Summary
Background
We report clinical safety and biochemical efficacy from a dose-ranging study of intravenously administered AVI-4658 phosphorodiamidate morpholino oligomer (PMO) in patients with Duchenne muscular dystrophy.
Method
We undertook an open-label, phase 2, dose-escalation study (0·5, 1·0, 2·0, 4·0, 10·0, and 20·0 mg/kg bodyweight) in ambulant patients with Duchenne muscular dystrophy aged 5–15 years with amenable deletions in DMD. Participants had a muscle biopsy before starting treatment and after 12 weekly intravenous infusions of AVI-4658. The primary study objective was to assess safety and tolerability of AVI-4658. The secondary objectives were pharmacokinetic properties and the ability of AVI-4658 to induce exon 51 skipping and dystrophin restoration by RT-PCR, immunohistochemistry, and immunoblotting. The study is registered, number NCT00844597.
Findings
19 patients took part in the study. AVI-4658 was well tolerated with no drug-related serious adverse events. AVI-4658 induced exon 51 skipping in all cohorts and new dystrophin protein expression in a significant dose-dependent (p=0·0203), but variable, manner in boys from cohort 3 (dose 2 mg/kg) onwards. Seven patients responded to treatment, in whom mean dystrophin fluorescence intensity increased from 8·9% (95% CI 7·1–10·6) to 16·4% (10·8–22·0) of normal control after treatment (p=0·0287). The three patients with the greatest responses to treatment had 21%, 15%, and 55% dystrophin-positive fibres after treatment and these findings were confirmed with western blot, which showed an increase after treatment of protein levels from 2% to 18%, from 0·9% to 17%, and from 0% to 7·7% of normal muscle, respectively. The dystrophin-associated proteins α-sarcoglycan and neuronal nitric oxide synthase were also restored at the sarcolemma. Analysis of the inflammatory infiltrate indicated a reduction of cytotoxic T cells in the post-treatment muscle biopsies in the two high-dose cohorts.
Interpretation
The safety and biochemical efficacy that we present show the potential of AVI-4658 to become a disease-modifying drug for Duchenne muscular dystrophy.
Funding
UK Medical Research Council; AVI BioPharma.
Introduction
Duchenne muscular dystrophy is a progressive, severely disabling neuromuscular disease that affects one in 3500 newborn boys and causes premature death.1 In Duchenne muscular dystrophy, the open reading frame of the X-linked dystrophin gene (DMD) is disrupted by deletions (roughly 65%), duplications (10%), point mutations (10%), or other smaller rearrangements. Dystrophin is located underneath the sarcolemma and assembles with sarcolemmal proteins such as dystroglycan, α-sarcoglycan, and neuronal nitric oxide synthase (NOS) to form the dystrophin-associated glycoprotein complex. The essential function of dystrophin in muscle is to connect the subsarcolemmal cytoskeleton to the sarcolemma by binding N-terminally to F-actin and C-terminally to β-dystroglycan. Loss of dystrophin results in inflammation, muscle degeneration, and replacement of muscle with fibroadipose tissue.2
In the milder allelic Becker muscular dystrophy, dystrophin mutations do not disrupt the open reading frame, a shortened but functional dystrophin protein is produced, and most patients are able to walk into late adulthood and have a normal lifespan.3 Therefore, induction of exon skipping to restore the open reading frame is an attractive therapeutic strategy in Duchenne muscular dystrophy that can be achieved with splice-switching oligomers. These oligomers are typically 20–30 nucleotides in length and are complementary in sequence to regions of the pre-mRNA transcript relevant for targeted DMD exon skipping.4 Splice-switching oligomers targeting dystrophin exons have been successfully used to restore dystrophin expression in vitro and in various animal models of Duchenne muscular dystrophy.5,6 In the mdx mouse, administration of 2′O-methyl-ribooligonucleoside-phosphorothioate (2′OMe) and phosphorodiamidate morpholino oligomers (PMOs) identified PMOs as more effective for induction of exon skipping and restoration of long-lasting dystrophin production after intramuscular or intravenous administration.7 In the X-linked muscular dystrophy dog, PMO administration was followed by dystrophin restoration and clinical benefit without adverse reactions.6
Two proof-of-principle clinical trials in patients with Duchenne muscular dystrophy, who received one intramuscular administration of either 2′OMe8 or PMO9 targeted to skip exon 51, showed efficient dystrophin restoration. More recently, in an open-label, dose-escalation study in 12 boys with Duchenne muscular dystrophy,10 weekly subcutaneous injections of PRO051, a 2′OMe splice switching oligomer, at 0·5, 2, 4, and 6 mg/kg bodyweight for 5 weeks induced skipping of exon 51 and increased dystrophin concentrations. A 12-week extension with a dose of 6 mg/kg bodyweight of PRO05110 was well tolerated and was followed by stabilisation of muscle function, but no significant improvement in a 6-min walk test. We report biochemical efficacy and clinical safety from a dose-ranging study of the first intravenous systemically administered PMO, AVI-4658, in patients with Duchenne muscular dystrophy.
Methods
Study design and participants
This open-label phase 2 study was approved by the UK Medicines and Healthcare Products Regulatory Agency. The UK Gene Therapy Advisory Committee provided ethics approval and site-specific approval under the number GTAC157 (EudraCT number 2007-004695-39) for both active trial sites in London and Newcastle, UK. Additionally, the study was adopted by the institutional review boards at both sites. Participants were aged 5–15 years and had genetically confirmed diagnosis of Duchenne muscular dystrophy with an out-of-frame deletion eligible for correction by skipping of exon 51. The absence of additional deletions and variants in the splice switching oligomer duplex formation region was confirmed in all participants by dystrophin exonic multiplex ligation-dependent probe amplification and DNA sequencing. Participants were enrolled after written informed assent from the child and written informed consent of a parent or legal guardian was obtained. Further study details are shown in the webappendix pp 1–3; the protocol is available online. If no original diagnostic muscle biopsy sample was available, a sample was obtained from the biceps brachii muscle (16 participants) and a post-treatment sample was taken from the contralateral biceps 2 weeks after the last dose of the study drug.
Figure 1 shows the dose escalation and cohort assignment. The data safety monitoring board met with clinical investigators and the sponsor to review safety before dose escalations took place. Each cohort led with a single patient and, after three doses, a safety monitoring committee consisting of clinical investigators, the sponsor medical monitor, and the medical study monitor reviewed all safety data from that patient before enrolment of subsequent patients to expand that cohort. Dose escalation occurred after informed discussion of the data safety monitoring board when all patients in the previous cohort had received at least 3 weeks of treatment.
Figure 1.
