Abstract
In rodents, many exogenous cannabinoid agonists including Δ9-THC and WIN 55,212-2 (WIN-2) have been shown to impair short-term memory (STM) by inhibition of hippocampal neuronal assemblies. However, the mechanisms by which endocannabinoids such as anandamide and 2-arachidonylglycerol (2-AG) modulate STM processes are not well understood. Here the effects of anandamide on performance of a Delayed Non-Match to Sample (DNMS) task (i.e., STM task) and concomitant hippocampal ensemble activity were assessed following administration of either URB597 (0.3, 3.0mg/kg), an inhibitor of the Fatty Acid Amide Hydrolase (FAAH), AM404 (1.5, 10.0mg/kg), a putative anandamide uptake/FAAH inhibitor, or R-methanandamide (3.0, 10.0mg/kg), a stable analogue of anandamide. Principal cells from hippocampal CA3/CA1 were recorded extracellularly by multi-electrode arrays in Long-Evans rats during DNMS task (1–30s delays) performance and tracked throughout drug administration and recovery. Both R-methanandamide and URB597 caused dose- and delay-dependent deficits in DNMS performance with suppression of hippocampal ensemble activity during the encoding (sample) phase. R-methanandamide induced effects were not reversed by capsaicine excluding a contribution of TRPV-1 receptors. AM404 produced subtle deficits at longer delay intervals but did not alter hippocampal neuronal activity during task-specific events. Collectively, these data indicate that endocannabinoid levels affect performance in a STM task and their pharmacological elevation beyond normal concentrations is detrimental also for the underlying physiological responses. They also highlight a specific window of memory processing, i.e. encoding, which is sensitive to cannabinoid modulation.
Keywords: delayed non-match to sample, electrophysiology, endocannabinoid, hippocampus, short-term memory, single-unit recording, ensemble code
1. Introduction
Systemic administration of phytocannabinoids or synthetic receptor agonists such as Δ9-Tetrahydrocannabinol (Δ9-THC), WIN55,212-2 and HU-210 impair working memory (WM) and short-term memory (STM) in rodents (Heyser et al., 1993; Lichtman et al., 1995; Ferrari et al., 1999; Hampson and Deadwyler, 2000; Mishima et al., 2001; Varvel and Lichtman, 2002; Hampson et al., 2003; Goonawardena et al., 2010a, 2010b; Robinson et al., 2010). Unexplored, however, remains the role(s) played by endogenous cannabinoids such as arachidonoyl ethanolamide (anadamide) and 2-arachidonyl glycerol (2-AG) on these different memory processes. Their presence in memory-relevant areas of the rodent brain (Di Marzo et al., 2001) together with the localization of cannabinoid (CB1) receptors on different cell types (Marsicano and Lutz, 1999; Domenici et al., 2006) in hippocampus and pre-frontal cortex (Herkenham et al., 1991) suggests that the endocannabinoid system could play a pivotal role in modulating both WM and STM processes. Typically, endocannabinoid function has been probed by administration of SR141716A, a well-known CB1 receptor antagonist. When given alone SR141716A enhanced STM in a radial-arm maze at long, but not short delays (Lichtman, 2000; Wolff & Leander, 2003), short-term social recognition memory (Terranova et al., 1996) and passive avoidance retention (Mazzola et al., 2003). More recently, we provided evidence for an intriguing prolongation of long-term spatial memory in rats (Robinson et al., 2008) suggesting that the time course of memory is normally curtailed by the activation of CB1 receptors. This is in line with evidence that SR141716A impairs extinction learning in the Morris water maze (Varvel et al., 2005) and extinction of conditioned freezing either to a tone or context (Marsicano et al., 2002; Suzuki et al., 2004) suggesting that an endocannabinoid tone is crucial for various forms of learning and memory.
Anandamide, the first endocannabinoid to be isolated (Devane et al., 1992) produces WM deficits in rats trained to perform a non-match-to-position operant conditioning task (Mallet and Beninger, 1998). Around the same time, Murillo-Rodriguez and co-workers (1998) confirmed that intra-cerebroventricular (icv) administration of anandamide weakens memory consolidation. Such a direct role of the hippocampal endocannabinoid system was proven in long-term memory since intra-hippocampal infusions of anandamide produced anterograde amnesia in rats trained to perform a one trial, step-down inhibitory avoidance task (Barros et al., 2004), and chronic infusions of rimonabant (CB1 receptor antagonist/inverse agonist) via minipumps enhanced acquisition learning and prolonged spatial memory possibly by preventing extinction learning (Robinson et al., 2008).
Since anandamide is rapidly metabolized and/or recycled into the cell, other studies have used R-methanandamide, a metabolically stable analogue of anandamide, to better understand how this particular endocannabinoid modulates learning and memory. R-methanandamide dose-dependently disrupted operant conditioning through a CB1 receptor mediated mechanism (Brodkin and Moerschbaecher, 1997), impaired recognition memory in rats (Kosiorek et al., 2003), and disrupted WM in the water maze in mice (Varvel and Lichtman, 2002; Varvel et al., 2006). These data strongly implicate endogenous cannabinoids in several forms of memory and warrant its use in this study. However, R-methanandamide may also activate transient receptor potential vanilloid type-1 (TRPV-1) receptors in the hippocampus (Chavez et al., 2010; Bhaskaran and Smith, 2010) and any drug-related action may not be specific to CB1 receptor mediated functions. Consequently, R-methanandamide was also assessed in the presence of capsazepine in an attempt to define a putative involvement of the TRPV-1 receptor in this STM task.
An alternative approach to modulate endocannabinoid levels is by interference with the metabolism of anandamide. It is metabolized by Fatty Acid Amide Hydrolase (FAAH), an integral membrane protein widely distributed in the rodent brain including the hippocampus (Freund et al., 2003). Various FAAH inhibitors have been developed including the well characterized URB597, known to deactivate FAAH and increase anandamide levels in the rodent brain at a dose of 0.3mg/kg (Fegley et al., 2004). The same dose caused WM deficits in a delayed alternation task in a T-maze (Seillier et al., 2010) without producing side effects (e.g. hypomotility, hypothermia, etc.) that often accompany exogenously administered CB1 receptor agonists. Such a pharmacological approach is thus preferred over synthetic cannabinoids.
A third possibility for pharmacological elevation of brain levels of anandamide is via the inhibition of its putative transporter protein. AM404 is an inhibitor of this transporter and lacks any direct action on cannabinoid receptors (Kelley and Thayer, 2004; Fegley et al., 2004; Fowler et al., 2004). However, AM404 may also act as a FAAH inhibitor (Glaser et al., 2003; Abush and Akirav, 2010) thereby inhibiting two possible mechanisms that physiologically can curtail the actions of anandamide. Systemic injections of AM404 (5 mg/kg) impaired spatial learning in the water maze (Abush and Akirav, 2010), but it dose-dependently (2 – 10mg/kg) enhanced extinction (i.e. block reconsolidation) of conditioned fear memory in rodents (Chatwal et al. 2005; Pamplona et al., 2008) suggesting effectiveness of treatment in terms of mnemonic systems.
Here, we reasoned that if these compounds were applied to rats trained in a delayed nonmatch-to-sample (DNMS) task, the resulting effects on STM and hippocampal ensemble firing in DNMS relevant phases (Hampson and Deadwyler, 2000; Goonawardena et al., 20010b) could shed new light on the physiological role of the endocannabinoid system in behaviorally defined situations.
