Abstract
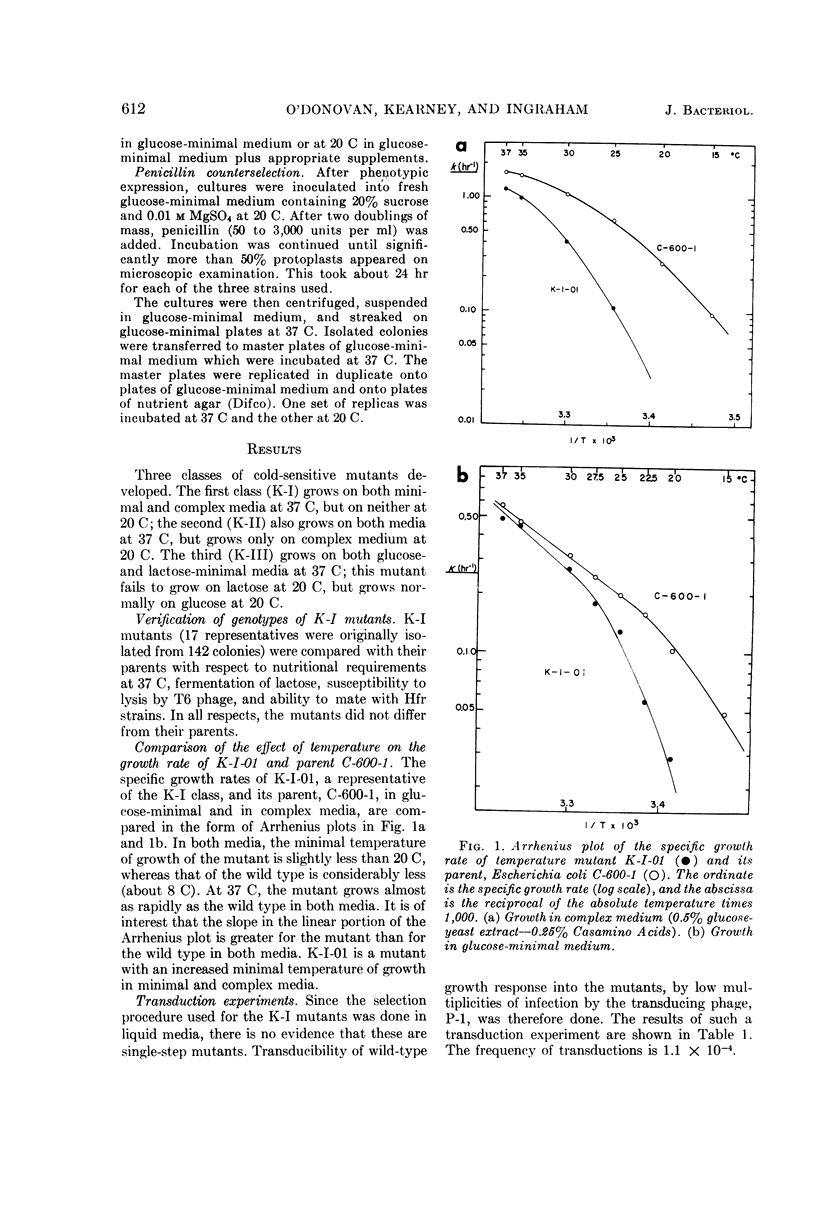
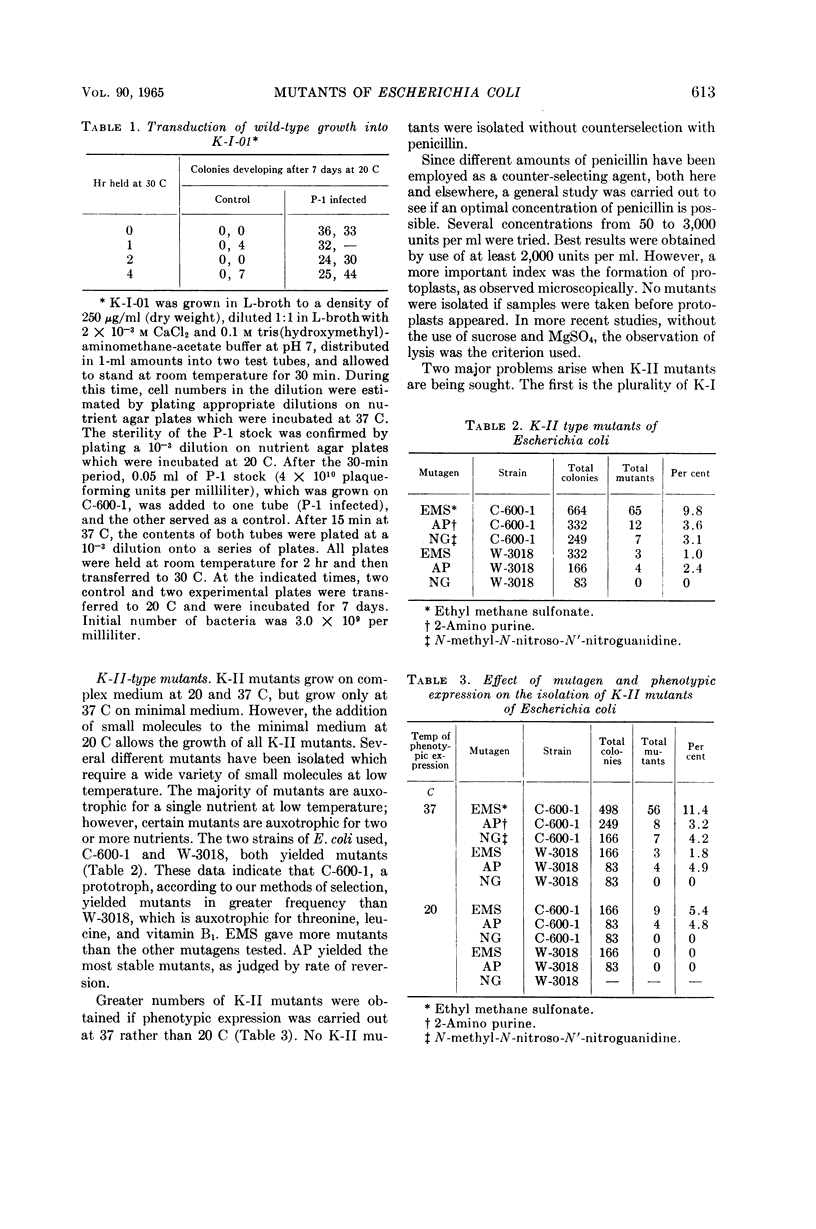
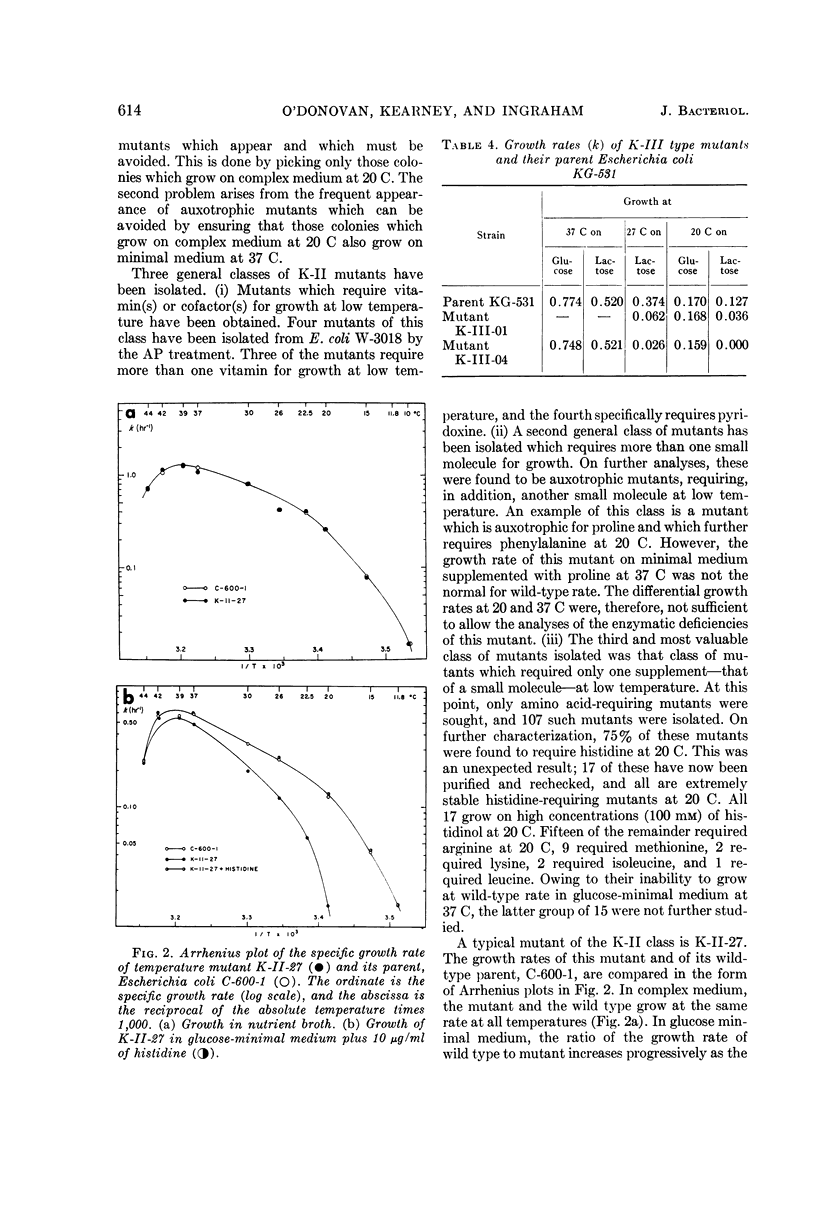
O'Donovan, Gerard A. (University of California, Davis), Catherine L. Kearney, and John L. Ingraham. Mutants of Escherichia coli with high minimal temperatures of growth. J. Bacteriol. 90:611–616. 1965.—Three general classes of mutants showing increased minimal temperatures of growth have been isolated from Escherichia coli. These mutants do not grow at temperatures below 20 C, although their parents can grow at temperatures as low as 8 C. The first class of mutants (K-I) cannot grow below 20 C in either complex or minimal medium, but grows at nearly normal rates at 37 C on both types of media. Normal growth rate at 20 C can be conferred on these mutants by infection at a low multiplicity with a transducing phage grown on the parent. The second class of mutants (K-II) fails to grow