Abstract
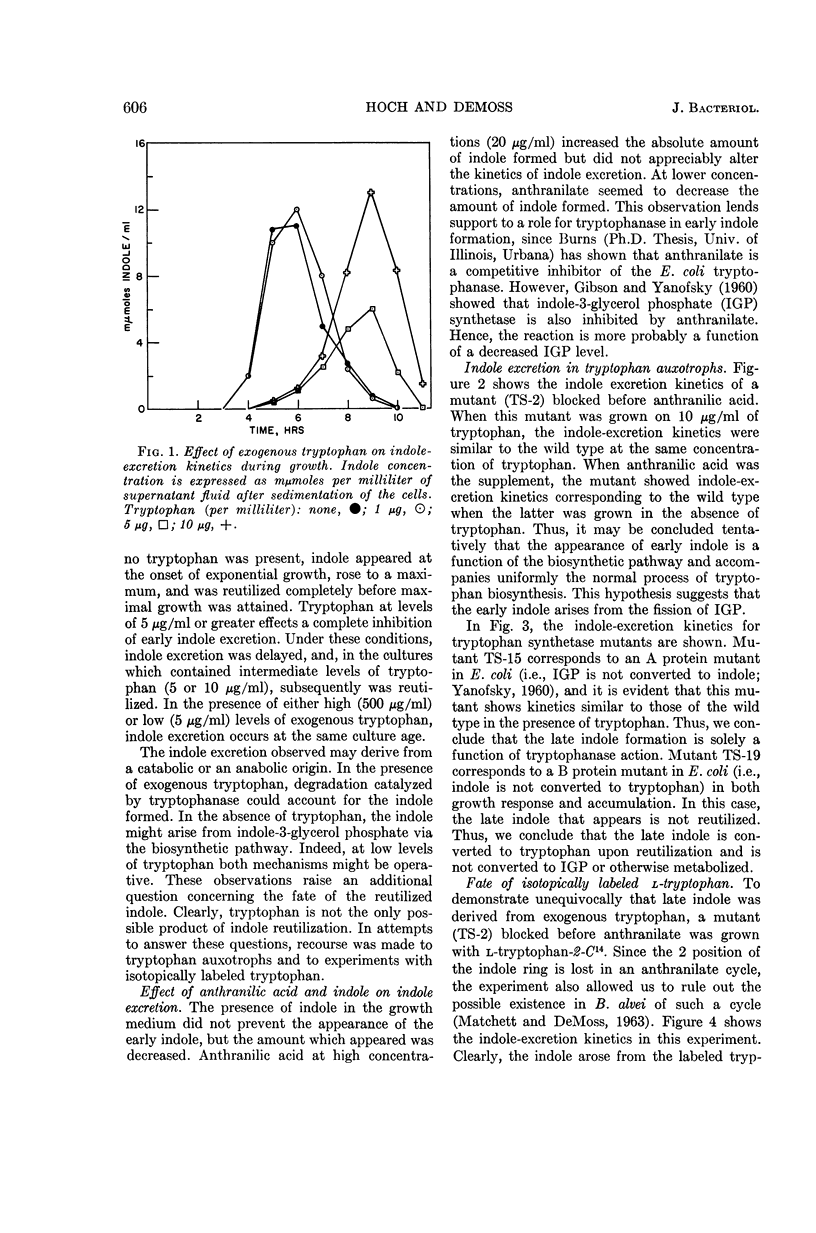
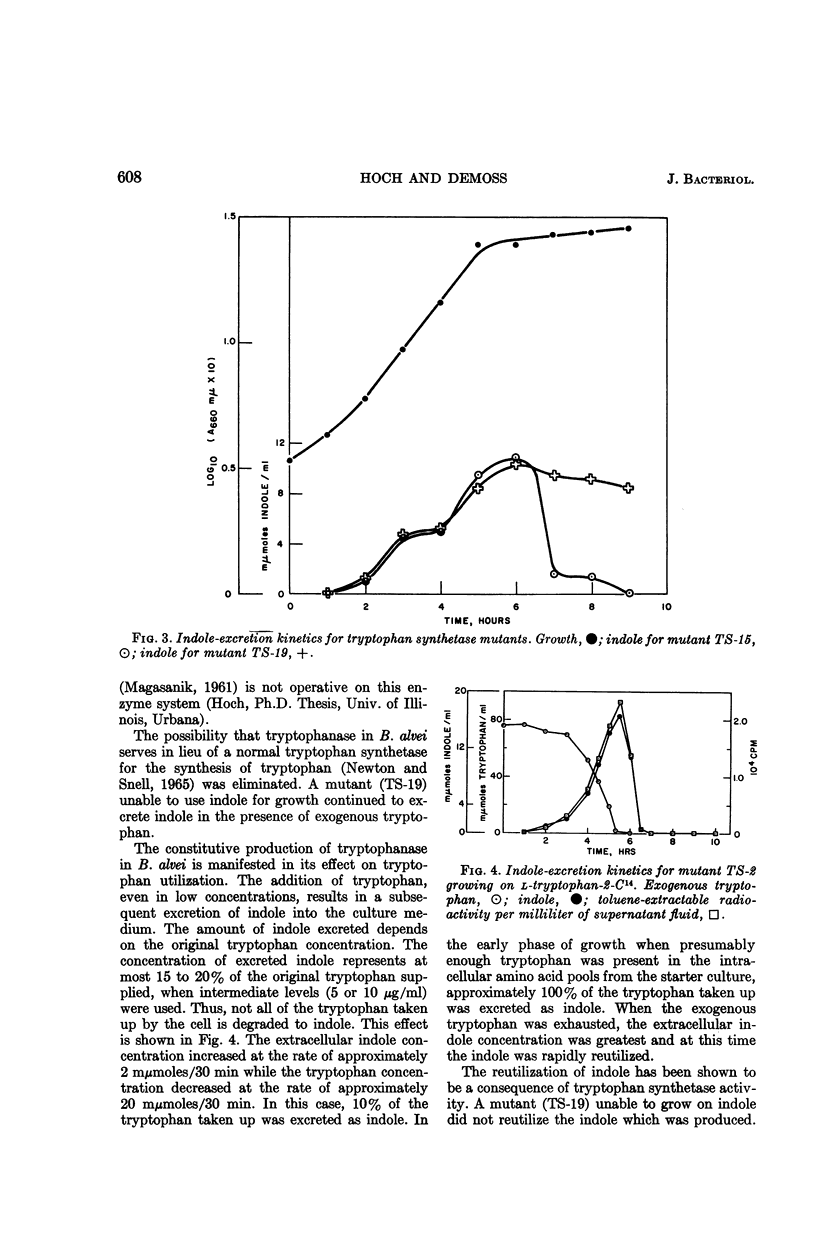
Hoch, J. A. (University of Illinois, Urbana), and R. D. DeMoss. Physiological effects of a constitutive tryptophanase in Bacillus alvei. J. Bacteriol. 90:604–610. 1965.—Tryptophanase synthesis in B. alvei is not under the control of tryptophan and is not subject to catabolite repression. Exogenously supplied tryptophan was converted to indole by tryptophanase, and was excreted into the culture medium. The amount of indole excreted was dependent upon the concentration of tryptophan supplied. At intermediate levels of tryptophan (5 to 15 μg/ml), the excreted indole was completely reutilized by the cell, in contrast to the result with higher levels. Indole reutil zation was shown to be dependent upon a functional tryptophan synthetase. In the absience of exogenous tryptophan, indole was excreted into the culture medium at an earlier physiological age. The early indole was shown not to be a consequence of tryptophanase action. The early indole accompanied uniformly the normal process of tryptophan biosynthesis, and the fission of indole-3-glycerol phosphate was suggested as the origin of the excreted indole.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- BURNS R. O., DEMOSS R. D. Properties of tryptophanase from Escherichia coli. Biochim Biophys Acta. 1962 Dec 4;65:233–244. doi: 10.1016/0006-3002(62)91042-9. [DOI] [PubMed] [Google Scholar]