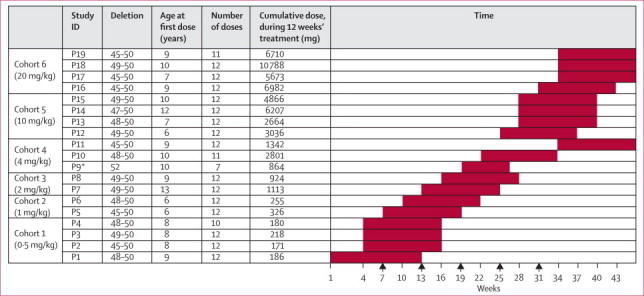
Patients recruited to the trial, their assignment to cohorts, and the dose-escalation scheme
Each full red box represents a time interval of 12 weeks' dosing. Arrows show the timepoints at which the data safety monitoring board met with clinical investigators and the sponsor to review safety before subsequent dose escalations. *Patient withdrawn from study after seven doses.
Procedures
AVI-4658 (sequence CTCCAACATCAAGGAAGATGGCATTTCTAG),11 an exon 51-targeting PMO, was provided by AVI BioPharma (Bothell, WA, USA) at 100 mg/mL in phosphate-buffered saline (1 mL/vial). It was diluted in up to 50 mL saline (NaCl 0·9% v/w) for intravenous infusion over 1 h.
Exon 51 skipping in muscle biopsy samples was assessed by RT-PCR.9 Dystrophin expression in pretreatment and post-treatment muscle was investigated first by immunohistochemical detection of dystrophin with MANDYS10612 (MDA Monoclonal Antibody Resource, Glenn Morris, Oswestry, UK) and Dys2 (Novacastra, UK) antibodies, initially assessed by two masked investigators. To control for the presence of trace levels of dystrophin in the pretreatment muscle, as well as revertant fibres,13,14 we set a baseline using sections of pretreatment muscles from each patient when counting dystrophin-positive fibres in post-treatment muscles, so that only any revertant fibres were seen as positive. We then used this threshold to count the dystrophin-positive fibres in sections of post-treatment muscle of that patient.2 The investigator had to be unmasked to which section came from the pretreatment and post-treatment biopsies. The semiquantitative measurements of dystrophin, α-sarcoglycan, and neuronal NOS expression levels were done as previously described.13 Finally, western blotting was done with the antidystrophin antibody Dys115 and quantified as explained in the webappendix p 4.
Inflammatory infiltrates in muscle biopsy samples were examined by immunohistochemistry with antibodies against CD3 pan T cells, CD4 T-helper cells, and CD8 cytotoxic T cells (webappendix p 3). Antidystrophin antibody induction in patients' sera was tested at week 1 and week 12.9 Pharmacokinetic parameters of AVI-4658 were established from plasma and urine taken over 24 h at the first, sixth, and 12th doses. Experimental details are provided in the webappendix p 3; pharmacokinetic parameters were calculated with WinNonlin Professional (version 5.2.1). Muscle function testing (North Star ambulatory assessment,16 myometry,17 step activity monitoring,18 and 6-min walk test19) was undertaken to identify any dose-dependent changes.
The primary objective of this study was to assess the safety of systemic administration of escalation of doses of AVI-4658. The secondary objectives were to investigate the pharmacokinetics of AVI-4658 and the biochemical efficacy as assessed by induction of dystrophin expression in muscle. The functionality of restored dystrophin was assessed by study of both the relocalisation of dystrophin-associated glycoprotein complex proteins to the sarcolemma and the effect of restored dystrophin on muscle inflammation.
Statistical analysis
No formal sample size calculations were done. All descriptive statistical analyses were done with Graph Pad Prism 4 statistical software. We calculated p values using a paired t test to compare pretreatment versus post-treatment samples. The exact Cochran-Armitage trend test was used to show the dose response to AVI-4658.
The study was registered at ClinicalTrials.gov, number NCT00844597.
Role of the funding source
The UK Medical Research Council funded the study teams, patients' travel, and the acquisition and analysis of the clinical and biochemical outcome measures. AVI BioPharma funded the regulatory submission, the study sponsorship including clinical and medical monitoring, data collection, and provision of the study drug. FM, KB, SC, and SBS had complete access to the data and FM as corresponding author was responsible for submission for publication.
Results
19 patients were enrolled within the UK: 12 at Great Ormond Street Hospital for Sick Children (London) and seven at the Royal Victoria Infirmary (Newcastle). Participants fulfilled eligibility criteria, had a mean age of 8·7 years (range 6–13), and had the clinical phenotype of Duchenne muscular dystrophy (table 1). A pretreatment muscle biopsy confirmed less than 5% revertant fibres in all patients. Adverse events were generally mild (63%) to moderate (32%), consistent with complications related to the disorder and to the paediatric age range, and showed no dose-dependent increase in frequency or severity (webappendix p 7). Difficult venous access largely due to cushingoid features resulted in failure to administer four of the planned 228 doses. Five other doses were not administered because of an adverse event in participant P9 leading to discontinuation of treatment after the seventh dose. In total, 219 doses of AVI-4658 were administered. Participant P11 was enrolled to ensure that at least two participants in each cohort (dose) completed the dosing period and had a post-treatment muscle biopsy.
Table 1.
