Abstract
The Galoka mountain chain, comprising principally the Galoka and Kalabenono massifs, situated at the northern edge of the Sambirano Region in NW Madagascar is an area that was virtually unknown botanically. It was visited three times between 2005 and 2007 as part of a floristic inventory. Both massifs contain the last remaining primary forests in the Galoka chain, which extends parallel to the coastline from South of Ambilobe to North of Ambanja. Several new species have been discovered amongst the collections, eight of which are described here.
Keywords: ANNONACEAE, ARALIACEAE, MALVACEAE, MELIACEAE, OLEACEAE, Xylopia, Polyscias, Hibiscus, Nesogordonia, Trichilia, Noronhia, Galoka, Kalabenono, Sambirano, Madagascar, Taxonomy, Conservation, IUCN Red List
Introduction
The island of Madagascar is renowned for its exceptional biodiversity, with extraordinary levels of species diversity and endemism found in many groups (Goodman & Benstead, 2005). Madagascar’s native vascular plant flora is estimated to comprise circa 12–14000 species, with well over 90% species endemism (Schatz, 2001; Phillipson & al., 2006), among the highest biodiversity levels on Earth. In the face of continuing deforestation and environmental degradation, it is crucial to document the island’s floristic diversity, especially at sites outside the network of protected areas that still contain primary vegetation (Callmander & al., 2005). It is estimated that around 90000 km2 of closed canopy primary forest and woodland remained in Madagascar as of 2000, with an average rate of loss during the 1990s of 0.9% per year (Steininger & al., 2003). Assuming that 90% of the country was once forested (Perrier de la Bathie, 1936), this suggests that only about 17% of the original primary vegetation may remain today.
Starting in 2005, with the aim of documenting the flora of Madagascar’s Northern Highlands, Missouri Botanical Garden, in partnership with the University of Antananarivo, the Parc Botanique et Zoologique de Tzimbazaza, the University of Neuchâtel and the Muséum National d’Histoire Naturelle of Paris conducted a three-year botanical inventory aimed at generating baseline data on the plant diversity of this inadequately known area. The results should provide valuable information for setting conservation priorities and identifying key areas for the establishment of new protected areas as part of Madagascar’s ongoing SAPM (Système d’aire protégée de Madagascar) process. The inventory work will also fill a large gap in the botanical dataset available for more focused studies and taxonomic revisions. Moreover, the botanical information generated will be critical for an ongoing examination of the complex biogeography of northern Madagascar. Among the mountainous regions that were explored in the Northern Highlands, the southern part of the Galoka mountain chain proved to possess a particularly high level of biodiversity (Solo & al., 2008) and yielded several plant species that were new to science.
The Galoka chain, which extends parallel to the western coastline of Madagascar from S of the town of Ambilobe to N of the city of Ambanja (Fig. 1), comprises two small massifs that contain some of the region’s last remaining primary forests: the Galoka Massif (1148 m) and the rocky Kalabenono Massif (1028 m). Until our exploration began, these massifs were virtually unknown and few scientists had visited the area. Perrier de la Bâthie conducted limited field work on two occasions between 1908 and 1923 while in the Sambirano region and Gachet (1958) apparently also worked in the region, although we have not been able to locate his herbarium material. Nine of the ten collections made by Perrier de la Bâthie that we have succeeded in locating in the Paris herbarium have already served as types of new species, seven of which have also been recorded in the nearby Manongarivo massif as indicated recently by Gautier (2002). The remaining two species based on Perrier de la Bâthie’s material, both palms (Dypsis caniculata (Jum.) Beentje & J. Dransf. and D. ligulata (Jum.) Beentje & J. Dransf.), appear to be endemic to the Galoka chain and were not recollected during our inventory.