2. Material and methods
2.1. Animals
Adult male, Long-Evans rats (Harlan, USA) aged between 6 – 8 months (280–350g) were individually housed in a temperature-controlled (20–22°C) environment with a 12hr light/dark cycle (lights on 7 am – 7 pm). All animals were water-restricted throughout training and drug testing, but allowed free access to food (regular rat-chow). The volume of water consumed and weights were monitored daily to maintain ~83% of ad libitum body weight. Water consumption during behavioral testing was recorded, and a supplemental volume given immediately after the session to provide 20–22hrs of water deprivation. All animal care and experimental procedures including water regulation and surgery were approved by the Wake Forest University Institutional Animal Care and Use Committee and were in concordance with the National Institutes of Health (NIH) Guide for the Care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.2. Apparatus
The apparatus as described previously (Heyser et al., 1993; Deadwyler et al., 2007; Goonawardena et al., 2010a, 2010b) consisted of a 43 × 43 × 50cm Plexiglass behavioral testing chamber with two retractable levers (left and right) positioned on either side of a water trough in the front panel and a nose-poke device mounted at the center of the back panel. A cue light (28-V) was located immediately above the nose-poke device. The test chamber was illuminated by two 28-V incandescent house lights mounted into the ceiling next to a video camera which recorded the behavior of rats continuously. The entire apparatus was computer-controlled and housed inside a commercially built sound-attenuated cubicle (Industrial Acoustics Co., Bronx, New York, USA).
2.3. Behavioral training procedure
All animals were trained to perform the DNMS task (Deadwyler and Hampson, 2004; Deadwyler et al., 2007; Goonawardena et al., 2010a, 2010b). Each trial consisted of three main phases: Sample, Delay and Nonmatch. It was initiated at the Sample phase in which either the left or right lever was selected at random and extended (counterbalanced design). The animal responded by pressing the lever (Sample Response; SR), which was immediately retracted, thereby initiating the Delay phase of the task (1–30s). In the Delay phase a cue light over the nose-poke device on the opposite wall was illuminated and the animal was required to respond with at least one nose poke to terminate the delay phase. The last nose-poke at the end of the delay phase also turned out the cue light and extended both levers in the front panel, signaling the onset of the Nonmatch phase. Only a Nonmatch Response (NR) on the lever opposite to the position of the SR was rewarded. Reinforcement consisted of a drop of water (40µL) delivered to the trough immediately after the NR occurred. At the termination of the Nonmatch phase both levers were retracted for a 10s inter-trial interval (ITI) with house lights on, after which one lever was extended again and the next trial started with the onset of the Sample phase. An error consisting of a ‘Match Response’ resulted in no water reward and caused the house lights to be turned off for 5s with both levers retracted, after which house lights were re-illuminated for another 5s, and the next trial started. All animals were trained to criterion of 90% correct responding on trials with delays of 1–5s in sessions of 100 trials; delays varied from 1–30s prior to surgery (i.e. implantation of electrode arrays), and animals were re-trained to the same level after surgical recovery and before drug testing.
2.4. Surgery
Following training, animals were anesthetized under a constant flow of isoflurane (3% for induction, 1.5% for maintenance)/O2 (100%) mixture and placed in a stereotaxic frame. A craniotomy (5mm diameter) was performed over the dorsal hippocampus so that the center pair of array electrodes consisting of sixteen stainless steel micro-wires (diameter: 40 µm each; Neurolinc Corp., NJ, USA) was positioned 3.4 mm posterior and 3.0 mm lateral to Bregma (Paxinos and Watson, 1998). The longitudinal axis of the array was angled 30° to the midline, with posterior electrodes more lateral than anterior sites. The array was lowered in 25–100 µm steps to a depth of 3.0 – 4.0 mm from the cortical surface for CA3 leads (eight long wires), and CA1 leads (eight short wires) automatically positioned 1.2 mm higher along the longitudinal axis of the hippocampus. Electrodes were spaced 200 µm apart within each row and 400 µm between rows. Recordings from all micro-wire electrodes were monitored throughout surgery to ensure correct placement in appropriate hippocampal cell layers and the exposed cortex was kept moist with 0.9% sodium chloride. After array placement, the cranium was sealed with bone wax and dental cement (Hampson and Deadwyler, 2000; Deadwyler et al., 2007; Goonawardena et al., 20010a, 2010b) and given 0.3 mg/kg analgesic (buprenorphine). All animals were allowed to recover for 3–4 days with ad libitum food and water before retraining. All efforts were made to minimize animal suffering.
2.5. Drug preparation and experimental design
AM404 (N-(4-hydroxyphenyl)arachidonoylethanolamide) and R-methanandamide (Cayman chemicals, Ann Arbor, MI) were obtained as solutions in ethanol. URB597 ([3 fenilo (3-carbamoylphenyl)]-N-cyclohexylcarbamate), Capsaicin (N-[(4-hydroxy-3-methoxyphenyl)methyl]-6E-8-methyl-nonenamide) and Capsazepine (N-[2-(4-chlorophenyl)ethyl]-1,3,4,5-tetrahydro-7,8-dihydroxy-2H-2-benzazepine-2-carbothioamide) (Cayman chemicals, Ann Arbor, MI) were purchased as crystalline solids and subsequently mixed in ethanol. Sufficient drug to make up the required concentration was mixed in Cremophor EL (Sigma, St Louis, MO) and saline (0.9%) at a ratio – ethanol (1 part) : cremophor (1 part) : saline (18 parts). The solution was stirred rapidly and placed under a steady stream of nitrogen gas to evaporate the ethanol (approx. 10 minutes). The resulting drug/detergent/saline suspension was sonicated prior to intraperitoneal (i.p.) injection. Vehicle solutions were prepared in the same manner, with the drug omitted.
During Experiment 1, each subject (n = 6) received both a low (3.0 mg/kg) and high (10.0 mg/kg) dose of R-methanandamide on separate days alternated with vehicle-only control days (i.e. day 1: vehicle; day 2: low dose; day 3: vehicle; day 4: high dose). Experiment 2 assessed the effects of R-methanandamide (10.0 mg/kg), Capsaicin (0.01 mg/kg) and R-methanandamide + Capsazepine (0.1 mg/kg) on separate days alternated with vehicle-only control days (n = 14). Experiments 2 and 3 (n = 6 each), examined the effects of URB597 (low dose: 0.3mg/kg; high dose: 3.0mg/kg) and AM404 (low dose: 1.5mg/kg; high dose: 10.0mg/kg) similar to that described in Experiment 1. Note that all animals in each experiment were administered with vehicle in between drug(s) to allow behavioral performance to return to baseline prior to subsequent drug exposure. All injections were administered 20 – 30 min prior to the start of each behavioral and electrophysiological test session.
2.6. Analyses of behavioral data
Performance under the influence of each treatment was assessed using three primary measures: 1) mean percentage correct trials; 2) mean percentage correct trials at each 5s delay interval epoch, and 3) response latency. Overall statistical significance for the first measure was calculated by one-way repeated measures analysis of variance (ANOVA) across each treatment (i.e. vehicle, low and high dose) followed by Tukey’s multiple comparison (post-hoc) to compare between treatments of interest. Two-way repeated measures ANOVA using treatment and delay interval as factors was employed for the second measure. Further planned paired t-tests were performed between treatments of interest to assess differences across each 5s delay interval epoch. Moreover, the mean latency to response levers (i.e. locomotor activity) was compared using one-way repeated measures ANOVA across treatment groups followed by Tukey’s multiple comparison tests between treatment groups of interest. p’s < 0.05 were considered statistically significant.
2.7. Multi-neuronal recording
Extracellular action potentials (spikes) and behavioral events within each DNMS trial were recorded. Single neurons recorded from each of the 16 micro-wire electrodes were digitized at 40 KHz and time-stamped along with behavioral events for computer-processing within each DNMS session (Hampson and Deadwyler, 2000; Hampson et al., 2003; Goonawardena et al., 2010a, 2010b). Action potentials of individual neurons were isolated by time-amplitude window discrimination and computer-identified individual waveform characteristics using a multi-neuron acquisition processor (MAP; Plexon, Dallas, TX). Single-units from each electrode were recorded daily using waveform and firing characteristics for each trial following respective treatments. Only spike waveforms with associated discharge rates consistent with behavioral correlates across sessions were included in the analysis.
