Abstract
Introduction
Interleukin-1 (IL-1) and interleukin-18 (IL-18) are potent pro-inflammatory cytokines in inflammation-related diseases. Their actions are regulated by IL-1 receptor antagonist (IL-1ra) and IL-18 binding protein (IL-18bp). This study was designed to 99mTc-radiolabel an IL-1ra and IL-18bp dual-domain cytokine ligand, IL-18bp-Fc-IL-1ra, for specific inflammation targeting.
Methods
99mTc-IL-18bp-Fc-IL-1ra was obtained by direct labeling via 2-iminothiolane reduction. Competitive binding of 99mTc-labeled and unlabeled IL-18bp-Fc-IL-1ra to rat polymorphonuclear leukocytes was assessed in vitro. A mouse ear edema model was used to evaluate specific targeting properties of 99mTc-IL-18b-Fc-IL1ra in vivo. The correlation between 99mTc-IL-18bp-Fc-IL-1ra uptake and 111In-labeled polymorphonuclear neutrophil infiltration was studied using ischemic-reperfused rat hearts.
Results
Direct 99mTc-labeling yielded a stable dual-domain cytokine radioligand with radiochemical purity greater than 95% after gel filtration. Competitive binding studies showed specific targeting of 99mTc-IL-18bp-Fc-IL-1ra to inflammatory cells. 99mTc-IL-18bp-Fc-IL-1ra uptake was 1.80 ± 0.17 % injected dose per gram (%ID/g) in the inflamed ear without blocking, whereas uptake in the presence of IL-18bp-Fc-IL-1ra was 1.09 ± 0.08 %ID/g (P < 0.05). The amounts of IL-1β and IL-18 were significantly increased in the inflamed ears compared to the vehicle controls. A significant correlation of 99mTc-IL-18bp-Fc-IL-1ra with 111In-labeled neutrophil distribution was observed in the ischemic-reperfused hearts (P < 0.001).
Conclusion
Targeting pro-inflammatory cytokines with 99mTc-IL-18bp-Fc-IL-1ra may provide a suitable approach for specific detection of inflammatory sites.
Keywords: 99mTc, Interleukin-1, Interleukin-18, Inflammation, SPECT
1. Introduction
Pro-inflammatory cytokines are associated with many systemic diseases, such as arthritis, atherosclerosis, and myocardial ischemia-reperfusion injury. Interleukin (IL) 1 (IL-1) is one of the most pleiotropic cytokines and causes a wide variety of biological effects related to inflammation, infection and autoimmune processes [1, 2]. The action of IL-1 is regulated by the structurally-related IL-1 receptor antagonist (IL-1ra), which is primarily produced by activated monocytes and tissue macrophages, as well as a variety of other cell types [3, 4]. IL-1ra is a specific receptor antagonist for two IL-1 gene family members (IL-1α and IL-1β); it binds to cell membrane IL-1 type I receptor and prevents IL-1 from binding to the same IL-1 receptor [4, 5]. IL-1ra has no agonist activity and does not initiate signal transduction. A recombinant, nonglycosylated form of the human IL-1ra, anakinra (Kineret®), has been approved by FDA for the treatment of rheumatoid arthritis (RA) [6]. IL-1ra has been shown great potential as a probe for experimental investigation and as a treatment for IL-1 mediated inflammatory disease [6, 7].
IL-18 is another pro-inflammatory cytokine that possesses significant inflammatory activity [2, 8, 9]. It is also known as interferon-gamma (IFN-γ) inducing factor and is produced by a wide range of human cell types, including macrophages, dendritic cells, keratinocytes, Kupffer cells and epithelial cells [2, 10–12]. IL-18 induces production of tumor necrosis factor alpha (TNF-α) and IL-1β in mononuclear cells [13] and initiates a cytokine cascade with concomitant expression of pro-inflammatory markers. Interestingly, IL-18 is a pro-inflammatory cytokine closely related to IL-1 and shares numerous properties with IL-1. For example, both inactive precursor forms of IL-1 and IL-18 are cleaved by caspase-1 to mature biologically active forms; IL-1 and IL-18 regulate each other by stimulating the production of caspase-1; and the IL-18 receptor chains (IL-18Rα and IL-18Rβ) are also members of the IL-1 receptor family [14, 15].
The action of IL-18 is regulated by IL-18 binding protein (IL-18bp), which is a secreted extracellular protein that acts as a decoy receptor. IL-18bp has a strong binding affinity to IL-18 and thereby inactivates IL-18, thus preventing activation of the cell surface receptors [16–18]. Binding of IL-18bp to IL-18 further forms an inactive complex with IL-18Rβ, which prevents the IL-18Rα chain from activating the cell [2].
Knowledge of cytokine pathways associated with inflammation may identify opportunities to detect the inflammatory response using radioligands. A specific cytokine radioligand that serves as a biomarker is clinically desirable in inflammatory diseases to noninvasively detect an inappropriate level of inflammation and promote timely therapeutic intervention. Because key cytokine combinations can work together in an organ or tissue to produce coordinated effects [19–23], strategies that target multiple proinflammatory pathways simultaneously, may be more effective than those targeting a single pathway. IL-1 and IL-18 share numerous properties in terms of targeting receptors and signal transduction pathways. The effects of IL-1 and IL-18 are additive or synergistic in promoting pathophysiological processes observed in many inflammation-related diseases, such as depressing myocardial function in ischemic myocardium and mediating endotoxemic myocardial dysfunction. The potential for using the synergistic effects of cytokines motivated us to develop radiolabeled dual-domain cytokine ligands for detecting inflammatory sites, and for predicting and evaluating therapies in inflammatory and autoimmune diseases. This study was designed to validate a technetium-99m-labeled dual-domain cytokine ligand, 99mTc-IL-18bp-Fc-IL-1ra, for inflammation imaging. IL-18bp-Fc-IL-1ra is a recombinant human fusion protein. It contains an amino-terminal segment that specifically binds to IL-18 and a carboxy-terminal sequence of IL-1ra that binds to the IL-1 receptor. The Fc portion of human IgG1 in the fusion protein links the two segments and is capable of dimerizing.
2. Materials and Methods
2.1. Production of IL-18bp-Fc-IL-1ra
The dual-domain protein IL-18bp-Fc-IL-1ra, with a sequence of 576 amino acids, was developed by AmProtein Corporation (Camarillo, CA). It is a recombinant fusion protein that is derived from recombinant DNA. In brief, the fusion protein was created through the joining of three genes which originally coded for human IL-18bp, IL-1ra, and IgG1 Fc fragment. Translation of this fusion gene resulted in a single polypeptide with functional properties derived from each of the original proteins. Production of IL-18bp-Fc-IL-1ra was accomplished by the large-scale culturing of Chinese hamster ovary (CHO) cells that have been "cloned" to express the recombinant DNA construct. The cells were cultured in a serum-free suspension system with CHO-CD4 medium (Irvine Scientific, Santa Ana, CA) and in-house feed medium, and scaled up in a 3-liter bioreactor (Applikon Biotechnology, Foster City, CA). The protein was purified by Protein-A direct capture, followed by ion-exchange and hydrophobic chromatography. The purified protein was further processed and analyzed by size-exclusion and high-performance liquid chromatography (SEC-HPLC).