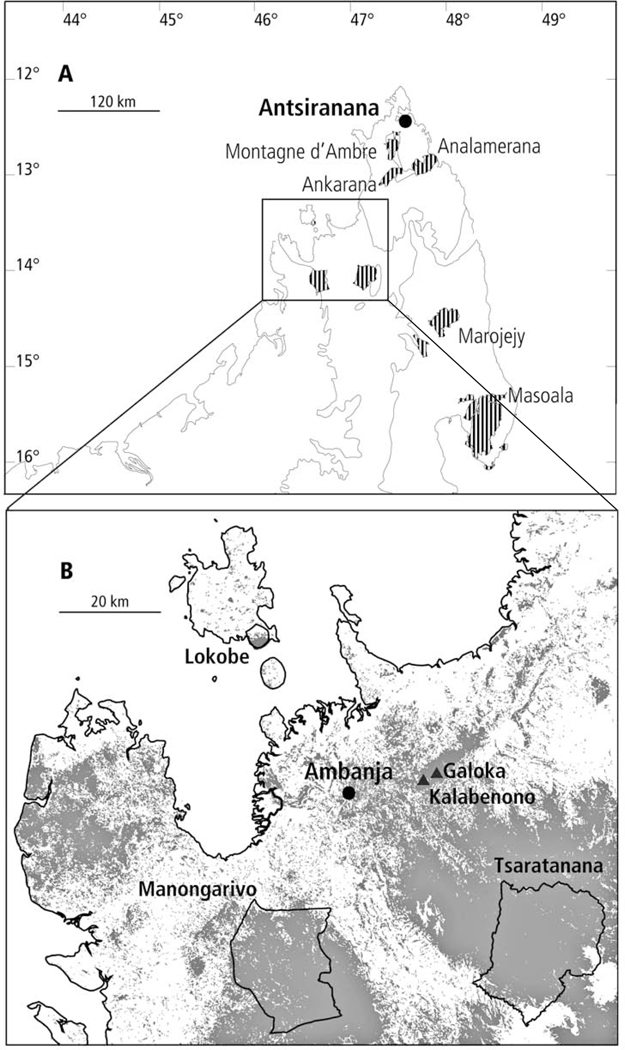
Fig. 1.
Map of Northern Madagascar. A. Current Protected Areas in Northern Madagascar (hatched) and frame (enlarged in B); B. Geographical situation of the Galoka and Kalabenono massifs with remaining forest in grey.
Of the circa 700 collections made in the Galoka-Kalabenono area during our field work in February 2005, November 2006 and November-December 2007, several clearly represented new species. Two have already been described elsewhere, Micronychia benono Randrian. & Lowry (Anacardiaceae) (Randrianasolo & Lowry, 2009) and Pandanus sermolliana Callm. & Buerki (Pandanaceae) (Callmander & al., 2008), whereas others in the genera Ravenea (Arecaceae), Schefflera (Araliaceae), Diospyros (Ebeneceae), Coffea (Rubiaceae) and Rinorea (Violaceae) are now being prepared for publication. In the present article we describe eight new species belonging to the five following families: Annonaceae, Araliaceae, Malvaceae s.l., Meliaceae and Oleaceae. All these novelties have restricted ranges and are therefore potentially at risk. We have included preliminary “IUCN Red List assessments”, which have proven valuable for ongoing conservation efforts (see Callmander & al., 2005).
Material & Methods
Study site
The southern part of the Galoka chain, situated at the northern edge of the Sambirano Region (Gachet, 1958), falls within Madagascar’s subhumid bioclimatic region (Cornet, 1974). It includes circa 6300 ha of primary vegetation (Moat & Smith, 2007) and lies between 13°33’30”–13°41’S and 48°36’–48°45’E. The chain is composed of Isalo Sandstone (Besairie, 1936) that has eroded in the Kalabenono area to form a remarkable landscape dominated by a pair of distinctly shaped domes (Gachet, 1958). The hydrographic network is of regional importance, with 3 main watercourses, the Antaramaizina and Andranomifafy in the West, and the Ambalihabe in the East. The people living in the area mostly belong to the Sakalava ethnic group, with numerous Tsimihety migrants, who are primarily responsible for increasing pressure being placed on local forests as a result of slash and burn agriculture. The Kalabenono massif, with its two rounded summits, is sacred for the local Sakalava (Solo & al., 2008), and its name refers indirectly to a legendary queen. Similarly, the name Galoka refers to a local king. According to legend, the queen embodied in the Kalabenono Massif gave birth to the nearby Manongarivo massif, fathered by the king at Galoka (see Solo & al., 2008).