only in minimal medium at 20 C. These mutants are characterized by their singular response to specific nutrients in minimal medium at 20 C. The third class of mutants (K-III) grows normally in minimal medium at all temperatures with either glucose or glycerol as the carbon source, but does not grow at 20 C with lactose as the carbon source.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. The thermophilic aerobic sporeforming bacteria. Bacteriol Rev. 1953 Jun;17(2):125–173. doi: 10.1128/br.17.2.125-173.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., LIELAUSIS I. TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4D: THEIR ISOLATION AND GENETIC CHARACTERIZATION. Genetics. 1964 Apr;49:649–662. doi: 10.1093/genetics/49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT J., STAPLETON R. PROPERTIES OF A TEMPERATURE-SENSITIVE REGULATORY SYSTEM. Proc Natl Acad Sci U S A. 1963 Aug;50:348–355. doi: 10.1073/pnas.50.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H., Fling M. Genetic Determination of Tyrosinase Thermostability in Neurospora. Genetics. 1953 Jul;38(4):360–374. doi: 10.1093/genetics/38.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAHAM J. L. Growth of psychrophilic bacteria. J Bacteriol. 1958 Jul;76(1):75–80. doi: 10.1128/jb.76.1.75-80.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- Maas W. K., Davis B. D. Production of an Altered Pantothenate-Synthesizing Enzyme by a Temperature-Sensitive Mutant of Escherichia Coli. Proc Natl Acad Sci U S A. 1952 Sep;38(9):785–797. doi: 10.1073/pnas.38.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG H., INGRAHAM J. L., MARR A. G. Damage and derepression in Escherichia coli resulting from growth at low temperatures. J Bacteriol. 1962 Aug;84:331–339. doi: 10.1128/jb.84.2.331-339.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