2.2. Radiolabeling IL-18bp-Fc-IL-1ra with 99mTc
IL-18bp-Fc-IL-1ra was radiolabeled with 99mTc using a modified non-specific labeling protocol as previously described [24–26] in which glucoheptonic acid serves as a transfer agent to chelate tin-reduced 99mTcO4 − to thiolated proteins. Glucoheptonic acid and 2-iminothiolane were purchased from Sigma Aldrich, Inc. (St Louis, MO). A total of 100µg protein in 200 µL PBS was incubated with 20 µL 2-iminothiolane (10 mg/mL in saline) at 37°C for 30 minutes. A freshly prepared 5µL SnCl2 solution (1 mg/mL in nitrogen-purged 0.1 N HCl) was mixed with 200 µL of glucoheptonic acid solution (2 mg/mL in nitrogen-purged saline). Three hundred µL of 99mTcO4 − (30 mCi) in 0.9% NaCl was added to the stannous glucoheptonate mixture and incubated at room temperature for 5 minutes to produce 99mTc-glucoheptonate. The thiolated protein was added into the vial of 99mTc-glucoheptonate and incubated at room temperature for 30 minutes. The reaction mixture was purified using a disposable PD-10 Desalting Column pre-packed with Sephadex G-25 Medium (GE Healthcare, Piscataway, NJ). The column was pre-equilibrated and eluted with PBS, pH 7.4. Protein concentrations of collected fractions were determined using a Genesys 10 Bio spectrophotometer and BCA™ Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL).
Radiolabeling efficiency (radiochemical purity, RCP) and stability were determined by instant thin-layer chromatography (ITLC-SG), ascending paper chromatography on Whatman 3MM paper, SG-81 paper, and size-exclusion high performance liquid chromatography (SEC-HPLC). The mobile phases for ITLC and paper chromatography were 0.9% saline and acetone. In this saline/acetone solvent system, free 99mTcO4 − moves with the solvent front, leaving labeled and reduced/hydrolyzed (R/H) 99mTc at the point of application. R/H 99mTc was separated by using 5% bovine serum albumin (BSA)-impregnated ITLC-SG strips as the stationary phase and solvent mixture ethanol:ammonia:water = 2:1:5 (v/v) as the mobile phase. In this system, free 99mTc and labeled proteins move with the solvent front, whereas R/H 99mTc remains at the point of application. Radiochromatograms of the ITLC-SG and paper strips were obtained using digital autoradiograph imaging. The strips were exposed to Fujifilm phosphor imaging plates for 1–5 minutes. A Fujifilm BAS-5000 Bio-Imaging Analysis System (Fujifilm Medical Systems USA, Stamford, CT) was used to scan the plates for digital autoradiograph collection. The strips were cut into 1-cm pieces and counted by a CRC-15W Dose Calibrator/Well Counter (Capintec, Ramsey, NJ).
Additional quality control was performed by SEC-HPLC using a Shodex KW 802.5 column (7.8 × 300 mm, 5 µm particle size) (Thomson Instrument Co., Oceanside, CA) and a Waters Breeze system (Waters Technologies Corp., Milford, MA) equipped with a Waters 1525 Binary HPLC Pump and a Waters 2489 dual absorbance detector (280 nm) in line with a Flow-Count™ Radio HPLC Flow Through Detector (Bioscan Inc., Washington, DC). The radiolabeled protein was eluted with 0.1mol/L KH2PO4 at pH 7.0 at a flow rate of 0.8 mL/min.
In vitro stability study was carried out in saline at room temperature and rat serum at 37°C, respectively. In vivo stability study was performed in two Sprague-Dawley rats that received intravenous injection of 99mTc-labeled protein purified by the PD-10 gel filtration (5–7 mCi). Blood samples were collected at 30, 60, and 180 minutes post-injection. The samples were centrifuged at 5000 rpm for 5 min to separate plasma and cellular fractions, and individual fractions were measured with the CRC-15W Dose Calibrator/Well Counter. The plasma was analyzed by SEC-HPLC.
2.3. In vitro cell binding studies
99mTc-IL-18bp-Fc-IL-1ra competitive binding assays were carried out using isolated rat polymorphonuclear leukocytes to determine the ligand concentration that inhibits 50% of the maximum specific binding (IC50) to the cells. The polymorphonuclear leukocytes were collected by blood sampling from three healthy male Sprague-Dawley rats (350–450 grams) using the hydroxyethyl-starch exchange transfusion method described previously [27–29]. The rats were anesthetized by 1.2% isoflurane and heparinized with intra-peritoneal injection of 1 mL heparin (1000 units). The right carotid artery and tail vein were catheterized. A total of 80–90 mL of blood was exchanged using 6% hydroxyethyl-starch-saline (DuPont Chemical Co., Dorval, Quebec, Canada). The leukocyte-rich plasma was separated from the blood by 40-minute sedimentation of red blood cells at room temperature. The leukocytes were recovered by centrifugation at 3,500g for 5 min and resuspended in 5 mL calcium-free and magnesium-free Tyrode's solution with 10% plasma and 25 units heparin per milliliter. The polymorphonuclear leukocytes were separated using sequential centrifugation and suspended in Roswell Park Memorial Institute (RPMI) medium with 10% fetal bovine serum (FBS) and 25 units heparin/mL and stored overnight at 2–8°C.
A total of 5 × 105 cells suspended in Eppendorf tubes with 1 mL of binding solution (cell culture medium containing 0.1% bovine serum albumin) were incubated with 2 nM 99mTc-IL-18bp-Fc-IL-1ra at varying concentrations of unlabelled IL-18bp-Fc-IL-1ra for 2 hours at room temperature. The cells were centrifuged at 3,500g for 5 min, the supernatant was removed, and the pellet was washed twice with 1 mL of ice cold PBS. After removing supernatant, the radioactivity bound to the cell pellet and the free radioactivity in the supernatant were counted using the CRC-15W Dose Calibrator/Well Counter. The results were calculated using the averages of triplicate determinations. Data were analyzed with non-linear regression analyses using SigmaPlot 11.0 Software (Systat Software Inc., Chicago, IL).
2.4. Biodistribution measurements
Biodistribution studies of 99mTc-IL-18bp-Fc-IL-1ra were performed in five healthy male Sprague-Dawley rats (250–300 g) and three healthy ICR mice. Three hours after intravenous injection of the radioligands (3.5–5.5 mCi for rats, 2.0–2.5 mCi for mice), the animals were euthanized with an overdose of sodium pentobarbital. Blood, skeletal muscle, heart, lung, stomach, intestine, liver, spleen, and kidneys were harvested, weighed, and measured using the CRC-15W Dose Calibrator/Well Counter.
2.5. Blood clearance
99mTc-IL-18bp-Fc-IL-1ra (3.5–5.5 mCi, 0.2–0.3 mL) was intravenously injected in four male Sprague-Dawley rats (250–300 g). Serial blood samples (200 µL) were collected from a carotid artery catheter at 0, 1, 3, 6, 10, 30, 60, 120, and 180 minutes after injection. Each volume of sample withdrawn was replaced with an equal volume of warm physiological saline solution. The samples were weighed and measured with the CRC-15W Dose Calibrator/Well Counter to determine percent injected dose per gram (%ID/g) at each time point. Blood-clearance curves were modeled using a non-linear Curve-Fit Kinetic Equation by TableCurve 2D® software (Systat Software Inc., Chicago, IL).