2.8. Analysis of neuronal data
Hippocampal principal cells (mean firing rate: 0.25 – 6 Hz) identified from selected wires in CA3 and CA1 sub-fields were recorded. Task specific activity during correct DNMS trials was characterized based on peak z-scores calculated from peri-event histograms (+/− 1.5s) around sample and nonmatch responses as follows: (1) the peak bin (histogram maximum) was found (bin size: 100 ms); (2) the mean (M) and standard deviation (SD) of background bins (those bins that are equal or less than peak bin / 2) were calculated; (3) peak z-score = (peak bin - M) / SD. All cells were selected only if the peak z-score around any task-specific event was equal or greater than 3.19 (P < 0.001). Thereafter, activity of these neurons was determined following vehicle and drug treatments. Peak z-scores of neurons that showed significant increases in firing during SR and NR were averaged across all sessions. Statistical significance was calculated using one-way repeated-measures ANOVA followed by Tukey’s multiple comparison tests for differences between treatments of interest. Again, p’s < 0.05 were accepted as significant.
3. Results
3.1. Effects of R-methanandamide, URB597 and AM404 on delayed-nonmatch-to-sample performance
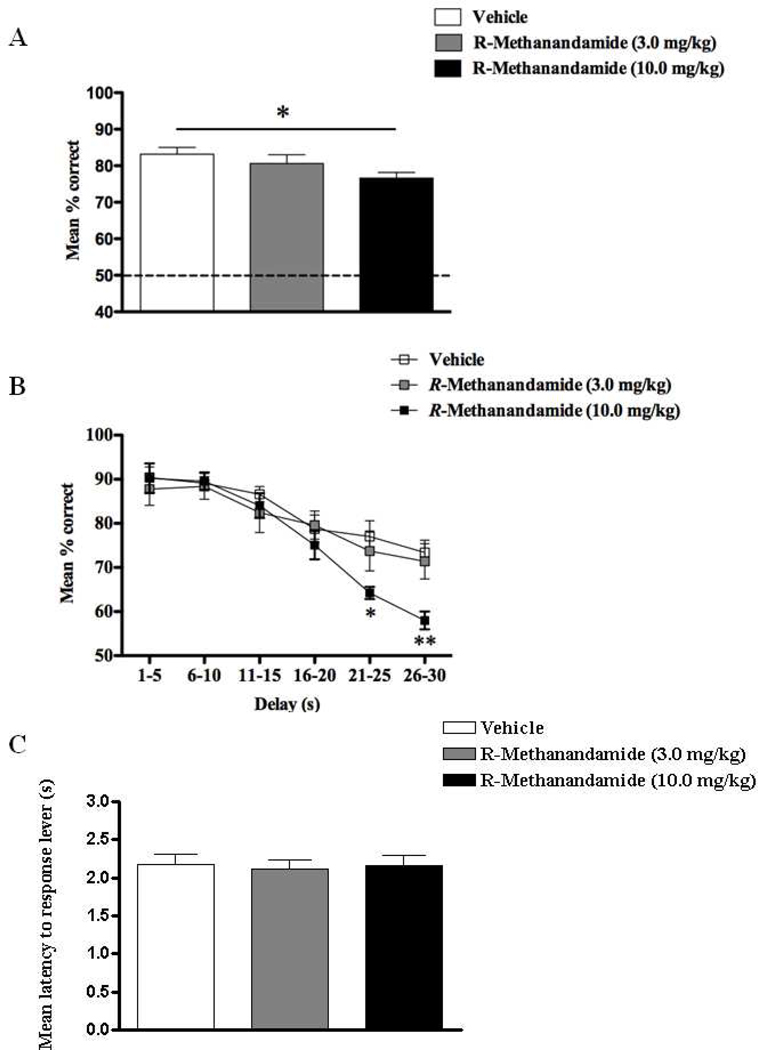
The mean overall DNMS performances (% correct responses) following administration of different doses of R-methanandamide in Exp.1, revealed a main effect of dose (F(1,17) = 3.95, p < 0.05; Fig.1A) owing to a reliable impairment when animals were treated with 10 mg/kg of R-methanandamide relative to vehicle (p < 0.05). The 100 trials of each DNMS session were sorted by % correct responses and delay epochs averaged across 5s intervals for the respective treatments (Fig.1B). Intriguingly, only when administered with 10mg/kg at very long delays were animals impaired leading to a dose × delay interaction (F(10,75) = 2.45, p < 0.01), apart from the normal delay effect (F(5,75) = 39.83, p < 0.001). This delay-dependence was confirmed for 10 mg/kg of R-methanandamide at both 21–25s (t = 2.95, p < 0.05) and 25–30s (t = 3.55, p < 0.01) delay intervals in comparison to vehicle, and was not present at any other delay interval (t’s < 0.84, p’s > 0.05), suggesting that the higher but not the lower dose of R-methanandamide was able to induce deficits in DNMS performance at delays greater than 20s. Moreover, the overall response latency was unaffected (F < 1), suggesting no effect of R-methanandamide on locomotor activity (Fig.1C).
Figure 1.
Overall percentage correct responses of DNMS performance across all delay intervals ranging from 1–30s (A), percentage correct DNMS responses sorted by length of delay in increments of 5sec (B), and latency to response levers (s) during DNMS performance (C) following the exposure of either vehicle, R-methanandamide (3.0 mg/kg) or R-methanandamide (10.0 mg/kg) (n = 6). Deficits in DNMS performance were observed only with the higher dose (10.0 mg/kg) and were most apparent at 21–25 and 26–30s delay intervals. Both doses had no effect on general locomotor activity. All values are represented as mean ± s.e.m. * p < 0.05; ** p < 0.01.
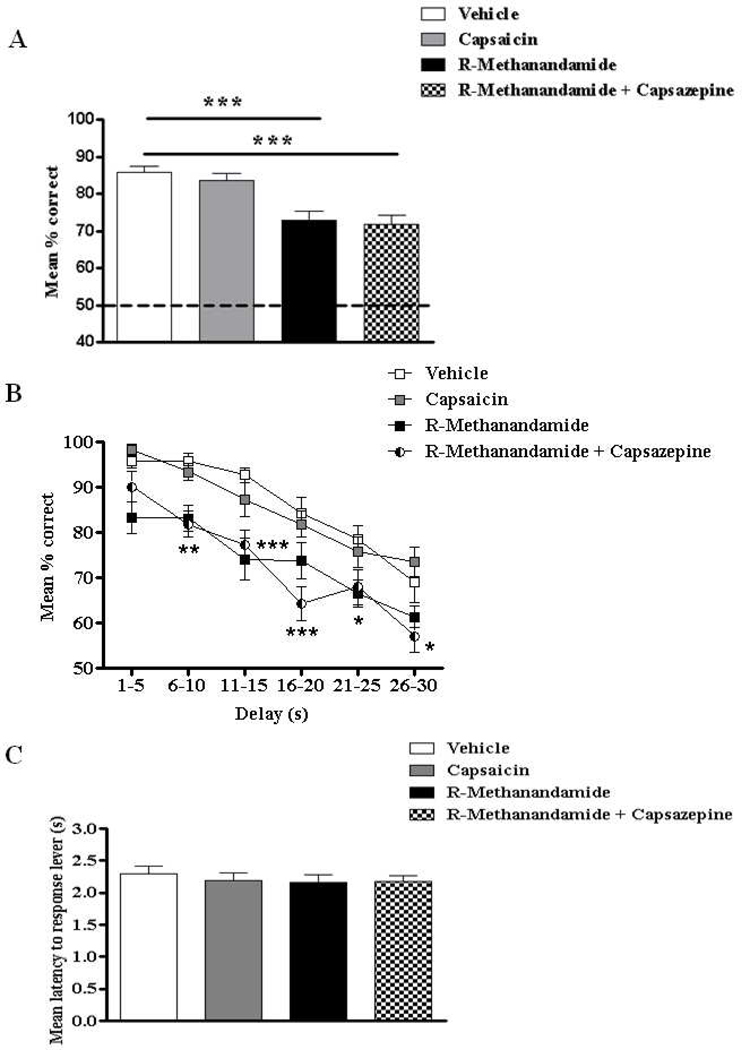
Since R-methanandamide is not specific to CB1, but also stimulates TRPV1 (vanilloid) receptors, Exp.2 explored whether R-methanandamide-induced deficits in STM can be reversed by blockade of TRPV1 with capsazepine. At the same time, a more specific activation of TRPV1 was probed using the agonist capsaicin to evaluate one selective contribution of the vanilloid system to DNMS performance. Data are depicted in Fig. 2 and confirm that i) the vanniloid system alone seems to play no part in DNMS performance since capsaicin did not affect STM (Fig.2A and B); ii) the impairment of R-methanandamide was confirmed; and iii) any TRPV1 receptor mediated action of R-methanandamide was excluded given that capsazepine did not reverse deficits. Consequently, we observed an overall main effect of treatment (F(3,55) = 14.90, p < 0.001; Fig.2A) on DNMS performance (% correct responses) owing to reliable deficits produced by R-methanandamide (p < 0.001) and R-methanandamide + capsazepine (p < 0.001) treated groups relative to vehicle; capsaicin failed to cause significant impairments. A main effect of drug (F(3,260) = 11.01, p < 0.001) and delay (F(5,260) = 58.83, p < 0.001) was evident when each trial was sorted by 5s delay intervals (Fig.2B) and post-hoc analysis revealed that capsaicin failed to impair DNMS performance at all delay interval epochs (all p’s > 0.05). Moreover, the R-methanandamide + capsazepine treated group produced delay-dependent deficits at all delay intervals greater than 6s (i.e. 6–10s: t = 4.02, p < 0.01; 11–15s: t = 4.89, p < 0.001; 16–20s: t = 4.50, p < 0.001; 21–25s: t = 2.47, p < 0.05; 26–30s: t = 3.29, p < 0.01). These results suggest that co-administration of capsazepine was not able to reverse R-methanandamide induced deficits in DNMS performance. Furthermore, the overall response latency (Fig.2C) was unaffected (F < 1), suggesting that none of the treatments in Exp.2 had any influence on locomotor activity.