Clinical summary
| Mutation* | Age (years)† | Weight (kg)† | 6-min walk test at baseline (m)‡ | Cardiomyopathy at recruitment | Dose and regimen of corticosteroid treatment | Other regular treatments taken at study entry | Serious or severe adverse events and relation to AVI-4658 | |
|---|---|---|---|---|---|---|---|---|
| Cohort 1 (0·5 mg/kg) | ||||||||
| P1 | Del 48–50 | 9 | 31 | 410 | No | Prednisolone 25 mg, intermittent (0·81 mg/kg per day) | .. | .. |
| P2 | Del 45–50 | 8 | 29 | 254 | Yes | Prednisolone 12·5 mg, daily (0·43 mg/kg per day) | Perindopril, calcium, vitamin D | .. |
| P3 | Del 49–50 | 8 | 38 | 437 | No | Prednisolone 25 mg, intermittent (0·66 mg/kg per day) | Omeprazole | .. |
| P4 | Del 48–50 | 8 | 36 | 139 | No | Prednisolone 20 mg, intermittent (0·55 mg/kg per day) | Calcium, vitamin D | .. |
| Cohort 2 (1 mg/kg) | ||||||||
| P5 | Del 45–50 | 6 | 26 | 250 | No | Prednisolone 22·5 mg, intermittent (0·86 mg/kg per day) | .. | .. |
| P6 | Del 48–50 | 6 | 21 | 371 | No | Prednisolone 12·5 mg daily (0·6 mg/kg per day) | Ranitidine | .. |
| Cohort 3 (2 mg/kg) | ||||||||
| P7 | Del 49–50 | 13 | 47 | 375 | No | Prednisolone 15 mg daily (0·32 mg/kg per day) | Risedronate, calcium, vitamin D | .. |
| P8 | Del 49–50 | 9 | 38 | 350 | No | Prednisolone 15 mg daily (0·4 mg/kg per day) | Ranitidine | Post-anaesthesia hospitalisation for 1 day due to vomiting, unrelated |
| Cohort 4 (4 mg/kg) | ||||||||
| P9 | Del 52 | 10 | 30 | 301 | Regional wall hypokinesia, normal FS | Prednisolone 15 mg daily (0·49 mg/kg per day) | Risedronate, calcium, vitamin D | Cardiomyopathy, possible |
| P10 | Del 48–50 | 10 | 62 | 146 | No | Not on steroids because of side-effects | .. | Ankle fracture, unrelated |
| P11 | Del 45–50 | 9 | 28 | 477 | No | Prednisolone 15 mg daily (0·54 mg/kg per day) | Risedronate, calcium, vitamin D | .. |
| Cohort 5 (10 mg/kg) | ||||||||
| P12 | Del 49–50 | 6 | 25 | 317 | No | Prednisolone 20 mg, intermittent (0·8 mg/kg per day) | Calcium, vitamin D | .. |
| P13 | Del 48–50 | 7 | 22 | 443 | No | Prednisolone 15 mg daily (0·68 mg/kg per day) | .. | .. |
| P14 | Del 47–50 | 12 | 52 | 138 | Yes | Deflazacort 30 mg daily (0·58 mg/kg per day) | Perindopril, bisoprolol, risedronate, calcium, vitamin D | .. |
| P15 | Del 49–50 | 10 | 39 | 169 | No | Prednisolone 20 mg daily (0·5 mg/kg per day) | .. | .. |
| Cohort 6 (20 mg/kg) | ||||||||
| P16 | Del 45–50 | 9 | 31 | 515 | No | Prednisolone 20 mg daily (0·65 mg/kg per day) | .. | .. |
| P17 | Del 45–50 | 7 | 25 | 492 | No | Prednisolone 17·5 mg daily (0·6 mg/kg per day) | .. | .. |
| P18 | Del 49–50 | 10 | 45 | 265 | Yes | Deflazacort 30 mg daily (0·66 mg/kg per day) | Lisinopril | .. |
| P19 | Del 45–50 | 9 | 30 | 405 | No | Prednisolone 15 mg daily (0·5 mg/kg per day) | Calcium, vitamin D | .. |
Intermittent=10 days on and 10 days off treatment. FS=fractional shortening.
Deleted exons in the dystrophin gene.
At the first dose of AVI-4658.
Assessed a week before the first dose.
The 219 intravenous PMO administrations, including cannulation, were generally well tolerated. Laboratory safety assessments did not show any effect of AVI-4658 on pulmonary, kidney, liver, or bone-marrow functions. We did not identify any widespread rash or other clinical signs indicating an adverse reaction; four infusions were associated with a local rash due to application of local anaesthetic cream before venepuncture. Two serious adverse events were reported, both unrelated to the study drug (table 1). Participant P9 had normal cardiac fractional shortening (34%) before study entry, although mild regional impairment of the left ventricular inferior segment was detected on Doppler imaging. Over the first few weeks of study treatment, mild intermittent sinus tachycardia (maximum 125 beats per min) was noted. After the seventh dose, an echocardiogram revealed that his fractional shortening had fallen to 22%, and treatment with ACE inhibitor and β blocker was started. The investigators and study sponsor agreed to discontinue PMO administration and that this patient should not undergo general anaesthesia for the post-treatment muscle biopsy. At the final visit at week 26, his heart function had stabilised with a fractional shortening of 26%. This complication was judged most likely to be part of the well described pattern of cardiomyopathy associated with Duchenne muscular dystrophy. Cardiac investigations in the remaining participants were consistent with findings in the disorder. Forced vital capacity did not change significantly during the study (webappendix pp 10–11, 24).
Creatine kinase concentrations did not show any dose-dependent trends. There was no induction of antidystrophin antibodies after treatment (data not shown). Muscle function testing did not reveal any dose-dependent changes (webappendix pp 12–21, 25–27). These assessments showed that most patients remained stable during the study period; four lost ambulation during follow-up, as was expected on the basis of their ambulatory stage at study entry (table 1).
The plasma half-life of AVI-4658 was short (1·62–3·60 h; figure 2), and no accumulation between doses was recorded. Clearance was 233–615 mL/h per kg, and volume of distribution was 450–981 mL/kg. As a result of the small sample size, a clear relation between dose and clearance and volume of distribution could not be established. Renal clearance of AVI-4658 ranged between 116 and 229 mL/h per kg and was between 32·3% and 46·2% of total (plasma) clearance at doses between 0·5 and 4 mg/kg. At 10 and 20 mg/kg doses, renal clearance accounted for 60·5% and 63·8% of total clearance from the plasma in the first 24 h after dosing.
Figure 2.
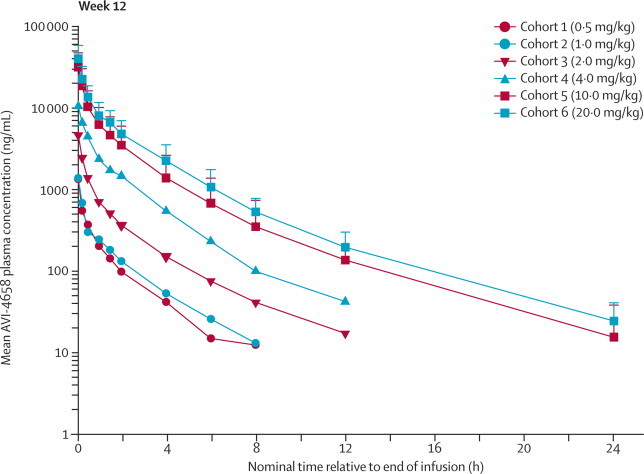
Plasma pharmacokinetics of AVI-4658
Mean plasma concentrations of AVI-4658 versus nominal elapsed time averaged across weeks 1, 6, and 12. Area under the curve (AUC) over 24 h accounted for greater than 95% of AUC0–∞, suggesting that most of the drug eliminated from the plasma was cleared within 24 h. AVI-4658 plasma exposure increased in a nearly proportional manner with dose for maximum concentration, AUC0–24, and AUC0–∞. Error bars show SDs.
Experiments on MyoD-converted patient fibroblasts treated with a 2′OMe congener of AVI-4658 were done in all participants11 to confirm before the trial that the patients were able to correctly skip exon 51. This preliminary step was done with a 2′OMe antisense oligonucleotide because PMO cannot be used to transfect cells in culture, whereas 2′OMe can be efficiently delivered by transfection. The results of this experiment were positive in all patients' cells and no qualitative differences in exon skipping between patients were noted (data not shown). When post-treatment muscle biopsies were assayed by RT-PCR, all patients had variable AVI-4658 induced skipping of exon 51, confirmed by sequencing (webappendix p 22).
