Figure 1.
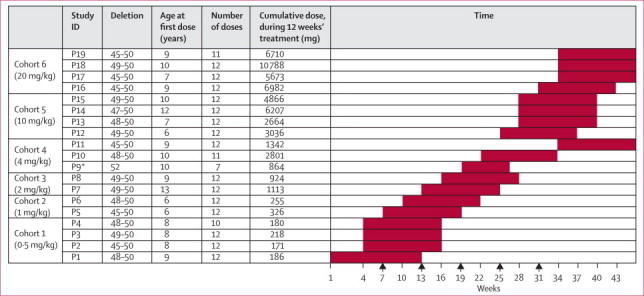
Patients recruited to the trial, their assignment to cohorts, and the dose-escalation scheme
Each full red box represents a time interval of 12 weeks' dosing. Arrows show the timepoints at which the data safety monitoring board met with clinical investigators and the sponsor to review safety before subsequent dose escalations. *Patient withdrawn from study after seven doses.