Figure 2.
Overall percentage correct responses of DNMS performance across all delay intervals ranging from 1–30s (A), percentage correct DNMS responses sorted by length of delay in increments of 5sec (B), and latency to response levers (s) during DNMS performance (C) following the exposure of either vehicle, or capsaicin (0.01 mg/kg), or R-methanandamide (10.0 mg/kg), or capsazepine (0.1 mg/kg), or R-methanandamide + capsazepine (n = 14). The TRPV-1 receptor agonist, capsaicin had no effect on DNMS performance. The R-methanandamide induced deficits in DNMS performance were not reversed by the TRPV-1 receptor antagonist, capsazepine. No effect on general locomotor activity was observed following each of the treatments. All values are represented as mean ± s.e.m. *# p < 0.05; ** p < 0.01; *** p < 0.001.
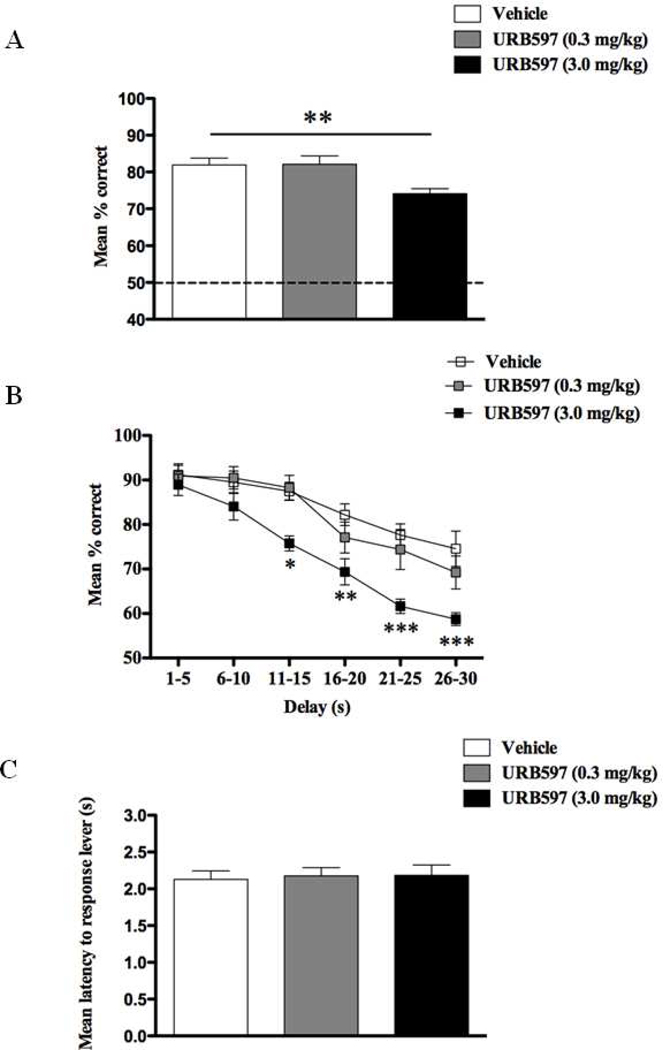
These results were further supported in Exp.3 following the treatment of different doses of the FAAH inhibitor, URB597, in which we also observed a dose-dependent impairment in DNMS performance (F(2,17) = 10.31, p < 0.001; Fig.3A) due to deficits after administration of the 3.0 mg/kg dose (p < 0.01). There was, however, no dose × delay interaction but we obtained main effects of dose (F(2,75) = 10.04, p < 0.001) and delay (F(5,75) = 46.31, p < 0.001; Fig 3B). Further planned comparisons confirmed that treatment with 3.0 mg/kg URB597 was detrimental for DNMS performance for all delays longer than 11s (all t’s > 2.1; p’s < 0.05), but not for shorter delay epochs (t’s < 1.33; p’s > 0.05). These URB597 induced deficits were not due to their effects on locomotion (F (2,17) < 1, p > 0.05; Fig.3C).
Figure 3.
Overall percentage correct responses of DNMS performance across all delay intervals ranging from 1–30s (A), percentage correct DNMS responses sorted by length of delay in increments of 5sec (B), and latency to response levers (s) during DNMS performance (C) following the exposure of either vehicle, URB597 (0.3 mg/kg) or URB597 (3.0 mg/kg) (n = 6). DNMS deficits were observed only with the higher dose (3.0 mg/kg) and most evident at delays intervals greater that 10s. Both doses had no effect on general locomotor activity. All values are represented as mean ± s.e.m. * p < 0.05; ** p < 0.01; *** p < 0.001.
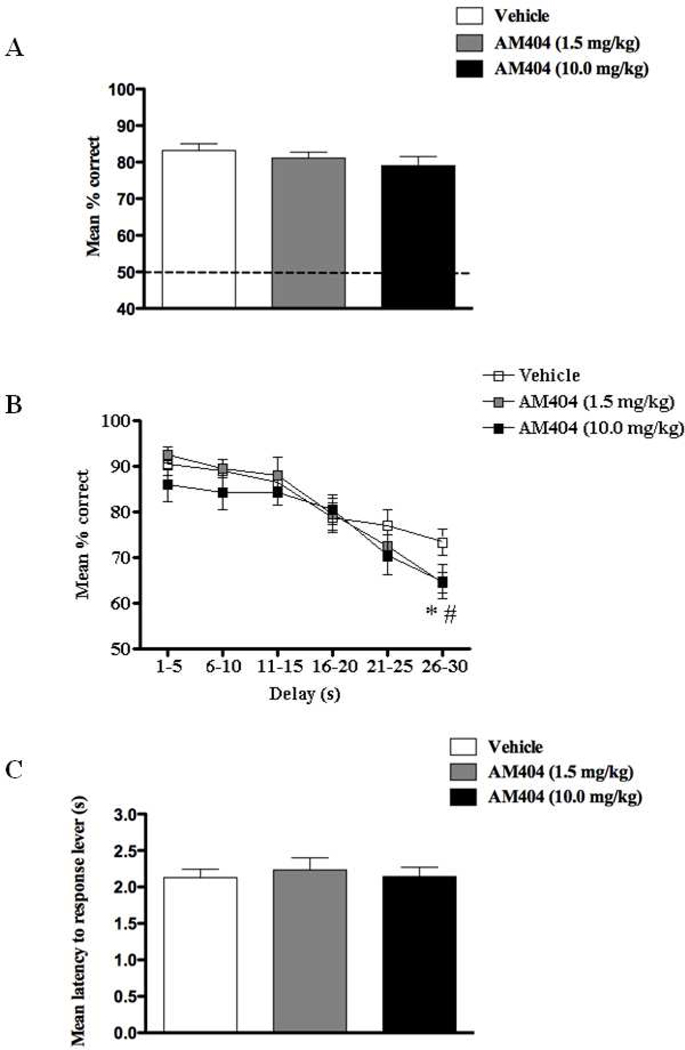
In contrast and despite a small reduction in performance, AM404 failed to cause an overall main effect of treatment (F(2,17) = 2.09, p > 0.05; Fig 4A) at doses that have been shown to affect behavior in vivo (Exp.4). This was also confirmed in terms of delay dependence; there was no effect with drug dose as a factor (F’s < 1.2), but a main effect of delay (F(5,75) = 34.78, p < 0.001; Fig 4B) confirmed an overall decline in performance accuracy for longer delay intervals. A planned comparison, however, detected a small yet reliable reduction in correct responses at 25–30s delay intervals following both the 1.5 mg/kg (t = 2.97, p < 0.05) and 10 mg/kg dose of AM404 (t = 2.56, p < 0.05) treatments. Again, these effects were seen in the absence of any motor deficits (F(2,17) = 1.22, p > 0.05; Fig 4C).