Seven patients had a post-treatment increase in dystrophin protein expression compared with their pretreatment biopsies, evidenced by at least two of the three methods of quantification: number of dystrophin-positive fibres, western blotting, and semiquantitative immunocytochemistry measurements (figure 3, webappendix p 23). In these seven patients, mean dystrophin fluorescence intensity increased from 8·9% (95% CI 7·1–10·6) to 16·4% (10·8–22·0) of normal control after treatment (p=0·0287). In the low-dose cohorts 1 to 4, there was no increase in dystrophin expression, with the exception of participant P7 in cohort 3. However, six of eight patients in the two high-dose cohorts 5 and 6 showed an increase in protein expression. Three patients, one in each of cohorts 3 (P7), 5 (P15), and 6 (P18), had a very substantial response to AVI-4658, having 21%, 15%, and 55% dystrophin-positive fibres, respectively. These three patients had also 314%, 198%, and 110% increases in dystrophin intensity compared with pretreatment biopsy on semiquantitative immunocytochemistry.13 Western blot analysis of these patients also showed an increase after treatment of protein levels from 2% to 18%, from 0·9% to 17%, and from 0% to 7·7% of normal muscle, respectively (table 2).
Figure 3.
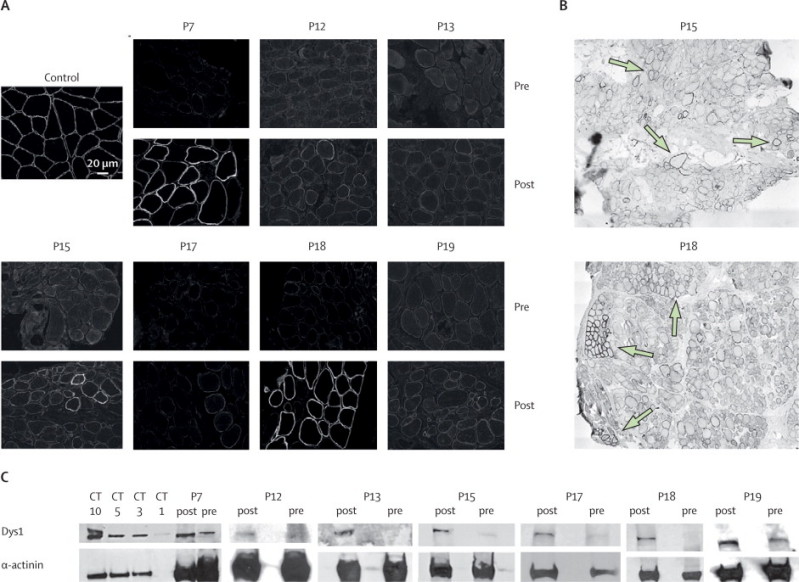
Dystrophin protein expression in the seven patients who responded to treatment
(A) Transverse sections of treated (post) and untreated (pre) muscle specimens immunolabelled with MANDYS106 antibody. (B) Post-treatment biopsy samples from participants P15 and P18; low-magnification images showing widespread and patchy dystrophin expression (arrows). (C) Western blotting of pretreatment and post-treatment muscle biopsy samples with antidystrophin Dys1 (exon 26–30) and antisarcomeric α-actinin antibodies; an average of 150 μg of total patient proteins was loaded per lane. CT=μg control muscle extract.
Table 2.
Summary of response to AVI-4658
|
Dystrophin-positive fibres (% of total muscle fibres) |
Mean fluorescence intensity per fibre* |
Dystrophin expression assessed by western blotting |
Response to AVI-4658† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Post-treatment | Pretreatment (% of control; SD) | Post-treatment (% of control; SD) | p value‡ | Increase (%) | Pretreatment (% of control) | Post-treatment (% of control) | ||
| Cohort 1 | |||||||||
| P1 | 1% | 1% | 5% (2·2) | 8% (4·5) | 0·0101 | 57% | None | None | + |
| P2 | 3% | 0% | 5% (2·3) | 5% (1·6) | 0·5120 | .. | None | None | + |
| P3 | 1% | 7% | 5% (5·4) | 5% (2·1) | 0·9761 | .. | None | None | + |
| Mean (SD) | 1·7% (1·2) | 2·7% (3·8) | 5·0% (0·0) | 6·0% (1·7) | .. | .. | .. | .. | .. |
| Cohort 2 | |||||||||
| P5 | 0% | 0% | 4% (1·9) | 4% (1·3) | 0·3902 | .. | None | None | + |
| P6 | 5% | 1% | 8% (4·2) | 6% (2·7) | 0·0231 | .. | Trace | Trace | + |
| Mean (SD) | 2·5% (3·5) | 0·5% (0·7) | 6·0% (2·8) | 5·0% (1·4) | .. | .. | .. | .. | .. |
| Cohort 3 | |||||||||
| P7 | 1% | 21% | 5% (1·5) | 19% (28) | 0·0021 | 314% | 2% | 18% | +++ |
| P8 | 1% | 5% | 7% (3·4) | 5% (2) | 0·0275 | .. | None | None | + |
| Mean (SD) | 1·0% (0·0) | 13·0% (11·3) | 6·0% (1·4) | 12·0% (9·9) | .. | .. | .. | .. | .. |
| Cohort 4 | |||||||||
| P10 | 5% | 4% | 9% (7·2) | 10% (6·7) | 0·0800 | 13% | None | None | + |
| P11 | 1% | 1% | 8% (4·3) | 11% (13) | 0·0949 | 30% | 1·1% | 0·7% | + |
| Mean (SD) | 3·0% (2·8) | 2·5% (2·1) | 8·5% (0·7) | 10·5% (0·7) | .. | .. | .. | .. | .. |
| Cohort 5 | |||||||||
| P12 | 3% | 6% | 9% (9·8) | 17% (27) | 0·0015 | 87% | None | 7% | ++ |
| P13 | 2% | 6% | 11% (4·5) | 10% (4·2) | 0·1611 | .. | None | 9·6% | ++ |
| P14 | 0% | 7% | 10% (13) | 13% (12) | 0·1125 | 30% | Trace | Trace | + |
| P15 | 1% | 15% | 9% (3) | 27% (24) | <0·0001 | 198% | 0·9% | 17% | +++ |
| Mean (SD) | 1·5% (1·3) | 8·5% (4·4) | 9·8% (1·0) | 16·8% (7·4) | .. | .. | .. | .. | .. |
| Cohort 6 | |||||||||
| P16 | 3% | 5% | 11% (1·6) | 13% (1·4) | 0·3496 | 16% | 0·5% | None | + |
| P17 | 3% | 8% | 9% (6) | 10% (7) | 0·1661 | 16% | 0·7% | 2·6% | ++ |
| P18 | 3% | 55% | 9% (4·2) | 19% (1·7) | <0·0001 | 110% | None | 7·7% | +++ |
| P19 | 5% | 7% | 10% (5·5) | 13% (10) | 0·0359 | 24% | 5% | 12·3% | ++ |
| Mean (SD) | 3·5% (1·0) | 18·8% (24·2) | 9·8% (1·0) | 13·8% (3·8) | .. | .. | .. | .. | .. |
Pretreatment muscle biopsy samples from participants P1, P3, and P6 were diagnostic quadriceps samples at the time of diagnosis, all other samples were obtained from the biceps muscle. Participant P4, who had missed two PMO doses because of cannulation challenges, declined the post-treatment muscle biopsy, but completed the rest of the study. Participant P9 did not undergo general anaesthesia for the post-treatment muscle biopsy because of an adverse event (cardiomyopathy) and was discontinued from treatment after week 7. Participant P11 was enrolled in cohort 4 to substitute for P9. Therefore, only 17 of 19 post-treatment muscle biopsy samples were obtained and participants P4 and P9 are not listed in this table. PMO=phosphorodiamidate morpholino oligomer.