2.6. Accumulations of 99mTc-IL-18bp-Fc-IL-1ra in TPA-induced inflammation in mouse ear
We assessed the ability of 99mTc-IL-18bp-Fc-IL-1ra to target inflammation in vivo in a mouse model of skin inflammation [30–32]. Ear edema was induced in ICR mice (10–15 weeks old) by topical application of 2.0 µg 12-O-tetradecanoylphorbol-13-acetate (TPA) (Sigma Aldrich, Inc., St Louis, MO) dissolved in 20 µL of acetone administered through a micropipette to the right ear (10 µL on both the inner and outer surface of the ear. The TPA treatment was repeated at 24 hours. The left ear was treated with 20 µL of acetone (vehicle) topically as a negative control. The thickness of each ear was measured using a digital caliper before each TPA administration and radiotracer injection. Three hours after the second TPA (or vehicle) treatment, 99mTc-IL-18bp-Fc-IL-1ra (2.0–2.5 mCi, 0.2 mL) was injected intravenously in 6 mice. To determine the specificity of 99mTc-IL-18bp-Fc-IL-1ra uptake in the inflamed area, blocking tests were carried out in 5 mice by intravenous injection of 500 µg (0.2 mL) non-radiolabeled IL-18bp-Fc-IL-1ra 30 minutes before radioligand administration. The animals were imaged using a small-animal SPECT imager, FastSPECT II, at 2–3 hours after radiotracer injection. The ears were localized in the center of the field of view. The animals were sacrificed at the end of the imaging session. The ears were excised for radioactive measurement using the CRC-15W Dose Calibrator/Well Counter and digital autoradiograph imaging using the Fujifilm phosphor imaging plates and the BAS-5000 system. Major organs and tissues in three TPA-treated mice were harvested and measured to determine the biodistribution of 99mTc-IL-18bp-Fc-IL-1ra.
2.7. Measurements of IL-1β and IL-18 cytokines in homogenized mouse ear skin
The mouse ears treated with TPA or vehicle was harvested, snap frozen in liquid nitrogen, stored in a −80° C freezer. Ear tissues were homogenized in a cell extraction buffer (Invitrogen Corporation, Camarillo, CA) containing 10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM, Na4P2O7, 2 mM, Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 0.5% deoxycholate. This cell extraction buffer was supplemented with 1 mM PMSF and protease inhibitor cocktail (Sigma-Aldrich Corp., St. Louis, MO) just prior to use. The tissue mixture was placed on ice, sonicated for 30 seconds, and incubated at 4°C for 10 minutes. The final homogenate was centrifuged at 20,000g at 4°C for 15 minutes. The supernatant fraction was aliquoted and frozen at −80°C until assays were performed for determination of cytokine protein levels.
The levels of IL-1β and IL-18 were quantified using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA). Mouse IL-1β ELISA kits were purchased from Invitrogen Corp. (Camarillo, CA) and mouse IL-18 ELISA kits were obtained from MBL (Medial & Biological Laboratories Co., Ltd., Nagoya, Japan). The IL-1β and IL-18 ELISA assays were performed according to the manufacturer’s recommended protocol. Briefly, diluted samples and standards at 50–100 µL were transferred to the antibody-coated wells in a 96-well polyvinyl plate. The plates were incubated for 60–120 minutes at room temperature and the wells were washed with wash solution. A solution of enzyme-conjugated detection antibody was added. After a second incubation and washing, a substrate solution containing tetramethylbenzidine/H2O2 was added. After 30-min incubation, the stop solution (0.5 mol/L H2SO4) was added and the plates were read using a DTX 880 Multimode Detector (Beckman Coulter, Inc., Fullerton, CA) at a wavelength of 450 nm.
2.8. Correlation of 99mTc-IL-18bp-Fc-IL-1ra distribution with polymorphonuclear leukocyte accumulation
An ischemic-reperfused rat heart model was used to determine the correlation between 99mTc-IL-18bp-Fc-IL-1ra distribution and 111In-oxine labeled neutrophil infiltration. Three Sprague-Dawley rats (male, 250–300 g) were intubated and anesthetized with 1.2% isoflurane. The animals were ventilated using a volume-controlled Inspira Advanced Safety Ventilator (Harvard Apparatus, Holliston, MA) with a mixture of oxygen and room air. A left thoracotomy incision was made at the fifth intercostal space and a ligature was placed around the left coronary artery (LCA), which was occluded for 45 minutes, followed by 2-hour reperfusion after ligature release. After ischemia-reperfusion treatment, the chest of was closed in layers with sutures. The surgical procedures were performed under sterile conditions.
Polymorphonuclear leukocytes were isolated from 3 healthy male Sprague-Dawley rats (350–450 grams) using the hydroxyethyl-starch exchange transfusion technique described above. The cells were suspended in RPMI medium with 10% FBS and 25 units heparin/mL and stored overnight at 2–8°C. The purified leukocytes were labeled with 111In-oxine (GE HealthCare, Phoenix, AZ). In brief, the cells were washed with PBS, mixed and suspended with 111In-oxine (100 µCi per million cells), followed by 20-minute incubation at room temperature. Subsequently, the cells were washed twice, 111In-oxine labeling efficiency was measured, and cell viability was determined by trypan blue exclusion. The 111In-labeled leukocytes (250 µCi, 0.2 mL) were injected intravenously in three rats treated with ischemia-reperfusion. Twenty-four hours after the administration of 111In-labeled leukocytes, 99mTc-IL-18bp-Fc-IL-1ra (3.0 mCi, 0.3 ml) was intravenously injected. The animals were euthanized 3 hours after the injection of 99mTc-IL-18bp-Fc-IL-1ra and the hearts were excised for tissue radioactivity measurement. After the right ventricle and great vessels were removed, the left ventricle was sliced into 1-cm-thick rings from apex to base and further cut into 20–24 tissue samples (46.8 ± 2.7 mg). All tissue samples were assayed in a Captus 3000 Well System with 1024 Multichannel Analyzer (Capintec, Ramsey, NJ) for 99mTc and 111In activity. Results were expressed as counts per minute per gram (cpm/g) for each radionuclide. These counts were corrected for isotope decay and cross-talk (downscatter) of 111In counts into the 99mTc window.
2.9. Data analysis
All quantitative results were expressed as mean ± S.E.M. Comparisons between two variables were performed with one-way analysis of variance. Probability values less than 0.05 were considered significant.
2.10. Ethics
The animal experiments were performed in accordance with Principles of Laboratory Animal Care from the National Institutes of Health (NIH Publication 85-23, revised 1985) and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arizona.
3. Results
3.1. 99mTc-labeling of IL-18bp-Fc-IL-1ra
The radiolabeling yield of 99mTc-IL-18bp-Fc-IL-1ra was typically greater than 75%. After gel filtration purification, RCP was greater than 95%. ITLC showed that 99mTc-labeled protein remained at the loading position when saline/acetone was used as the mobile phase. There was no significant movement of free 99mTc with the solvent front. When ethanol:ammonia:water was used as the mobile phase, labeled protein moved with the solvent front, and no significant reduced/hydrolyzed 99mTc was found at the loading point. A representative HPLC chromatogram of 99mTc-IL-18bp-Fc-IL-1ra is shown in Fig. 1. The specific activity of 99mTc-IL-18bp-Fc-IL-1ra was 7.4–8.3 MBq/µg.