Figure 4.
Overall percentage correct responses of DNMS performance across all delay intervals ranging from 1–30s (A), percentage correct DNMS responses sorted by length of delay in increments of 5sec (B), and latency to response levers (s) during DNMS performance (C) following the exposure of either vehicle, AM404 (1.5 mg/kg), or AM404 (10.0 mg/kg) (n = 6). A subtle deficit in DNMS performance was observed with both low (1.5 mg/kg) and high (10.0 mg/kg) doses at delay intervals greater than 25s. No effect on general locomotor activity was observed following the treatment of both doses. All values are represented as mean ± s.e.m. *# p < 0.05.
3.2. Effects of R-methanandamide, URB597 and AM404 on hippocampal ensemble activity during delayed-nonmatch-to-sample task specific events
Recordings and analyses of hippocampal ensemble activity in rodents performing the DNMS short-term memory task have been conducted over a number of years and have provided substantial insight into the functional nature of task-specific ensemble firing patterns (Deadwyler et al., 1996, 2007; Deadwyler and Hampson, 2004; Hampson et al., 2003; Goonawardena et al., 2010a,b). The DNMS task requires the rat to make a ‘Sample’ lever response (SR) at the start of the trial and memorize the lever position in order to make the correct ‘Nonmatch’ response (NR) choice after a temporal delay of 1–30s on different trials during the session. Here hippocampal neuronal activity (i.e. peak z-score) around SR and NR was assessed in conjunction with behavior (see 3.1 above) following drug treatment.
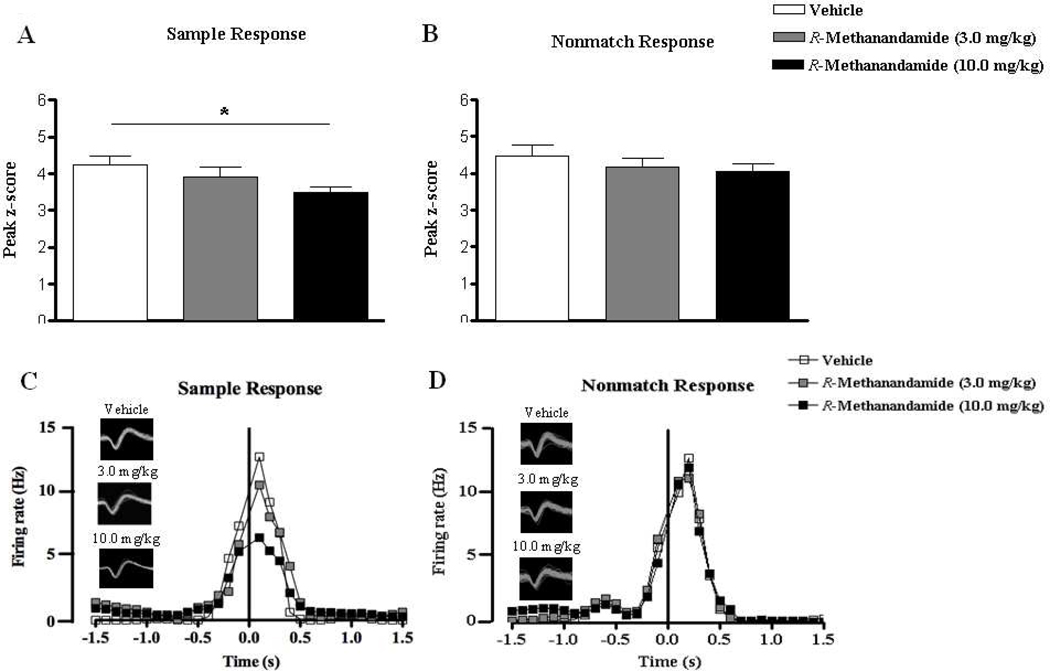
In line with behavioral performance, 10mg/kg R-methanandamide suppressed the z-score and its peak during the SR, but not the NR phase of the trial (Fig. 5). This was confirmed statistically in n = 13 hippocampal pyramidal neurons yielding a main effect of dose (F(2,38) = 3.93, p < 0.05; Fig.5A) due to firing rate reductions in the high dose (t = 2.79, p < 0.05). This was different during the NR (F < 1; n = 15; Fig. 5B). These results confirm that R-methanandamide alters hippocampal ensemble activity at the high dose affecting specifically the encoding of the sample lever position. This is also exemplified by the firing rates of a principal cell which responded during the SR (Fig. 5C) and another cell which fired during the NR (Fig. 5D) that were tracked over days when different doses of R-methanandamide were administered.
Figure 5.
Peak z-scores ((peak bin – mean firing rate of background bins) / standard deviation of background bins) of hippocampal principal cells that demonstrated increased firing during the sample (A) and nonmatch (B) response under vehicle conditions and following acute treatments of low (3.0 mg/kg) or high (10.0 mg/kg) doses of R-methanandamide. The firing rates and waveforms of two separate cells that responded to the sample and nonmatch events following each treatment are represented in (C) and (D) respectively. Note that the higher dose of R-methanandamide was able induce a suppression in firing frequency around the sample phase. All values in (A) and (B) are represented as mean ± s.e.m. * p < 0.05.