Mean fluorescence signal intensity of MANDYS10612 as percentage of control muscle (details in webappendix p 23).
Response to AVI-4658: + shows response at RNA level (exon 51 skipping), but without detectable increase of dystrophin expression in post-treatment muscle biopsy; ++ shows response at RNA level and increase of dystrophin expression in post-treatment muscle; and +++ shows response at RNA level and increase in post-treatment muscle biopsy sample with all three methods of dystrophin quantification.
Two-tailed t test comparing the mean fluorescence intensity in the pretreatment versus post-treatment biopsy sample for each patient; we assessed the dose response to AVI-4568 across cohorts using the Cochran-Armitage method and confirmed a significant linear trend of dose response leading to increase in dystrophin expression (responders with ++ or +++) with increasing dose (p=0·0203).
A dose-dependent significant linear increase in dystrophin expression was noted (table 2; exact Cochran-Armitage trend test, p=0·0203). Additionally, AVI-4658 administration induced significant increase of dystrophin expression on immunocytochemistry pretreatment versus post-treatment in cohorts 5 and 6 (paired two-sided t test, p=0·04). Participant P19 had dystrophin levels on blot of 12·3% of control muscle, but a smaller increase in fluorescence intensity and percentage of dystrophin-positive fibres, probably reflecting variability in different blocks of the muscle biopsy sample studied with the different techniques (table 2). The functional properties of restored dystrophin were confirmed by quantification of α-sarcoglycan and neuronal NOS expression. Dystrophin-positive fibres had roughly a 30% average increase in α-sarcoglycan expression compared with dystrophin-negative fibres in the patient with the best response to treatment, P18 (deletion 49–50). Dystrophin restoration was followed by restoration of neuronal NOS at the sarcolemma, more so in patients with exon 49–50 deletions than in those with 45–50 deletions (figure 4A), which is consistent with the observation that the neuronal NOS binding domain is located in dystrophin exons 42–45.20,21
Figure 4.
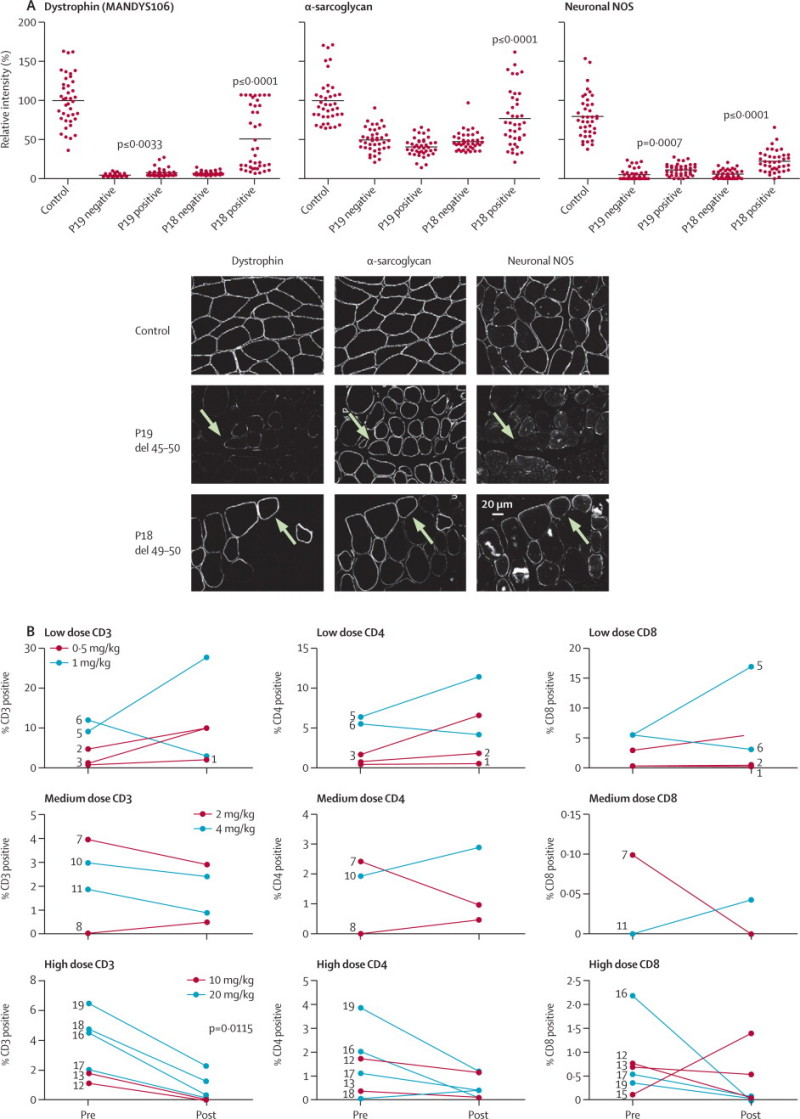
Functional analysis of restored dystrophin
(A) Expression of dystrophin, α-sarcoglycan, and neuronal NOS in post-treatment muscle biopsy samples from participants P18 and P19 was quantified13 relative to control muscle in 40 dystrophin-positive or dystrophin-negative muscle fibres and normalised to β-spectrin expression. To overcome high background seen with the neuronal NOS antibody, the average background intensity of neuronal NOS-negative membranes was subtracted from control and patient values. For participant P19, there was no difference in α-sarcoglycan intensity between dystrophin-positive and dystrophin-negative fibres in the post-treatment muscle biopsy sample and neuronal NOS showed only a small increase in dystrophin-positive fibres (paired two-tailed t test, p=0·0007). For participant P18, neuronal NOS and α-sarcoglycan intensity was significantly increased in the dystrophin-positive fibres (neuronal NOS mean intensity as percentage of control: dystrophin-negative fibres 7% [SD 7], dystrophin-positive fibres 28% [SD 17], paired two-tailed t test, p≤0·0001; α-sarcoglycan mean intensity as percentage of control: dystrophin-negative fibres 45% [SD 16], dystrophin-positive fibres 75% [SD 31], p<0·0001). (B) Sarcolemmal restoration of the dystrophin-associated glycoprotein complex by AVI-4658. Post-treatment muscle biopsy samples from participants P19 and P18 were stained with antibodies against dystrophin (exon 43, MANDYS106), α-sarcoglycan, neuronal NOS, and β-spectrin. The arrows show the same dystrophin-positive fibre in each panel. In P19 (deletion of exons 45–50) the fibre shown by the arrow has increased α-sarcoglycan sarcolemmal expression, but not neuronal NOS because this patient is deleted for part of the dystrophin neuronal NOS binding site. (C) Inflammatory infiltrates quantification on pretreatment and post-treatment muscle samples. Muscle sections were incubated with antibodies (DAKO, UK) raised against human CD3 (pan T cell), CD4 (helper T cell) and CD8 (killer T cell). For each section, the number of CD-positive cells was represented as a percentage of the total number of muscle fibres. Patients with pretreatment and post-treatment values of zero are not represented. NOS=nitric oxide synthase.