Fig. 1.
A representative HPLC-chromatogram of 99mTc-labeled IL-18bp-Fc-IL-1ra product using a 2-step thiolation protocol immediately after gel purification.
Stability tests of 99mTc-IL-18bp-Fc-IL-1ra using thin-layer chromatography and HPLC showed no significant degradation of the product up to 5 hours in either saline at room temperature or serum at 37°C. After 6 hours, more than 90% of the radioactivity was still associated with IL-18bp-Fc-IL-1ra. At 24 hours, RCP was 86.9% in saline and 92.2% in serum.
The inv vivo stability study showed that that 87.4%, 84.1%, and 86.4% of the radioactivity in blood was found in the plasma at 30, 60, and 180 minutes post-injection, respectively, with the remaining amounts associated with the cell fraction. HPLC analysis of plasma showed that the radioactivity associated with IL-18bp-Fc-IL-1ra was 91.7% at 3 hours post-injection. There was no significant amount of free 99mTc in the plasma.
3.2. In vitro binding assays
Binding of 99mTc-IL-18bp-Fc-IL-1ra to leukocytes gradually decreased with addition of increasing amounts of non-radiolabeled IL-18bp-Fc-IL-1ra, as shown in Fig. 2. The 50% inhibitory concentration (IC50) for 99mTc-IL-18bp-Fc-IL-1ra was 73.56 nM (n=3).
Fig. 2.
Results of in vitro cell binding competitive experiments of 99mTc-IL-18bp-Fc-IL-1ra using isolated rat leukocytes.
3.3. Blood clearance and biodistribution measurements
Blood radioactivity (%ID/gm) and normalized clearance (% peak) over time are shown in Fig. 3. After rapid early clearance within ten minutes post-injection, the radioactive washout became relatively slower. 99mTc fractional retention at 180 minutes post-injection was 11.0 ± 0.7 % of peak activity. The blood clearance was best fit to a Two Second Order Independent Decay (Non-linear Equation 8153) with calculated half-lives of 6.0 minutes. The equation was Y=a+b/(1+b*c*X)+d/(1+d*e*X), where a = −0.145, b = 6.04, c = 0.221, d = 1.772, e = 0.007 (γ = 0.999).
Fig. 3.
Blood-clearance curves in healthy rats. The animals were anesthetized with 1.5% isoflurane. 99mTc-IL-18bp-Fc-IL-1ra (3.5–5.5 mCi, 0.2–0.3 mL) was intravenously injected. Left panel: Original blood clearance data in terms of %ID/g; Right panel: Normalized blood-clearance curves, in which radioactivity at each time point is given as percentage of peak activity at 2 minutes post-injection.
Tissue radioactive distributions in rats and mice are summarized in Table 1. The organs with greatest activity at 180 minutes post-injection include liver, spleen, and kidneys. There was low radioactive uptake in heart, skeletal muscle, and skin. In terms of %ID/g, the results of biodistribution measurements in the blood and other major organs/tissues of ICR mice were significantly higher than that of rats.
Table 1.
Biodistribution measurements of 99mTc-IL-18bp-Fc-IL-1ra (%ID/gm)
| Tissues | Rats (n=5) | Mice (n=3) | Mice/TPA* (n=3) |
|---|---|---|---|
| Blood | 0.36±0.03 | 1.15±0.07 | 1.07±0.02 |
| Heart | 0.11±0.02 | 0.41±0.05 | 0.38±0.06 |
| Lung | 0.20±0.02 | 0.80±0.13 | 0.79±0.14 |
| Liver | 3.47±0.24 | 23.12±1.51 | 23.08±1.55 |
| Stomach | 0.14±0.01 | 0.66±0.10 | 0.52±0.08 |
| Spleen | 1.12±0.16 | 3.91±0.43 | 3.30±0.46 |
| Intestine | 0.64±0.13 | 14.29±4.05 | 16.51±1.84 |
| Kidneys | 5.84±0.34 | 10.03±1.19 | 9.07±0.85 |
| Skin | 0.03±0.01 | 0.38±0.04 | 0.33±0.05 |
| Muscle | 0.02±0.01 | 0.10±0.02 | 0.09±0.02 |
Mice/TPA*: mice with TPA-induced ear edema
3.4. TPA-induced skin inflammation
Ear edema was observed in all animals that received TPA treatments. Ear thickness increased to 0.54 ± 0.08 mm from 0.21 ± 0.01 (P < 0.01) after TPA treatments. In the ears treated only with vehicle, ear thickness remained unchanged in comparison with the initial thickness (0.21 ± 0.01 mm vs. 0.23 ± 0.01, P > 0.05).
The inflamed ears in the TPA-treated animals exhibited high accumulation of 99mTc-IL-18bp-Fc-IL-1ra, which could be clearly detected by in vivo SPECT imaging and ex vivo autoradiograph imaging. The radioactive uptake in the control ear was minimal and typically invisible by SPECT imaging. The in vivo specificity of 99mTc-IL-18bp-Fc-IL-1ra binding was established by blocking of radioactive uptake in the inflamed ears with non-radiolabeled IL-18bp-Fc-IL-1ra. Representative FastSPECT II and autoradiograph images of the ears in animals with and without a blocking dose of unlabeled IL-18bp-Fc-IL-1ra are shown in Fig. 4. The ears with TPA-induced edema exhibited a significant reduction of radiotracer uptake on both in vivo and ex vivo images after the blocking dose.
Fig. 4.
A and C: Representative SPECT and autoradiograph images in a mouse with TPA-induced right ear edema. The SPECT images were acquired for 15 minutes at 3 hours post-injection of 2.21 mCi radiotracer (0.2 ml). The animal was anesthetized with 1.5% isoflurane. B and D: SPECT and autoradiograph images in a mouse with ear inflammation received unlabeled IL-18bp-Fc-IL-1ra blocking. The animal received 2.24 mCi radiotracer (0.2 ml) and 15-minute SPECT image acquisition. It is noted that the ears on C and D were exposed on the same phosphor imaging plate simultaneously and the scale was set at the same quantitative value. Based on computerized analysis, the ratio of inflamed ear to controlled ear on SPECT and autoradiograph images was 2.06 and 2.41 in the mouse with unlabeled IL-18bp-Fc-IL-1ra blocking and 4.61 and 6.45 without blocking, respectively.
Biodistribution results of 99mTc-IL-18bp-Fc-IL-1ra in the mice with TPA-induced inflammatory ears are presented in Table 1. There was no difference in biodistribution between TPA-treated mice and healthy control mice. Based on postmortem gamma well counting at 3 hours post injection, the uptake ratio of inflamed ears to contralateral vehicle-treated ears was 8.75 ± 2.20 in the animals that received saline and 3.84 ± 0.61 in the animals that received unlabeled IL-18bp-Fc-IL-1ra (P < 0.05). The average ear uptake of the radiopharmaceutical (%ID/g) was 1.80 ± 0.17 (right ears) vs. 0.48 ± 0.07 (left ears) (P < 0.001) in the animals without blocking, compared to 1.09± 0.08 (right ears) vs. 0.53 ± 0.13 (left ears) (P < 0.05) with blocking. The difference between the radioactivity of inflamed ears in the animals with and without blocking was significant (P < 0.05).