- FRANK L. H., DEMOSS R. D. Specific enzymic method for the estimation of L-tryptophan. Arch Biochem Biophys. 1957 Apr;67(2):387–397. doi: 10.1016/0003-9861(57)90293-x. [DOI] [PubMed] [Google Scholar]
- GIBSON F., YANOFSKY C. The partial purification and properties of indole-3-glycerol phosphate synthetase from Escherichia coli. Biochim Biophys Acta. 1960 Oct 7;43:489–500. doi: 10.1016/0006-3002(60)90471-6. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Direct evidence for a trytophan-anthranilic acid cycle in Neurospora. Biochim Biophys Acta. 1963 Jun 4;71:632–642. doi: 10.1016/0006-3002(63)91136-3. [DOI] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. PHYSIOLOGICAL CHANNELING OF TRYPTOPHAN IN NEUROSPORA CRASSA. Biochim Biophys Acta. 1964 Apr 4;86:91–99. doi: 10.1016/0304-4165(64)90162-x. [DOI] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. CATALYTIC PROPERTIES OF TRYPTOPHANASE, A MULTIFUNCTIONAL PYRIDOXAL PHOSPHATE ENZYME. Proc Natl Acad Sci U S A. 1964 Mar;51:382–389. doi: 10.1073/pnas.51.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. FORMATION AND INTERRELATIONSHIPS OF TRYPTOPHANASE AND TRYPTOPHAN SYNTHETASES IN ESCHERICHIA COLI. J Bacteriol. 1965 Feb;89:355–364. doi: 10.1128/jb.89.2.355-364.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



