less cognitive decline (p=0.06) and lower rates of brain atrophy in the occipital and temporal lobes, supporting previous reports.(Colcombe et al., 2003; Erickson et al., 2010; Yaffe et al., 2001) Additionally, worse decline in CR fitness over 2-years was associated with atrophy in frontal and subcortical regions in our nondemented group. Some frontal and temporal atrophy is common in aging,(Colcombe et al., 2003; Fjell et al., 2009) and moderate exercise has previously been shown to attenuate this change (Colcombe et al., 2006) and result in improvements in executive cognitive function.(Colcombe et al., 2004; Kramer et al., 1999)
An alternative possibility to explain our observed relationship between CR fitness and disease progression is that an underlying effect of AD pathology concomitantly drives decline in cognition, brain atrophy and CR fitness. For example, muscle mitochondria are responsible for a substantial degree of muscle oxygen consumption, and AD subjects have systemically reduced mitochondrial function.(Swerdlow and Khan, 2009; Swerdlow et al., 1997) Over the course of the 2-year study period, AD subjects whose oxygen uptake capacity (VO2peak) declined the most had the greatest degree of brain atrophy suggesting these factors may be inter-related. It is important to note that our cohort is composed only those individuals capable of satisfactorily completing an exercise test (RER > 1.0) and reasonably reflects near maximal physiological capacity for oxygen consumption rather than motivation during the test. Thus, our findings suggest the possibility that declining respiratory function is a manifestation of the AD process, and emphasizes an emerging recognition of systemic biochemical dysfunction in AD.
In conclusion, we report that lower CR fitness seen early in AD persists over 2 years. Those with the lowest CR fitness in the earliest stages of AD experienced more severe AD progression. Further, declining fitness over 2 years was associated with greater brain atrophy in regions affected by AD neuropathology. The results support prior findings of a link between fitness and brain health in AD and indicate the need to further investigate the interaction of CR health and function and mechanistic links between CR fitness and brain change in AD.
Acknowledgments
This study was supported by grant R03AG026374-01 from the National Institutes of Aging, grant K23NS058252 from the National Institute on Neurological Disorders and Stroke, and generous support from the University of Kansas Endowment Association, William and Carolie Hougland, and the Fraternal Order of Eagles. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, expertise, and nursing support. The Hoglund Brain Imaging Center is supported by grant C76 HF00201. EDV is supported in part by a fellowship from the Foundation for Physical Therapy. We thank Natalia Loskutova for her insight into early versions of the manuscript. We also appreciate the dedication of Brain Aging Project team and participants.
Footnotes
DISCLOSURE STATEMENT: The authors have no actual or potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. S1053-8119(07)00584-8 [pii] [DOI] [PubMed] [Google Scholar]
- Ashburner J, Andersson JL, Friston KJ. Image registration using a symmetric prior--in three dimensions. Hum Brain Mapp. 2000;9(4):212–25. doi: 10.1002/(SICI)1097-0193(200004)9:4<212::AID-HBM3>3.0.CO;2-#. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. S1053-8119(05)00110-2 [pii] [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–9. doi: 10.1001/archneurol.2009.307. 67/1/71 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Scahill RI, Boyes RG, Frost C, Lewis EB, Rossor CL, Rossor MN, Fox NC. Differentiating AD from aging using semiautomated measurement of hippocampal atrophy rates. Neuroimage. 2004;23(2):574–81. doi: 10.1016/j.neuroimage.2004.06.028. S1053-8119(04)00338-6 [pii] [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic Studies in Cognitively Healthy Aging and Alzheimer Disease: Relation of Histologic Markers to Dementia Severity, Age, Sex, and Apolipoprotein E Genotype. Archives of Neurology. 1998;55(3):326–35. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Nat Acad Sci USA. 1990;87(14):5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–6. doi: 10.1212/01.wnl.0000317094.86209.cb. 71/3/210 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE. Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(1):39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–84. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):M176–M80. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. 61/11/1166 [pii] [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Nat Acad Sci USA. 2004;101(9):3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC. Aerobic Exercise Training and Improved Neuropsychological Function of Older Individuals. Neurobiolf Aging. 1984;5(1):35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology. 2010;75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. WNL.0b013e3181f88359 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–31. doi: 10.1523/JNEUROSCI.3252-09.2009. 29/48/15223 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH, Petot GJ, Debanne SM. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proc Nat Acad Sci USA. 2001;98(6):3440–5. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 67(1):80–6. doi: 10.1001/archneurol.2009.297. 67/1/80 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Jr, Winters WL, Yanowitz FG, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Lewis RP, O’Rourke RA, Ryan TJ. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30(1):260–311. doi: 10.1016/s0735-1097(97)00150-2. S0735109797001502 [pii] [DOI] [PubMed] [Google Scholar]