Finally, the inflammatory infiltrate was investigated to establish whether dystrophin restoration had any effect on the prominent inflammatory response seen in Duchenne muscular dystrophy (figure 4B).22 A reduction in inflammatory infiltrates was recorded in cohorts 5 and 6, apart from in participant P15 in whom the CD8 cell count was increased, but not around dystrophin-positive fibres. Furthermore, this patient did not have antidystrophin antibodies. Despite the small number of samples, the significant reduction in CD3 cell count (paired two-tailed t test, p=0·0115) shows that restored dystrophin is tolerated by the immune system. The quotient between CD3% and dystrophin intensity in the seven patients who responded to treatment showed a significant reduction in the post-treatment muscle biopsy samples (one-tailed paired t test, p=0·0078), confirming the correlation with the increase in dystrophin expression.
Discussion
We show for the first time that repeated systemic administration of a PMO splice switching oligomer (AVI-4658) induces targeted exon skipping in skeletal muscle in patients with Duchenne muscular dystrophy, restoring correctly localised dystrophin at the sarcolemma (panel). The administration of AVI-4658 was very well tolerated, without clear drug-induced adverse events with single doses of up to 900 mg and cumulative exposure exceeding 10 000 mg. The absence of drug-related adverse events after 12 weeks is encouraging, but caution is still needed because any splice switching oligomer would need to be given lifelong.
Panel. Research in context.
Systematic review
We searched PubMed in March, 2011, using the keywords “Duchenne”, “antisense”, “exon skipping”, and “clinical trial”. Splice-switching oligomers have been tested previously after intramuscular injection in animal models and in patients affected by Duchenne muscular dystrophy.6,8,9 An open-label, dose-escalation study10 in 12 boys with Duchenne muscular dystrophy receiving weekly subcutaneous injections of the 2′OMe PRO051 at 0·5, 2, 4, and 6 mg/kg bodyweight for 5 weeks induced skipping of exon 51. Low dystrophin levels were reported after treatment, although the absence of pretreatment samples makes precise quantification of the biochemical efficacy of PRO051 difficult. This study was followed by a 12-week extension study using a dose of 6 mg/kg bodyweight of PRO051, with stabilisation of muscle function, but no significant improvement in a 6-min walk test.
Interpretation
We report for the first time that the systemic administration of a splice-switching oligomer based on PMO chemistry (AVI-4658) induced restoration of dystrophin expression in skeletal muscle of boys with Duchenne muscular dystrophy. The clinical and laboratory safety data in our open-label, dose-escalation, repeated intravenous administration study showed that AVI-4658 was well tolerated. Seven patients had a significant dose response, six of whom were in the two high-dose cohorts, showing restoration of dystrophin protein expression. This finding was associated with increased expression of proteins associated with dystrophin, such as α-sarcoglycan and neuronal nitric oxide synthase, the sarcolemmal localisation of which is disrupted in Duchenne muscular dystrophy. Additionally, we showed a dose-dependent reduction in the inflammatory infiltrate in muscles of boys with Duchenne muscular dystrophy in whom dystrophin expression was restored. This finding is encouraging because it suggests that the restored dystrophin attenuates the inflammation that is a hallmark of the disease's pathology; it also suggests that the newly produced dystrophin does not produce novel immunogenic epitopes.
A clear and significant dose response was recorded in terms of dystrophin protein expression, leading to seven patients who responded to treatment at higher doses. This finding was accompanied by a significant reduction of inflammatory infiltrates in patients in the two highest dose cohorts. Patients with the highest levels of dystrophin also had increased sarcolemmal expression of proteins of the dystrophin-associated glycoprotein complex. This outcome included restoration of neuronal NOS to the sarcolemma in patients with deletions that did not disrupt the NOS binding site20 localised in spectrin repeats 16/17 of the rod domain. The restoration of neuronal NOS is beneficial for patients in whom ongoing muscle damage is compounded by paradoxical exercise-induced vasoconstriction23 as a result of dysfunctional fine-tuning of blood flow.20
The reduced inflammation in muscle could be related to reduced necrosis due to improved sarcolemmal function with better resistance to mechanical load, induced by the restored dystrophin.24 We noted variable levels of protein restoration in the seven patients who responded to treatment and considered possible reasons for this variability. Variability due to specific deletions and intronic breakpoints of individual patients could not be detected in vitro, because the response to splice switching oligomer in all patients' myotubes was qualitatively similar. The stability of the resulting protein might be relevant21 since the patients with the three greatest responses to treatment all had 49–50 deletions and mildly affected patients with Becker muscular dystrophy or even asymptomatic individuals with deletion of exons 49–51 have been described,25 suggesting that this shortened protein is highly functional. However two patients who did not respond to treatment (cohort 1, P3, who had no increase in either dystrophin-positive fibres or dystrophin expression, and cohort 3, P8, who had an increase in dystrophin-positive fibres, but not in dystrophin expression) and one with a small response (cohort 5, P12) also have the deletion 49–50; additionally, patients with exon 45–50 deletions did not have more protein than did patients with other genotypes, although asymptomatic individuals with 45–51 deletions are on record.26–28 An aspect to consider is the genetic background, which in humans is variable, including the intronic deletions breakpoints. A further variable could be differences in pharmacodynamics of PMO, although our analysis suggests that there was not a clear correlation between response and maximum concentration and area under the curve for AVI-4658. When concomitant treatments, age, and extent of muscle pathological changes were taken into account, no obvious pattern emerged. Immune response to novel dystrophin epitopes induced by exon skipping could be another reason for the variability; however, this explanation seems unlikely because we did not detect humoral immunity in any of the patients and furthermore we documented a reduction in T cells in muscle biopsy samples in cohorts 5 and 6, who showed the highest dystrophin levels.
In a recent systemic exon skipping trial in Duchenne muscular dystrophy using the 2′OMe chemistry, high numbers of dystrophin-positive fibres but low dystrophin levels were described.10 However, the study had no pretreatment muscle biopsy in patients in whom a dystrophin response was reported, making establishment of a proper baseline to distinguish minimally positive from negative fibres difficult. Indeed, in Duchenne muscular dystrophy, sections of muscle often have discernible trace levels after immunostaining with the antibodies to dystrophin used,9,13 and these pretreatment levels need to be taken into account to provide an accurate measurement of both number of positive fibres and dystrophin intensity.9,10,13 In our study, we first established the level of dystrophin in the pretreatment muscle biopsy sample, and regarded those levels as the baseline for that individual—ie, judged fibres as positive only if they exceeded the intensity levels of the pretreatment biopsy.