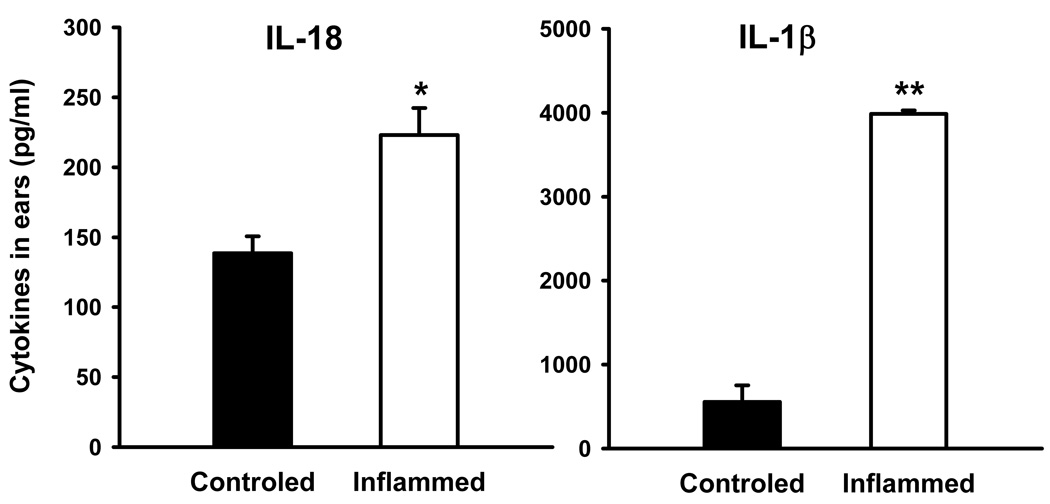
3.5. Levels of IL-1β and IL-18 in the inflamed mouse ears
ELISA results of cytokine measurements in homogenized ear tissue are shown in Fig. 5. The amounts of IL-1β and IL-18 (pg/ml) were significantly increased in the ears with TPA-induced inflammation compared with vehicle-treated ears (IL-1β: 3986.7 ± 40.6 vs. 555.4 ± 196.3, P < 0.001; IL-18: 223.1 ± 19.3 vs. 138.6 ± 12.1, P < 0.05). The ratio of inflamed ears to contralateral vehicle-treated ears was 12.87 ± 4.65 in IL-1β and 1.65 ± 0.18 in IL-18 (P < 0.05).
Fig. 5.
Expression of IL-1β and IL-18 in the mouse ear lesions of TPA-induced acute inflammation determined by measuring their concentration in the supernatant of ear homogenate by ELISA. Data are mean ± SEM. * P < 0.05, ** P < 0.001compared with corresponding vehicle-treated ears.
3.6. Correlation between 99mTc-IL-18bp-Fc-IL-1ra distribution and polymorphonuclear leukocyte accumulation
Cell-labeling efficiency of the donor rat leukocytes was 70–82%. The viability of labeled cells, determined by trypan blue exclusion, was greater than 96% at 3 hours. Fig. 6 shows a linear relationship between 99mTc-IL-18bp-Fc-IL-1ra uptake and 111In-labeled leukocyte infiltration in the ischemic-reperfused rat heart model. The correlation between the two was significant (γ = 0.921, P < 0.001).
Fig. 6.
Regression analysis of 99mTc-IL-18bp-Fc-IL-1ra versus 111In-oxine labeled leukocytes in ischemic-reperfused rat hearts.
4. Discussion
Conventional imaging methods typically rely on anatomical, physiological or metabolic changes to provide an indirect or non-specific demonstration of inflammation [33–35]. Scintigraphic imaging with radiolabeled leukocytes is a standard clinical procedure for detection of occult infectious and inflammatory diseases [36–39]. However, in vitro radiolabeling of leukocytes requires a complex procedure with potential risk of infection or misadministration. Nonspecific activation of leukocytes may interfere with targeted localization in vivo [37]. Fluorine-18 labeled 2-deoxyglucose, [18F]FDG, can also be used to image inflammation. [18F]FDG competes with glucose for uptake into metabolically active cells, where it accumulates in proportion to metabolic activity. [18F]FDG PET imaging can demonstrate the increased metabolic activity that accompanies inflammation, but the procedure offers low specificity for inflammation.
Molecular imaging using cytokines may hold promise for detection of infection and inflammation [40–42]. An ideal cytokine-based radiolabeled molecular probe should bind specifically to pro-inflammatory cytokines or receptors with high affinity and should have sufficient residence time at sites of inflammation to allow for imaging. The radiotracer should be sufficiently cleared from the circulation to provide adequate contrast between inflammatory lesions and normal surrounding tissues or blood pool. Factors affecting the behavior of a cytokine radioligand for imaging inflammation include choice of the molecular ligand, membrane receptor composition in the target cells, and biological availability of the radioligand. In this study, we investigated a dual-domain cytokine radioligand to specifically localize sites of inflammation in animal models.
IL-18bp is a constitutively secreted inhibitor of the biological activity of IL-18 with high-affinity and specificity. IL-18bp resembles a soluble receptor for IL-18, but it lacks a transmembrane and cytosolic domain [2, 16]. A soluble recombinant IL-18bp-Fc can be produced by attaching a sequence encoding an isoform-a of human IL-18bp to a sequence encoding human IgG1 Fc [43–45]. This hybrid IL-18bp-Fc has many advantages, including specificity, low immunogenicity and high affinity. Using recombinant DNA technology, IL-18bp-Fc can be secondarily fused with IL-1ra to produce a single two-domain polypeptide for potential inflammation targeting via IL-1 and IL-18 pathways bi-specifically. First, like anakinra, the carboxy-terminal sequence of IL-18bp-Fc-IL-1ra may act as a competitive receptor antagonist for binding of IL-1 to cell-membrane-bound IL-1 type I receptors on numerous cell types. The amino-terminal segment of IL-18bp-Fc-IL-1ra may specifically bind to IL-18 and function independently as a decoy to prevent the initiation of signal transduction at the cell membrane IL-18 receptor. When IL-18bp-Fc-IL-1ra is bound on the cell surface in inflammatory tissues, the IL-18bp domain may retain the ability to bind IL-18. Theoretically, when IL-18bp-Fc-IL-1ra is systemically administered, the IL-18bp domain can target circulating IL-18. However, because exogenous IL-1ra exhibits a rapid pharmacokinetic profile of blood clearance in humans and other species [46–48], the recombinant IL-1ra domain may guide the fusion protein to IL-1 receptor-rich inflammatory sites quickly.
IL-18bp-Fc-IL-1ra was radiolabeled with 99mTc using the 2-step thiolation protocol in this study. The direct 99mTc-labeling approach is simple and efficient. It involves the initial reduction of endogenous disulfide bonds to generate thiol binding sites. Using this approach, the dual-domain protein was 99mTc-labeled with high radiochemical purity and without significant 99mTc-colloid formation. We have also implemented an indirect labeling approach using 6-hydrazinopyridine-3-carboxylic acid (HYNIC) as a bifunctional chelator (data not shown). Indirect labeling is versatile and can be site-specific but is more complicated than direct labeling. Further studies are needed to compare inflammation targeting of fusion proteins labeled using thiolation and HYNIC methods.