- Hamalainen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28(12):1889–903. doi: 10.1016/j.neurobiolaging.2006.08.008. S0197-4580(06)00303-4 [pii] [DOI] [PubMed] [Google Scholar]
- Hassmen P, Koivula N. Mood, physical working capacity and cognitive performance in the elderly as related to physical activity. Aging. 1997;9(1–2):136–42. doi: 10.1007/BF03340139. [DOI] [PubMed] [Google Scholar]
- Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. J Gerontol. 1993;48(1):12–7. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill Exercise Testing in an Epidepiologic Study of Elderly Subjects. J Gerontol Biol Sci. 1998;53A(4):259–67. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(3):188–97. doi: 10.1097/WAD.0b013e31819cb8a2 00002093-200907000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51:993–9. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45(5):357–65. doi: 10.1016/j.exger.2010.02.005. S0531-5565(10)00076-8 [pii] [DOI] [PubMed] [Google Scholar]
- Kramer AF, Colcombe SJ, McAuley E, Scalf PE, Erickson KI. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26 (1, Supplement 1):124–7. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–9. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5(4):238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise Is Associated with Reduced Risk for Incident Dementia among Persons 65 Years of Age and Older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68(3):311–8. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412b–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Pereira A, Ribeiro S, Wiest M, Moore LC, Pantoja J, Lin SC, Nicolelis MA. Processing of tactile information by the hippocampus. Proc Natl Acad Sci U S A. 2007;104(46):18286–91. doi: 10.1073/pnas.0708611104. 0708611104 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters F, Collette F, Degueldre C, Sterpenich V, Majerus S, Salmon E. The neural correlates of verbal short-term memory in Alzheimer’s disease: an fMRI study. Brain. 2009;132(Pt 7):1833–46. doi: 10.1093/brain/awp075. awp075 [pii] [DOI] [PubMed] [Google Scholar]
- Pignatti F, Rozzini R, Trabucchi M, Yaffe K. Physical Activity and Cognitive Decline in Elderly Persons. Arch Int Med. 2002;162(3):361–2. doi: 10.1001/archinte.162.3.361. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–51. doi: 10.1093/aje/kwi092. 161/7/639 [pii] [DOI] [PubMed] [Google Scholar]
- Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer’s disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9(6):390–405. doi: 10.1016/j.jamda.2008.02.007. S1525-8610(08)00099-6 [pii] [DOI] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. S1474-4422(05)70198-8 [pii] [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Brickman AM, Cosentino S, Schupf N, Xin-Tang M, Gu Y, Stern Y. Physical Activity and Alzheimer Disease Course. Am J Geriatr Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181eb00a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherder EJA, Van Paasschen J, Deijen JB, Van Der Knokke S, Orlebeke JFK, Burgers I, Devriese PP, Swaab DF, Sergeant JA. Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment Health. 2005;9(3):272–80. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- Stummer W, Weber K, Tranmer B, Baethmann A, Kempski O. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke. 1994;25(9):1862–9. doi: 10.1161/01.str.25.9.1862. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218(2):308–15. doi: 10.1016/j.expneurol.2009.01.011. S0014-4886(09)00017-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, Davis RE, Parker WD., Jr Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49(4):918–25. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8(6):1046–56. doi: 10.1006/nbdi.2001.0427. S0969-9961(01)90427-9 [pii] [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Nat Acad Sci USA. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB. Leisure activities and the risk of dementia in the elderly. New Eng J Med. 2003;348(25):2508. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage. 2003;20(2):667–82. doi: 10.1016/S1053-8119(03)00361-6. S1053811903003616 [pii] [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA. 2004;292(12):1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Aust N Z J Public Health. 1997;21(1):45–52. doi: 10.1111/j.1467-842x.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A Prospective Study of Physical Activity and Cognitive Decline in Elderly Women: Women Who Walk. Arch Int Med. 2001;161(14):1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]