With respect to the variable dystrophin restoration we have reported, the most plausible conclusion is that stochastic events affect muscle splice switching oligomer targeting and contribute to variability. Animal models treated with both 2′OMe and PMO also showed substantial variability of dystrophin expression, even in contralateral muscles from the same animal.5,29 Because splice switching oligomers do not target skeletal muscle specifically, their uptake is partly dependent on local events such as muscle perfusion, damage, and inflammation. To obtain more uniform protein production, either high doses of PMO or prolonged frequent administration, or both, could be considered because PMOs seem to be well tolerated.30 The safety profile we noted with AVI-4658 at doses of 20 mg/kg, supported by animal testing at up to human equivalent doses of 100 mg/kg,30 is encouraging and bodes well for longer administration periods and higher clinical dose. Preclinical data suggest that repeated administration of even small doses over an extended time achieves more homogeneous restoration of dystrophin than does the same cumulative dose administered as a bolus injection of PMO.31 This finding suggests that a long period of administration will be necessary to achieve homogeneous dystrophin expression.
In terms of the clinical efficacy, the PRO051 study10 claims that eight of 11 boys who were ambulant at entry to the extension study showed improvement in the 6-min walking test of 35·2 m (SD 28·7) after 12 weeks' treatment; however, this change was not significant. Moreover, several of these children were younger than 7 years and, according to longitudinal observation,19 boys younger than 7 years with Duchenne muscular dystrophy gain motor function. Additional confounding factors are the variability in the walking test (SD 36 m19) and the powerful placebo effect of open-label studies. Despite these limitations, this observation is encouraging.
In our study, boys remained mostly stable during follow-up, but because the period during which AVI-4658 was administered was only 12 weeks, we did not observe any significant clinical improvement, and the lack of a study extension was a limitation of our study, since only extended exposure to the drug is able to affect progression of the disease. Nevertheless, our results are very encouraging because they prove that doses of 10 mg/kg and 20 mg/kg of AVI-4658, which were very well tolerated, consistently induced dystrophin expression in the seven patients who responded to treatment up to levels typically found in patients with Becker muscular dystrophy or disease of intermediate severity between Duchenne muscular dystrophy and Becker muscular dystrophy. The restoration of the dystrophin-associated glycoprotein complex suggests that the produced dystrophin is functional. Because of the variability of dystrophin restoration, MRI or spectroscopy of muscle are promising methods to assess the effect of systemic treatment in Duchenne muscular dystrophy. Recent studies have described the correlation between MRI and the degree of dystrophic muscle changes,32 the effect of exercise in Duchenne muscular dystrophy,33 and reduction of muscle inflammation after PMO treatment in dogs.6
On the basis of our data and recent preclinical data,31 we expect that extended administration of AVI-4658 at doses of 10 mg/kg or higher will result in sufficient dystrophin expression to have a positive effect on the prevention of muscle degeneration in Duchenne muscular dystrophy. Indeed, chronic administration (1 year) of doses of PMO similar to the one used in our study produced significant improvement in muscle pathology and function in mdx mice.34 AVI-4658 has the potential to ameliorate the progressive natural history of Duchenne muscular dystrophy and now needs to be investigated in clinical efficacy trials.
Acknowledgments
Acknowledgments
We thank the participating patients and their families, the charities Muscular Dystrophy Campaign, Action Duchenne, and the Duchenne Family Support Group for participating in the UK MDEX consortium, which undertook this study. We also thank the members of the MDEX Scientific Advisory Board chaired by Kay Davies (see consortium website for full membership) for their constructive criticism. We thank Matt Rogan, Kathy Smith, and Jim Balsley for their participation in the data safety monitoring board. We thank Kanagasabai Ganeshaguru for study coordination in London, Maria Kinali for constructive discussion in early stages about the trial design and Geoff Bell for patient coordination in Newcastle upon Tyne, Darren Chambers, Rita Barresi, and Richard Charlton for their excellent technical assistance in processing muscle samples, and Rivka Steinberg for the in-vitro testing of patients' fibroblasts. We thank Valeria Ricotti for her technical assistance in the CD cell counts and Glenn Morris, Oswestry, and the MDA Monoclonal Antibody Resource for MANDYS106. We are grateful for the support of the NIHR Biomedical Research Centre Funding Scheme and the Somers Clinical Research Facility at Great Ormond Street Hospital, UCL Institute of Child Health, and in particular thank Anna Massey, Katie Rees, and Elizabeth Leach and the physiotherapists Marion Main, Maria Ash, Michelle Eagle, and Anna Mayhew for their expert assessment of the study participants. This work was supported by the Newcastle NIHR Clinical Research Facility, in particular the study nurses Linda Smith and Dorothy Carman. We are very grateful for Mariacristina Scoto's help in the clinical care of the patients. We also acknowledge the collaborations of the North Star Clinical network, which contributed to the recruitment of participants via the clinical colleagues Adnan Manzur, Stephanie Robb, Helen Roper, Rosaline Quinlivan, and Louise Hartley. We are grateful to the surgeons for obtaining the muscle biopsies (at the London site: Joe Curry and Paolo De Coppi; at the Newcastle site: Anne Lawson). We are grateful to Ann S Le Couteur, Helen McConachie, and Nil Chakrabarti at the Newcastle site and to Sriranjan Sucharita at the London site for psychiatric interviews of the patients and their families. MEG is grateful for support from the ICHT Comprehensive Biomedical Research Centre. The study was supported by the MRC Centre for Neuromuscular Diseases at UCL and Newcastle including the MRC Neuromuscular Centre Biobank. JEM is supported by a Wellcome Trust University Award. FM is supported by Great Ormond Street Hospital Children's Charity. Newcastle University, UCL, and Oxford are partners in TREAT-NMD (EC036825). The study was sponsored by AVI BioPharma (Bothell, WA, USA).
Contributors
SC, FM, KB, MG, and SBS designed and wrote the clinical trial protocol together with the amendments. SC and MG identified patients and coordinated clinical teams, executed the trial procedures, collected data, and followed up the study participants, under the supervision of FM and KB. SC, VAG, and JEM coordinated the collaborative work between clinical and laboratory teams and the biochemical work-up of clinical trial samples under the supervision of FM. FM, SC, MG, and KB obtained patient consent. JB oversaw and interpreted cardiac tests for the Newcastle cohort. SA was responsible of the acquisition of the genetic data. FM and KB managed the study budget. SC, VAG, JEM, and FM drafted the first report and have seen and approved the final version. DJW, GD, MJAW, SDW, and VS contributed to the interpretation of results and drafting of the report. VAG and KA did RT-PCR, image capture, and dystrophin expression analysis. RK contributed to the design of the RT-PCR assay and to interpretation of results. ST did the western blot analysis. LF and CS processed muscle biopsy specimens and immunofluorescence staining and their analysis. MEG devised and oversaw the psychiatric assessments of the subjects and families. SC did the statistical analysis. SBS was responsible as sponsor's medical officer for overseeing safe conduct of the study and participated in all safety monitoring discussions and data reviews. All authors contributed to the interpretation of results and drafting of the report and have seen and approved the final version.