The radiopharmaceutical kinetic profile and inflammation targeting properties of 99mTc-IL-18bp-Fc-IL-1ra were investigated in rat and mouse models in this study. Human IL-1ra can bind to murine IL-1 receptors and inhibit IL-1-mediated responses with similar avidity [49]. Unlike antibody to IL-18, IL-18bp does not exhibit species specificity, and human IL-18bp neutralizes both human and murine IL-18 [16]. Thus, the in vivo data of 99mTc-IL-18bp-Fc-IL-1ra in rats and mice should provide clinically valuable information regarding the usefulness of this dual-domain cytokine ligand in targeting inflammation sites. In rats, 99mTc-IL-18bp-Fc-IL-1ra showed improved blood-clearance kinetics compared to other radiolabeled recombinant proteins reported previously [48, 50]. The moderately fast blood-clearance kinetics of 99mTc-IL-18bp-Fc-IL-1ra may enhance the delivery of the radiotracer to target sites and produce a favorable target to non-target ratio for inflammation imaging. Biodistribution results in rats and mice indicate that 99mTc-IL-18bp-Fc-IL-1ra was excreted via the kidneys and liver, similar to results reported in previous studies with 125I-labeled recombinant IL-1ra in Swiss mice [48]. Higher radioactive distribution of blood and organs in the mice relative to rats may be related to a kinetic difference between species. Overall. the biodistribution of 99mTc-IL-18bp-Fc-IL-1ra in this study is similar to the data of 125I-labeled recombinant IL-1ra [48]. This observation suggests that the IL-1ra domain of 99mTc-IL-18bp-Fc-IL-1ra may serve as ligand carrier and guide the dual-domain radioligand to tissue sites. Because the surface-bound IL-1ra does not undergo receptor-mediated internalization [51], the domain of IL-18bp in 99mTc-IL-18bp-Fc-IL-1ra anchored on the membrane can still function as a inhibitor of IL18. Reduction of 99mTc-IL-18bp-Fc-IL-1ra uptake following the blocking agent in the inflammatory ears could theoretically reflect the simultaneous blockade of two domains.
Our experimental results indicate that 99mTc-IL-18bp-Fc-IL-1ra may be a suitable candidate for specific imaging of inflammation. The specific binding of 99mTc-IL-18bp-Fc-IL-1ra to leukocytes was demonstrated in an in vitro competitive binding assay. This cell-based in vitro binding result indicates that the IL-1ra domain of 99mTc-IL-18bp-Fc-IL-1ra can target cell-membrane-bound IL-1 receptors because IL-18bp binds to IL-18, not IL-18 receptors. The binding specificity of 99mTc-IL-18bp-Fc-IL-1ra was further documented in vivo by blocking experiments in the TPA-induced skin-inflammation model. TPA-induced skin inflammation is a good model for investigating cytokine targeting of inflammation since it is characterized by high production of cytokines, including IL-1β, IL-6, IL-12, and TNFα, as well as epidermal hyperproliferation, T-cell infiltration, and increased angiogenesis [52, 53]. However, based on our results of cytokine quantification, IL-18 may not play a significant role in this acute mouse ear inflammation model. The increased radioactive uptake in the inflamed ears might result predominantly from the high expression of IL-1 receptors. Because we only used IL-18bp-Fc-IL-1ra as a blocker, it is unclear whether a single domain or two domains are responsible for 99mTc-IL-18bp-Fc-IL-1ra binding. Additional studies using unlabeled IL-1ra and IL-18bp are required to determine the specificities of two domains in targeting IL-1 receptors and IL-18.
The correlation between 99mTc-IL-18bp-Fc-IL-1ra and 111In-labeled leukocyte uptake in the ischemic-reperfused rat heart model provides further support for 99mTc-IL-18bp-Fc-IL-1ra as an inflammation-imaging agent. We selected the ischemic-reperfused rat heart model to explore the inflammation targeting properties of 99mTc-IL-18bp-Fc-IL-1ra because proinflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-18 (IL-18), and interferon γ (IFN-γ), are induced at the site of myocardial injury [22, 54–58].
99mTc-IL-18bp-Fc-IL-1ra may have clinical implications in cardiovascular diseases. In acute myocardial infarction, there is robust upregulation of intramyocardial cytokines in the infarct zone as well as in non-infarcted myocardium within the first hours to one day [54, 55]. The elaborated cytokines then regulate myocytes and non-myocytes to trigger additional cellular inflammatory response. 99mTc-IL-18bp-Fc-IL-1ra may be useful to assess myocardial ischemia-reperfusion injury and evaluate the intensity of inflammation via IL-1 and IL-18 pathways. This dual-domain cytokine radioligand may also be used to evaluate the inflammation accompanying other cardiovascular disorders such as atherosclerosis, cardiomyopathy, and myocarditis.
In RA, a plethora of pro-inflammatory cytokines, including TNF-α, IL-1, IL-6, IL-7, IL-17, IL-18, and interferon-γ (IFN-γ), are involved in synovial inflammation and the resulting joint destruction and cartilage degradation[59, 60]. Elevated expression of IL-18 increases production of TNF-α and IL-1 in the RA synovial membrane and correlates significantly with local inflammation [61]. A variety of cytokine-based strategies are being explored in RA patients. Recombinant human IL-1ra (anakinra) was the first biologic agent approved for RA treatment [6]. IL-18bp is currently in clinical trials for the treatment of RA and severe psoriasis [62]. An objective approach is needed to evaluate treatment response and disease activity because the presentation of the disease may vary considerably [60]. Targeting IL-1 and IL-18, as well as other cytokines, using cytokine radioligands may have a significant clinical impact on prediction and stratification in the individual patient [63].
There are several limitations in this study. As mentioned above, we only assessed in vitro competitive binding of 99mTc-IL-18bp-Fc-IL-1ra to rat polymorphonuclear leukocytes with unlabeled IL-18bp-Fc-IL-1ra. Although the specific targeting of 99mTc-IL-18bp-Fc-IL-1ra to inflammatory cells gives a clue that the IL-1ra domain may be able to target the cell membrane-bound IL-1 receptors, it is unclear if the IL-18bp domain is biologically involved in the in vivo binding to IL-18. Further in vitro studies are warranted to investigate the bi-specificity of this dual-domain cytokine radioligand. We have demonstrated that 99mTc-IL-18bp-Fc-IL-1ra has the ability to target inflammatory sites specifically. However, we have not yet obtained in vivo data to determine whether an individual cytokine radioligand, 99mTc-labeled IL-1ra-Fc or IL-18bp-Fc, would achieve the same uptake level (%ID/g) and binding specificity as the dual-domain radioligand. There are some shortcomings in the animal models used in this study. The mouse ear with TPA-induced acute inflammation might not be an optimal animal model for studies of inflammation targeting via the IL-18 pathway because it does not have high IL-18 expression. Increased capillary permeability at sites of inflammation in the mouse ear edema model may contribute to accumulation of radiolabeled proteins. Similarly, the pathophysiological changes in the ischemic-reperfused myocardium increase vascular permeability and also increase interstitial space [64]. As a result, the radioactivity detected in the inflamed lesions might be a combination of specific cytokine binding and nonspecific leakage of the tracer. The contribution of nonspecific binding and leakage needs to be quantitatively clarified in further studies using radiolabeled non-specific antibodies or proteins with similar molecular weight. Finally, it needs to be explored whether an elevated blood level of individual cytokines, such as IL-18, could prevent the dual-domain radioligand from reaching its target site.