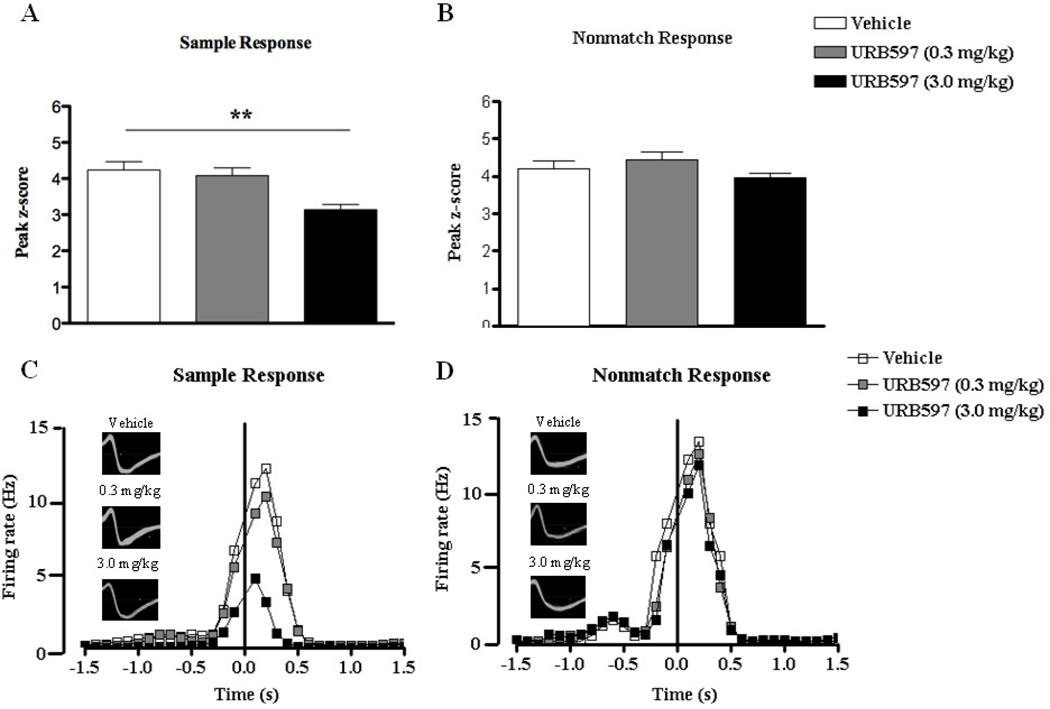
A similar analysis was conducted for URB597 and again the higher dose of 3mg/kg significantly reduced hippocampal cell firing (overall: F(2,38) = 8.84, p < 0.01; t = 3.88, p < 0.01 compared with vehicle; n = 13) around the SR (Fig. 6A). However, the lower dose of 0.3 mg/kg failed to produce any alterations in firing rate around SR (p > 0.05). No drug effect on NR firing was observed (F < 1.6; n = 15; Fig. 6B). Two representative example cells with selectively increased firing in response to the sample and nonmatch phases are presented in Fig. 6C and 6D respectively. They were tracked during control and drug conditions. Note the selective suppression of activity during SR, but not NR.
Figure 6.
Peak z-scores ((peak bin – mean firing rate of background bins) / standard deviation of background bins) of hippocampal principal cells that demonstrated increased firing during the sample (A) and nonmatch (B) response under vehicle conditions and following acute treatments of low (0.3 mg/kg) or high (3.0 mg/kg) doses of URB597. The firing rates and waveforms of two neurons that responded to sample and nonmatch events following each treatment are represented in (C) and (D) respectively. URB597 at the higher dose induced significant reductions in firing frequency around the sample phase. All values in (A) and (B) are represented as mean ± s.e.m. ** p < 0.01.
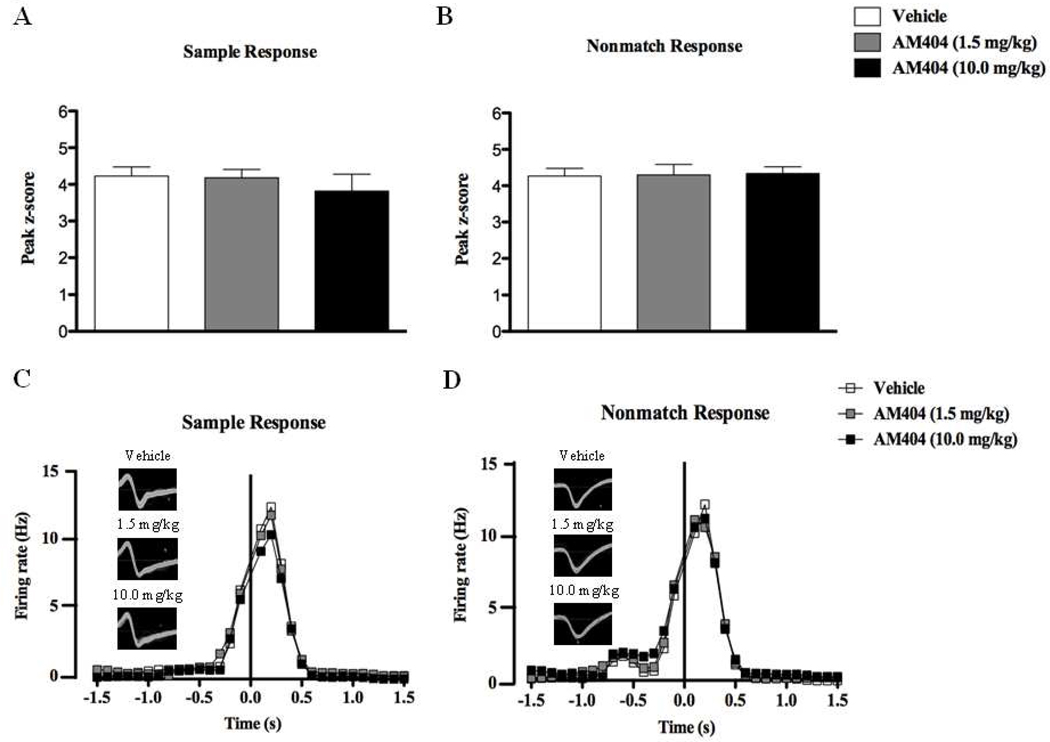
In contrast, AM404 failed to produce any changes to hippocampal ensemble activity (peak z-scores) either around SR (F < 1; n = 12, Fig.7A) or NR (F < 1, n = 15, Fig.7B) during DNMS performance. The firing frequencies of two individual cells that responded to the sample and nonmatch phases following respective AM404 dosing are depicted in Figs. 7C and 7D respectively.
Figure 7.
Peak z-scores ((peak bin – mean firing rate of background bins) / standard deviation of background bins) of hippocampal principal cells that demonstrated increased firing during the sample (A) and nonmatch (B) response under vehicle conditions and following the acute treatments of low (1.5 mg/kg) or high (10.0 mg/kg) doses of AM404. The firing rate and waveforms of two separate neurons that responded to sample and nonmatch events following each treatment are represented in (C) and (D) respectively. Both doses failed to produce significant alterations in event-related firing frequencies during DNMS performance. All values in (A) and (B) are represented as mean ± s.e.m.
Overall these electrophysiological results corroborate the behavioral outcome and provide plausible mechanistic explanations for the failures in performance observed in some drug doses.
4. Discussion
We have previously concentrated our research efforts on synthetic and plant-derived cannabinoid function as drugs of abuse. From this work (Goonawardena et al., 2010a,b; Robinson et al., 2008; 2010) we concluded that exogenously applied agonists exert their effects through CB1 and non-CB1 receptor mediated actions. It is therefore important to disentangle these two actions and this is best achieved through selective modulation of the endocannabinoid system. The current work is a first attempt in this direction.
Three compounds with different pharmacological profiles were administered in a within-subject design and effects on STM assessed. While both the stable anandamide analogue, R-methanandamide, and more prominently the FAAH inhibitor, URB597, impaired task performance and neuronal encoding of the sample event, an inhibitor of the anandamide transporter AM404 was not effective. This difference is attributed to the different pharmacological targets of our compounds, but at the same time highlights cannabinoid release during the sample event as a limiting factor for STM in the DNMS task. We propose that high levels of endocannabinoid release in response to the sample lever presentation curtails the length of STM so that correct performance is at risk for long, but not short delay intervals.