Conflicts of interest
FM serves on scientific advisory boards for Acceleron Pharma, Genzyme, AVI BioPharma, Debiopharma Group, GlaxoSmithKline, Prosensa, and Santhera Pharmaceutical, receives research support from AVI BioPharma, and has received funding for trials from AVI, Trophos, and PTC. KB has served on scientific advisory boards for Acceleron, AMT, AVI Biopharma, Debiopharm, Genzyme, GlaxoSmithKline, Prosensa, PTC, and Santhera and has received funding for trials from AVI and PTC. SBS is employed full time as Chief Medical Officer and Senior Vice President by AVI BioPharma and owns AVI stock. RK is employed full time as Senior Vice President and Distinguished Scientist by AVI BioPharma and owns AVI stock. VS has served on scientific advisory boards for Acceleron and Genzyme and has received funding for a trial from GlaxoSmithKline. GD and SDW hold patents in the area of exon skipping. MJAW serves as a scientific adviser for AVI BioPharma and Novartis. All other authors declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Bushby K, Finkel R, Birnkrant DJ, for the DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Bushby KM, Gardner-Medwin D, Nicholson LV. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. II. Correlation of phenotype with genetic and protein abnormalities. J Neurol. 1993;240:105–112. doi: 10.1007/BF00858726. [DOI] [PubMed] [Google Scholar]
- 4.Sazani P, Graziewicz MA, Kole R. Splice switching oligonucleotides as potential therapeutics. In: Crooke ST, editor. Antisense drug technology, principles, strategies and applications. CBC Press; Boca Raton, FL, USA: 2008. pp. 89–114. [Google Scholar]
- 5.Lu QL, Rabinowitz A, Chen YC. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokota T, Lu QL, Partridge T. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- 8.van Deutekom JC, Janson AA, Ginjaar IB. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 9.Kinali M, Arechavala-Gomeza V, Feng L. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goemans NM, Tulinius M, van den Akker JT. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 11.Arechavala-Gomeza V, Graham IR, Popplewell LJ. Comparative analysis of antisense oligonucleotide sequences for targeted skipping of exon 51 during dystrophin pre-mRNA splicing in human muscle. Hum Gene Ther. 2007;18:798–810. doi: 10.1089/hum.2006.061. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TM, Morris GE. Use of epitope libraries to identify exon-specific monoclonal antibodies for characterization of altered dystrophins in muscular dystrophy. Am J Hum Genet. 1993;52:1057–1066. [PMC free article] [PubMed] [Google Scholar]
- 13.Arechavala-Gomeza V, Kinali M, Feng L. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression. Neuropathol Appl Neurobiol. 2010;36:265–274. doi: 10.1111/j.1365-2990.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 14.Arechavala-Gomeza V, Kinali M, Feng L. Revertant fibres and dystrophin traces in Duchenne muscular dystrophy: implication for clinical trials. Neuromuscul Disord. 2010;20:295–301. doi: 10.1016/j.nmd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Neri M, Torelli S, Brown S. Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul Disord. 2007;17:913–918. doi: 10.1016/j.nmd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Mazzone ES, Messina S, Vasco G. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009;19:458–461. doi: 10.1016/j.nmd.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 17.Mayhew JE, Florence JM, Mayhew TP. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 18.McDonald CM, Widman LM, Walsh DD, Walsh SA, Abresch RT. Use of step activity monitoring for continuous physical activity assessment in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2005;86:802–808. doi: 10.1016/j.apmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.McDonald CM, Henricson EK, Han JJ. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve. 2010;42:966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 20.Lai Y, Thomas GD, Yue Y. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger CC, Bhasin N, Tewari M. Exon-skipped dystrophins for treatment of Duchenne muscular dystrophy: mass spectrometry mapping of most exons and cooperative domain designs based on single molecule mechanics. Cytoskeleton (Hoboken) 2010;67:796–807. doi: 10.1002/cm.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pescatori M, Broccolini A, Minetti C. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007;21:1210–1226. doi: 10.1096/fj.06-7285com. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi YM, Rader EP, Crawford RW. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- 25.Muntoni F, Di Lenarda A, Porcu M. Dystrophin gene abnormalities in two patients with idiopathic dilated cardiomyopathy. Heart. 1997;78:608–612. doi: 10.1136/hrt.78.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helderman-van den Enden AT, Straathof CS, Aartsma-Rus A. Becker muscular dystrophy patients with deletions around exon 51; a promising outlook for exon skipping therapy in Duchenne patients. Neuromuscul Disord. 2010;20:251–254. doi: 10.1016/j.nmd.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Morandi L, Mora M, Confalonieri V. Dystrophin characterization in BMD patients: correlation of abnormal protein with clinical phenotype. J Neurol Sci. 1995;132:146–155. doi: 10.1016/0022-510x(95)00147-t. [DOI] [PubMed] [Google Scholar]
- 28.Saengpattrachai M, Ray PN, Hawkins CE, Berzen A, Banwell BL. Grandpa and I have dystrophinopathy?: approach to asymptomatic hyperCKemia. Pediatr Neurol. 2006;35:145–149. doi: 10.1016/j.pediatrneurol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Aoki Y, Nakamura A, Yokota T. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52-deficient mdx mouse. Mol Ther. 2011;18:1995–2005. doi: 10.1038/mt.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sazani P, Weller DL, Shrewsbury SB. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int J Toxicol. 2010;29:143–156. doi: 10.1177/1091581809359206. [DOI] [PubMed] [Google Scholar]
- 31.Malerba A, Sharp PS, Graham IR. Chronic systemic therapy with low-dose morpholino oligomers ameliorates the pathology and normalizes locomotor behavior in mdx mice. Mol Ther. 2011;19:345–354. doi: 10.1038/mt.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinali M, Arechavala-Gomeza V, Cirak S. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology. 2010;76:346–353. doi: 10.1212/WNL.0b013e318208811f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrood P, Hollingsworth KG, Eagle M. MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J Magn Reson Imaging. 2009;30:1130–1138. doi: 10.1002/jmri.21941. [DOI] [PubMed] [Google Scholar]
- 34.Wu B, Xiao B, Cloer C. One-year treatment of morpholino antisense oligomer improves skeletal and cardiac muscle functions in dystrophic mdx mice. Mol Ther. 2010;19:576–583. doi: 10.1038/mt.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