5. Conclusions
IL-18bp-Fc-IL-1ra was successfully radiolabeled with 99mTc via 2-iminothiolane thiolation. The dual-domain radioligand, 99mTc-IL-18bp-Fc-IL-1ra, exhibited a favorable radiopharmaceutical kinetic profile. Targeting pro-inflammatory cytokines with the novel dual-domain 99mTc-IL18bp-Fc-IL-1ra appears to be a promising approach for specific detection of inflammatory sites and assessment of inflammatory response.
Acknowledgments
The authors are grateful to Dr. Lars Furenlid for support in in vivo imaging acquisition, Sara Lewis for assistance in neutrophil isolation and radiolabeling, as well as Li Wan for assistance in animal studies. We wish to thank Dr. Paul McDonagh for allowing use of his laboratory materials and equipment in tissue processing. This work was supported by NIH grants NHLBI R01-HL090716 and NIBIB P41-EB002035.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, et al. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 4.Bresnihan B. The prospect of treating rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. BioDrugs. 2001;15:87–97. doi: 10.2165/00063030-200115020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Efthimiou P, Markenson JA. Role of biological agents in immune-mediated inflammatory diseases. South Med J. 2005;98:192–204. doi: 10.1097/01.SMJ.0000153119.37032.8B. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RC, Dripps DJ, Eisenberg SP. Interleukin-1 receptor antagonist (IL-1ra) as a probe and as a treatment for IL-1 mediated disease. Int J Immunopharmacol. 1992;14:475–480. doi: 10.1016/0192-0561(92)90178-n. [DOI] [PubMed] [Google Scholar]
- 7.Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sims J, Towne J, Blumberg H. 11 IL-1 family members in inflammatory skin disease. Ernst Schering Res Found Workshop. 2006:187–191. doi: 10.1007/3-540-37673-9_11. [DOI] [PubMed] [Google Scholar]
- 9.Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol. 2002;14:117–122. doi: 10.1016/s0952-7915(01)00306-5. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar B, Colston JT, de la Rosa SD, Rao PP, Freeman GL. TNF-alpha and H2O2 induce IL-18 and IL-18R beta expression in cardiomyocytes via NF-kappa B activation. Biochem Biophys Res Commun. 2003;303:1152–1158. doi: 10.1016/s0006-291x(03)00496-0. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato Z, Jee J, Shikano H, Mishima M, Ohki I, Ohnishi H, et al. The structure and binding mode of interleukin-18. Nat Struct Biol. 2003;10:966–971. doi: 10.1038/nsb993. [DOI] [PubMed] [Google Scholar]
- 15.Azam T, Novick D, Bufler P, Yoon DY, Rubinstein M, Dinarello CA, et al. Identification of a critical Ig-like domain in IL-18 receptor alpha and characterization of a functional IL-18 receptor complex. J Immunol. 2003;171:6574–6580. doi: 10.4049/jimmunol.171.12.6574. [DOI] [PubMed] [Google Scholar]
- 16.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 17.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001;14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa Y, Akita K, Taniai M, Torigoe K, Mori T, Nishida Y, et al. Cloning and expression of interleukin-18 binding protein. FEBS Lett. 1999;445:338–342. doi: 10.1016/s0014-5793(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 19.Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Horton WE, Jr, Udo I, Precht P, Balakir R, Hasty K. Cytokine inducible matrix metalloproteinase expression in immortalized rat chondrocytes is independent of nitric oxide stimulation. In Vitro Cell Dev Biol Anim. 1998;34:378–384. doi: 10.1007/s11626-998-0019-8. [DOI] [PubMed] [Google Scholar]
- 21.Di Girolamo N, Lloyd A, McCluskey P, Filipic M, Wakefield D. Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral fibroblasts. Am J Pathol. 1997;150:653–666. [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello CA. Novel targets for interleukin 18 binding protein. Ann Rheum Dis. 2001;60 Suppl 3:iii18–iii24. doi: 10.1136/ard.60.90003.iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woldbaek PR, Tonnessen T, Henriksen UL, Florholmen G, Lunde PK, Lyberg T, et al. Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse; a potential role in cardiac dysfunction. Cardiovasc Res. 2003;59:122–131. doi: 10.1016/s0008-6363(03)00339-0. [DOI] [PubMed] [Google Scholar]
- 24.Tesic M, Sheldon KM, Ballinger JR, Boxen I. Labelling small quantities of monoclonal antibodies and their F(ab')2 fragments with technetium-99m. Nucl Med Biol. 1995;22:451–457. doi: 10.1016/0969-8051(94)00132-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhao M, Zhu X, Ji S, Zhou J, Ozker KS, Fang W, et al. 99mTc-labeled C2A domain of synaptotagmin I as a target-specific molecular probe for noninvasive imaging of acute myocardial infarction. J Nucl Med. 2006;47:1367–1374. [PubMed] [Google Scholar]
- 26.Mishra AK, Iznaga-Escobar N, Figueredo R, Jain VK, Dwarakanath BS, Perez-Rodriguez R, et al. Preparation and comparative evaluation of 99mTc-labeled 2-iminothiolane modified antibodies and CITC-DTPA immunoconjugates of anti-EGF-receptor antibodies. Methods Find Exp Clin Pharmacol. 2002;24:653–660. doi: 10.1358/mf.2002.24.10.802314. [DOI] [PubMed] [Google Scholar]
- 27.Williams JH, Jr, Moser KM, Ulich T, Cairo MS. Harvesting the noncirculating pool of polymorphonuclear leukocytes in rats by hetastarch exchange transfusion (HET): yield and functional assessment. J Leukoc Biol. 1987;42:455–462. doi: 10.1002/jlb.42.5.455. [DOI] [PubMed] [Google Scholar]
- 28.Issekutz AC, Nakazato S, Issekutz TB. Differential roles of VLA-4(CD49d/CD29) and LFA-1(CD11a/CD18) integrins and E-and P-selectin during developing and established active or adoptively transferred adjuvant arthritis in the rat. Immunol Cell Biol. 2003;81:397–408. doi: 10.1046/j.1440-1711.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara T, Miyamoto M, Takayama S, Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods. 1995;33:91–100. doi: 10.1016/1056-8719(94)00062-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim EJ, Park H, Kim J, Park JH. 3,3'-diindolylmethane suppresses 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin via the downregulation of inflammatory mediators. Mol Carcinog. 2010;49:672–683. doi: 10.1002/mc.20640. [DOI] [PubMed] [Google Scholar]
- 31.Updyke LW, Yoon HL, Chuthaputti A, Pfeifer RW, Yim GK. Induction of interleukin-1 and tumor necrosis factor by 12-O-tetradecanoylphorbol-13-acetate in phorbol ester-sensitive (SENCAR) and resistant (B6C3F1) mice. Carcinogenesis. 1989;10:1107–1111. doi: 10.1093/carcin/10.6.1107. [DOI] [PubMed] [Google Scholar]