4.1. Synaptic adaptation and long-delay performance are impaired by R-methanandamide
R-methanandamide caused delay-dependent deficits in DNMS performance following the administration of a high dose (10 mg/kg) as opposed to a low dose (3.0 mg/kg). This is in line with our previous work, which attests CB1 receptors in hippocampus (and adjacent brain regions) having a selective role in STM (Hampson & Deadwyler, 1999; 2000). It appears that they are functionally irrelevant in DNMS performance when the NR immediately follows SR, but gain relevance for longer delay intervals when the memory demand (i.e. cognitive workload) is high (Goonawardena et al., 2010b). These results are in agreement with other behavioral data that (i) R-methanandamide at similar doses impaired working memory in the water maze (Varvel and Lichtman, 2002; Varvel et al., 2006) or learning an operant conditioning task (Brodkin and Moerschbaecher, 1997), and (ii) anandamide itself can lead to memory deficits (Varvel et al. 2006; Castellano et al., 1997, 1999; Costanzi et al., 2003). Unexplored in all these studies were possible physiological responses to drug exposure. We here confirm that R-methanandamide indeed alters discharges during the sample event and thereby compromises encoding of the sample lever with the result that animals are more likely to fail in their NR and is thus in agreement with findings obtained following exposure to exogenous cannabinoids such as Δ9-THC and WIN-2 (Hampson & Deadwyler, 2000; Hampson et al., 2003; Goonawardena et al., 2010). Given that anandamide is able to inhibit hippocampal long-term potentiation (LTP) (Terranova et al., 1995; Lees & Dougalis, 2004) and block the formation of new synapses in hippocampal cultures via the activation of CB1 receptors (Kim and Thayer, 2001), it is tempting to speculate that an increase in firing rate during the sample (encoding) phase provides a strong high-frequency stimulus and induce LTP-like plastic changes that could survive the delay for accurate recall later, and that R-methanandamide disrupts this process. However, to date no concrete evidence suggests that this blockade of neuronal firing during encoding results from the ability of anandamide to limit LTP.
4.2 Heightened endocannabinoid levels affect DNMS performance and sample firing during encoding, but not during recall
The main focus of this study was on the function of endocannabinoids. Although R-methanandamide is quasi-identical with anandamide, its pharmacological actions are somewhat different and longer lasting. We therefore blocked the metabolism of anandamide by inhibition of its degradation by FAAH (URB597) or its putative re-uptake transporter (AM404). Although we expected both methods to yield similar outcome, results clearly indicate stronger efficacy of FAAH inhibition. URB597 produced both delay- and dose-dependent impairments in DNMS performance while AM404-induced deficits in DNMS performance were very subtle and not paralleled by physiological changes. This is despite doses used that have previously been confirmed to potently elevate anandamide levels in vivo (Fegley et al., 2004; Pamplona et al., 2008; Abush and Akirav, 2010; Glaser et al., 2005; Fowler et al., 2004) and may be interpreted in two ways: 1) URB597 is more potent than AM404 or 2) their differential pharmacology has different effects on anandamide levels and so the behavioral/physiological outcome. While our experiments do not allow conclusive distinction between these theories, the dose selection was based on previous work and for URB597, there is compelling evidence for efficacy as low as 0.3mg/kg which impaired working memory (i.e. delayed-alternation task) in the T-maze in rats (Seillier et al., 2010). Similarly, systemic administration of 10 mg/kg or direct intra-cerebroventricular injection of AM404 facilitated the extinction of short-term contextual fear memory in rats (Pamplona et al., 2008; Bitencourt et al., 2008). In addition, Chhatwal and co-workers (2005) reported that AM404 (2–10 mg/kg) dose-dependently enhanced extinction of conditioned fear memory in rats. And although we take this as strong evidence for drug efficacy in the doses selected in our study, it remains unexplored whether doses required for impairment of operant conditioning with positive reward are similar to extinction of fear. Alternatively, the negative result in exp.3 may be due to differential mechanisms activated in hippocampus and amygdala during the two tasks. Observed potent effects of URB597 relative to AM404 and R-methanandamide may lie with its (URB597) ability to inhibit FAAH and thereby prevent the breakdown of other endocannabinoids such as palmitylethanolamide (PEA) and oleoylethanolamide (OEA) that are also substrates of FAAH (Mazzola et al., 2009; Wise et al., 2008). PEA and OEA are co-synthesized with anandamide and can potentiate anandamide responses (so called ‘entourage effects’) via the activation of other cannabinoid-sensitive receptors such as GPR119 (G-protein coupled), TRPV-1 (Transient-Receptor-Potential-Vanilloid type-1) and PPARα (Peroxisome-Proliferator-Activated-Receptor-alpha) receptors (Hansen, 2010; Ho et al., 2008; Ning et al., 2008). In addition, there is considerable debate as to whether the uptake of anandamide is mediated via an active transporter or passive diffusion (Glaser et al., 2003, 2005). If anandamide is taken up by facilitated (passive) diffusion, it should mediate bidirectional transport and be involved in both its release and re-uptake (Hillard et al., 1997). In fact previous work has shown that effects of transport inhibitors can result in a blockade of endocannabinoid-mediated effects and intracellular accumulation of anandamide and 2-AG, possibly due to the disruption of endocannabinoid release (Ronesi et al., 2004; Ligresti et al., 2004; Pillolla et al., 2007). Thus, the blockade of anandamide release may functionally counteract the blockade of FAAH by AM404 resulting in a lack of effect by this drug. This would explain the failure of AM404 to alter behavior and event-related firing during this particular STM task.
The finding that higher doses of URB597 (3.0 mg/kg) suppressed hippocampal neuronal firing during the sample but not the nonmatch phase suggests that elevation of anandamide disrupted the encoding of trial relevant information during DNMS performance. Similar results from exp.1 with R-methanandamide therefore provide compelling evidence that anandamide modulation impinges on hippocampal neurophysiology and the alterations observed in single unit firing indeed underlie the behavioral impairment. They add to similar and existing data from studies on exogenous cannabinoid exposure such as Δ9-THC and WIN55,212-2 and DNMS performance, in which similar changes in discharge patterns have been observed (Hampson and Deadwyler, 2000; Hampson et al., 2003; Goonawardena et al., 2010b). Additionally, this is the first in vivo study to demonstrate URB597 induced alterations in hippocampal ensemble activity in awake, behaving animals. Coomber and co-workers (2008) reported a dose-dependent attenuation of kainate-induced firing and bursting rates of hippocampal neurons in vivo following URB597 administration, but under anesthesia conditions.
4.3 R-methanandamide induced deficits in DNMS performance are not mediated by TRPV-1 receptors
Like anandamide, which is considered to be both a cannabinoid and an endovanilloid, R-methanandamide also acts as a ligand for TRPV-1 receptors (Roberts et al., 2008; Breyne and Vanheel, 2006) and these receptors are highly expressed on cell bodies and dendrites of hippocampal neurons (Toth et al., 2005). Owing to the recent finding that anandamide mediates a TRPV-1 triggered form of long-term depression (LTD) in the hippocampus, (Chavez et al., 2010) we explored whether TRPV-1 receptors may also have a modulatory role in hippocampal-dependent learning and memory, and possibly contribute to the R-methanandamide and URB597 induced deficits in DNMS performance. However, our results suggest TRPV-1 receptors do not seem to play an active role in this particular STM task since DNMS performance because neither capsaicin alone, nor capsaicipine in conjunction with R-methanandamide exerted pharmacological efficiency.
Although the emphasis here was on anandamide, other endocannabinoids in the hippocampus, such as 2-AG (Stella et al., 1997) might also play important roles and thus explain the relatively weak potency of R-methanandamide and AM404. However, until recently no pharmacological tools were available to selectively modulate brain levels of 2-AG. The discovery of URB602, URB754 and JZL184 (King et al., 2007; Hillard et al., 2007; Long et al., 2009) as potent inhibitors of monoacylglycerol lipase (MAGL), the enzyme responsible in the metabolic breakdown of 2-AG, are of considerable significance in this regard, and require further examination.
In conclusion, this is the first study that reports STM deficits in an operant conditioning task with concurrent alterations in hippocampal neuronal activity following enhancement of brain anandamide levels via the blockade of its breakdown (URB597), but not re-uptake or release (AM404). We provide evidence for the congruent suppression of hippocampal single cell firing and task performance, which seems due to a lack of on-demand release of anandamide that normally enables encoding of task-relevant information. The differences in pharmacology between the applied compounds may explain the variations in results.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant DA08549 to R.E.H. and G.R. The authors thank Drs. Allyn Howlett and Bettina Platt for advice on earlier versions of this manuscript.