- 32.Cao Q, Cai W, Li ZB, Chen K, He L, Li HC, et al. PET imaging of acute and chronic inflammation in living mice. Eur J Nucl Med Mol Imaging. 2007;34:1832–1842. doi: 10.1007/s00259-007-0451-0. [DOI] [PubMed] [Google Scholar]
- 33.Jalilian AR, Bineshmarvasti M, Sardari S. Application of radioisotopes in inflammation. Curr Med Chem. 2006;13:959–965. doi: 10.2174/092986706776361049. [DOI] [PubMed] [Google Scholar]
- 34.Villanueva FS, Wagner WR, Vannan MA, Narula J. Targeted ultrasound imaging using microbubbles. Cardiol Clin. 2004;22:283–298. doi: 10.1016/j.ccl.2004.02.008. vii. [DOI] [PubMed] [Google Scholar]
- 35.Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004;94:433–445. doi: 10.1161/01.RES.0000119321.18573.5A. [DOI] [PubMed] [Google Scholar]
- 36.Rini JN, Bhargava KK, Tronco GG, Singer C, Caprioli R, Marwin SE, et al. PET with FDG-labeled leukocytes versus scintigraphy with 111In-oxine-labeled leukocytes for detection of infection. Radiology. 2006;238:978–987. doi: 10.1148/radiol.2382041993. [DOI] [PubMed] [Google Scholar]
- 37.Peters AM, Danpure HJ, Osman S, Hawker RJ, Henderson BL, Hodgson HJ, et al. Clinical experience with 99mTc-hexamethylpropylene-amineoxime for labelling leucocytes and imaging inflammation. Lancet. 1986;2:946–949. doi: 10.1016/s0140-6736(86)90601-x. [DOI] [PubMed] [Google Scholar]
- 38.Peters AM, Saverymuttu SH. The value of indium-labelled leucocytes in clinical practice. Blood Rev. 1987;1:65–76. doi: 10.1016/0268-960x(87)90021-x. [DOI] [PubMed] [Google Scholar]
- 39.Peters AM. Development of radiolabelled white cell scanning. Scand J Gastroenterol Suppl. 1994;203:28–31. doi: 10.3109/00365529409091393. [DOI] [PubMed] [Google Scholar]
- 40.Riou LM, Ruiz M, Sullivan GW, Linden J, Leong-Poi H, Lindner JR, et al. Assessment of myocardial inflammation produced by experimental coronary occlusion and reperfusion with 99mTc-RP517, a new leukotriene B4 receptor antagonist that preferentially labels neutrophils in vivo. Circulation. 2002;106:592–598. doi: 10.1161/01.cir.0000023878.04716.6d. [DOI] [PubMed] [Google Scholar]
- 41.van Eerd JE, Oyen WJ, Harris TD, Rennen HJ, Edwards DS, Corstens FH, et al. Scintigraphic imaging of infectious foci with an 111In-LTB4 antagonist is based on in vivo labeling of granulocytes. J Nucl Med. 2005;46:786–793. [PubMed] [Google Scholar]
- 42.van Eerd JE, Broekema M, Harris TD, Edwards DS, Oyen WJ, Corstens FH, et al. Imaging of infection and inflammation with an improved 99mTc-labeled LTB4 antagonist. J Nucl Med. 2005;46:1546–1551. [PubMed] [Google Scholar]
- 43.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, et al. IL-18-binding protein protects against lipopolysaccharide-induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol. 2001;167:5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- 44.Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, et al. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz MJ, Knapp S, Florquin S, Pater J, Takeda K, Akira S, et al. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun. 2003;71:1630–1634. doi: 10.1128/IAI.71.4.1630-1634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrera P, van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, van Lent PL, et al. Radiolabelled interleukin-1 receptor antagonist for detection of synovitis in patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;39:870–874. doi: 10.1093/rheumatology/39.8.870. [DOI] [PubMed] [Google Scholar]
- 47.Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 48.van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, Claessens RA, van der Meer JW, et al. Different behaviour of radioiodinated human recombinant interleukin-1 and its receptor antagonist in an animal model of infection. Eur J Nucl Med. 1996;23:1531–1535. doi: 10.1007/BF01254480. [DOI] [PubMed] [Google Scholar]
- 49.McCoy RD, Davidson BL, Roessler BJ, Huffnagle GB, Simon RH. Expression of human interleukin-1 receptor antagonist in mouse lungs using a recombinant adenovirus: effects on vector-induced inflammation. Gene therapy. 1995;2:437–442. [PubMed] [Google Scholar]
- 50.Waibel R, Alberto R, Willuda J, Finnern R, Schibli R, Stichelberger A, et al. Stable one-step technetium-99m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat Biotechnol. 1999;17:897–901. doi: 10.1038/12890. [DOI] [PubMed] [Google Scholar]
- 51.Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem. 1991;266:10331–10336. [PubMed] [Google Scholar]
- 52.Teige I, Hvid H, Svensson L, Kvist PH, Kemp K. Regulatory T cells control VEGF-dependent skin inflammation. J Invest Dermatol. 2009;129:1437–1445. doi: 10.1038/jid.2008.375. [DOI] [PubMed] [Google Scholar]
- 53.Hvid H, Teige I, Kvist PH, Svensson L, Kemp K. TPA induction leads to a Th17-like response in transgenic K14/VEGF mice: a novel in vivo screening model of psoriasis. Int Immunol. 2008;20:1097–1106. doi: 10.1093/intimm/dxn068. [DOI] [PubMed] [Google Scholar]
- 54.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55:329–340. doi: 10.1016/s0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 55.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146:419–428. [PMC free article] [PubMed] [Google Scholar]
- 56.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 57.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 58.Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci U S A. 2001;98:2871–2876. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malemud CJ. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging. 2010;27:95–115. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937–1949. doi: 10.2967/jnumed.110.076232. [DOI] [PubMed] [Google Scholar]
- 61.Joosten LA, Radstake TR, Lubberts E, van den Bersselaar LA, van Riel PL, van Lent PL, et al. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:339–347. doi: 10.1002/art.10814. [DOI] [PubMed] [Google Scholar]
- 62.Tak PP, Bacchi M, Bertolino M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. European journal of drug metabolism and pharmacokinetics. 2006;31:109–116. doi: 10.1007/BF03191127. [DOI] [PubMed] [Google Scholar]
- 63.Glaudemans AW, Dierckx RA, Kallenberg CG, Anzola Fuentes KL. The role of radiolabelled anti-TNFa monoclonal antibodies for diagnostic purposes and therapy evaluation. Q J Nucl Med Mol Imaging. 2010;54:639–653. [PubMed] [Google Scholar]
- 64.Saeed M, van Dijke CF, Mann JS, Wendland MF, Rosenau W, Higgins CB, et al. Histologic confirmation of microvascular hyperpermeability to macromolecular MR contrast medium in reperfused myocardial infarction. J Magn Reson Imaging. 1998;8:561–567. doi: 10.1002/jmri.1880080308. [DOI] [PubMed] [Google Scholar]