Abbreviations
- 2-AG
2-Arachidonylglycerol
- DNMS
Delayed Non-Match to Sample
- FAAH
Fatty Acid Amide Hydrolase
- STM
Short-Term Memory
- WIN-2
WIN55,212-2
- WM
working memory
- SR
sample response
- NR
non-match response
- TRPV-1
transient receptor potential vanilloid type-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abush H, Akirav I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus. 2010;20:1126–1138. doi: 10.1002/hipo.20711. [DOI] [PubMed] [Google Scholar]
- Barros DM, Carlis V, Maidana M, Silva ES, Baisch AL, Ramirez MR, Izquierdo I. Interactions between anandamide-induced anterograde amnesia and post-training memory modulatory systems. Brain Res. 2004;1016:66–71. doi: 10.1016/j.brainres.2004.04.067. [DOI] [PubMed] [Google Scholar]
- Bhaskaran MD, Smith BN. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp. Neurol. 2010;223:529–536. doi: 10.1016/j.expneurol.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur.Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Breyne J, Vanheel B. Methanandamide hyperpolarizes gastric arteries by stimulation of TRPV1 receptors on perivascular CGRP containing nerves. J. Cardiovasc. Pharmacol. 2006;47:303–309. doi: 10.1097/01.fjc.0000205053.53946.10. [DOI] [PubMed] [Google Scholar]
- Brodkin J, Moerschbaecher JM. SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J.Pharmacol.Exp.Ther. 1997;282:1526–1532. [PubMed] [Google Scholar]
- Castellano C, Cabib S, Palmisano A, Di Marzo V, Puglisi-Allegra S. The effects of anandamide on memory consolidation in mice involve both D1 and D2 dopamine receptors. Behav.Pharmacol. 1997;8:707–712. doi: 10.1097/00008877-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Castellano C, Ventura R, Cabib S, Puglisi-Allegra S. Strain-dependent effects of anandamide on memory consolidation in mice are antagonized by naltrexone. Behav.Pharmacol. 1999;10:453–457. doi: 10.1097/00008877-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Coomber B, O' Donoghue MF, Mason R. Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse. 2008;62:746–755. doi: 10.1002/syn.20547. [DOI] [PubMed] [Google Scholar]
- Costanzi M, Battaglia M, Populin R, Cestari V, Castellano C. Anandamide and memory in CD1 mice: effects of immobilization stress and of prior experience. Neurobiol.Learn.Mem. 2003;79:204–211. doi: 10.1016/s1074-7427(03)00006-6. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J.Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Goonawardena AV, Hampson RE. Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav. Pharmacol. 2007;18:571–580. doi: 10.1097/FBP.0b013e3282ee2adb. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, Zieglgansberger W, Lutz B, Rammes G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J. Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc.Natl.Acad.Sci. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Ottani A, Vivoli R, Giuliani D. Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacology Biochemistry and Behavior. 1999;64:555–561. doi: 10.1016/s0091-3057(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, Lopez-Rodriguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis--a difficult issue to handle. Eur.J.Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc.Natl.Acad.Sci. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Deutsch DG, Studholme KM, Zimov S, Yazulla S. Endocannabinoids in the intact retina: 3 H-anandamide uptake, fatty acid amide hydrolase immunoreactivity and hydrolysis of anandamide. Vis.Neurosci. 2005;22:693–705. doi: 10.1017/S0952523805226020. [DOI] [PubMed] [Google Scholar]
- Goonawardena AV, Robinson L, Hampson RE, Riedel G. Cannabinoid and cholinergic systems interact during performance of a short-term memory task in the rat. Learning & Memory. 2010a;17:502–511. doi: 10.1101/lm.1893710. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Robinson L, Riedel G, Hampson RE. Recruitment of hippocampal neurons to encode behavioral events in the rat: Alterations in cognitive demand and cannabinoid exposure. Hippocampus. 2010b;20:1083–1094. doi: 10.1002/hipo.20706. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and Memory. Life Science. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of Hippocampal neural encoding for short-term memory in rats. J.Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Kelly EJ, Deadwyler SA. Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus. 2003;13:543–556. doi: 10.1002/hipo.10081. [DOI] [PubMed] [Google Scholar]
- Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp. Neurol. 2010;224:48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J.Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J.Pharmacol.Exp.Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Hillard CJ, Ho WS, Thompson J, Gauthier KM, Wheelock CE, Huang H, Hammock BD. Inhibition of 2-arachidonoylglycerol catabolism modulates vasoconstriction of rat middle cerebral artery by the thromboxane mimetic, U-46619. Br. J. Pharmacol. 2007;152:691–698. doi: 10.1038/sj.bjp.0707468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem. Phys. Lipids. 2000;108:123–134. doi: 10.1016/s0009-3084(00)00191-2. [DOI] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. 'Entourage' effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br.J.Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BG, Thayer SA. Anandamide transport inhibitor AM404 and structurally related compounds inhibit synaptic transmission between rat hippocampal neurons in culture independent of cannabinoid CB1 receptors. Eur. J. Pharmacol. 2004;496:33–39. doi: 10.1016/j.ejphar.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J. Neurosci. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem.Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiorek P, Hryniewicz A, Bialuk I, Zawadzka A, Winnicka MM. Cannabinoids alter recognition memory in rats. Pol.J.Pharmacol. 2003;55:903–910. [PubMed] [Google Scholar]
- Lees G, Dougalis A. Differential effects of the sleep-inducing lipid oleamide and cannabinoids on the induction of long-term potentiation in the CA1 neurons of the rat hippocampus in vitro. Brain Res. 2004;997:1–14. doi: 10.1016/j.brainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404:175–179. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Morera E, van der Stelt M, Monory K, Lutz B, Ortar G, Di Marzo V. Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem. J. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct Neuronal subpopulations in the adult mouse forebrain. European Journal of Neuroscience. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn. Mem. 2009;16:332–337. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola C, Micale V, Drago F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J. Pharmacol. 2003;447:219–225. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Mishima K, Egashira N, Hirosawa N, Fujii M, Matsumoto Y, Iwasaki K, Fujiwara M. Characteristics of learning and memory impairment induced by delta 9-tetrahydrocannabinol in rats. Jpn.J.Pharmacol. 2001;87:297–308. doi: 10.1254/jjp.87.297. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Sanchez-Alavez M, Navarro L, Martinez-Gonzalez D, Drucker-Colin R, Prospero-Garcia O. Anandamide modulates sleep and memory in rats. Brain Res. 1998;812:270–274. doi: 10.1016/s0006-8993(98)00969-x. [DOI] [PubMed] [Google Scholar]
- Ning Y, O'Neill K, Lan H, Pang L, Shan LX, Hawes BE, Hedrick JA. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br.J.Pharmacol. 2008;155:1056–1065. doi: 10.1038/bjp.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol.Learn.Mem. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Ed. New York: Academic Press; 1998. [Google Scholar]
- Pillolla G, Melis M, Perra S, Muntoni AL, Gessa GL, Pistis M. Medial forebrain bundle stimulation evokes endocannabinoid-mediated modulation of ventral tegmental area dopamine neuron firing in vivo. Psychopharmacology (Berl) 2007;191:843–853. doi: 10.1007/s00213-007-0733-z. [DOI] [PubMed] [Google Scholar]
- Robinson L, Goonawardena AV, Pertwee R, Hampson RE, Platt B, Riedel G. WIN55,212-2 induced deficits in spatial learning are mediated by cholinergic hypofunction. Behav. Brain. Res. 2010;208:584–592. doi: 10.1016/j.bbr.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, McKillop-Smith S, Ross NL, Pertwee RG, Hampson RE, Platt B, Riedel G. Hippocampal endocannabinoids inhibit spatial learning and limit spatial memory in rats. Psychopharmacology (Berl) 2008;198:551–563. doi: 10.1007/s00213-007-1012-8. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J. Neuroscience. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int.J.Neuropsychopharmacol. 2010;13:373–386. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch.Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, et al. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology. 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology. 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Cravatt BF, Engram AE, Lichtman AH. Fatty acid amide hydrolase (−/−) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J.Pharmacol.Exp.Ther. 2006;317:251–257. doi: 10.1124/jpet.105.095059. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J.Pharmacol.Exp.Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of Fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2008;54:181–188. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol. 2003;477:213–217. doi: 10.1016/j.ejphar.2003.08.025. [DOI] [PubMed] [Google Scholar]