Collection and conservation status
We have examined all the available material of the genera involved at G, K, MO, NEU, P, TAN, TEF, with special attention paid to the specimens collected in the near by Manongarivo massif and cited in Gautier (2002). Historical collections lacking geographic coordinates were post facto georeferenced where possible using the “Gazetteer to Malagasy Botanical Collecting Localities” (Schatz & Lescot, 2008). These data are placed in square brackets in the citation of paratypes. The conservation status of each species was assessed following IUCN (2009) using the Red List criteria. The calculations of area of occupancy (AOO), extent of occurrence (EOO) and number of subpopulations are based on methods presented in Callmander & al. (2007). The grid cell size used to calculate AOO is 3 × 3 km. IUCN threat status (2009) assigned to the new species is based on criteria A to E and their corresponding subcriteria. The generation length has been estimated as 20 years for understory tree species described regarding “Criterion A” (reduction in population size). For assessments under “Criterion B” (geographic range size), the relevant consideration under subcriterion “a” is linked with “location”, which is a direct function of the magnitude of the most plausible threat. To qualify as “Critically Endangered” (CR), for example, there must be only a single location, i.e. a single threatening event that can rapidly affect all individuals of the taxon. We consider here the threat faced by species restricted to the Kalabenono or Galoka massifs massif to be unique (one location), characterized by the rapid forest clearance for timber and agriculture that is currently occurring there.
Results
Annonaceae
The genus Xylopia L. with circa 180 species is the only pantropical genus of Annonaceae. It is characterized by the combination of axillary inflorescences, narrow valvate petals that are pressed together in bud, septate anthers with a pollen tetrad or polyad in each compartment, fusion of the filaments to form a cone-like chamber within which sit the carpels, and dehiscent monocarps with seeds bearing a sarcotesta and/or an aril. Xylopia is represented in Madagascar by over 20 species belonging to two sections; all species are endemic to the island and several are, as yet, undescribed (Schatz & Phillipson, pers. comm.).
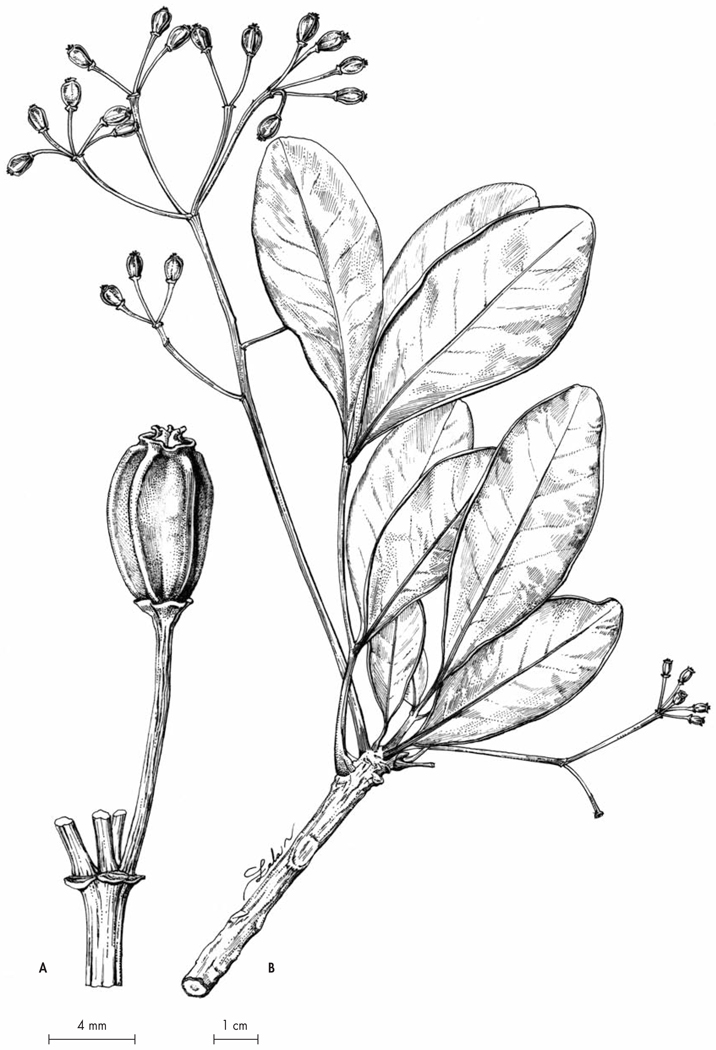
Xylopia kalabenonensis D. M. Johnson, Deroin & Callm., spec. nova (Fig. 2, 3, 4)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambilobe, commune de Beramanja, Anketrabe, forêt de Kalabenono, chaîne du Galoka, 7 km au SE d’Anketrabe, 13°38’23”S 48°40’06”E, 854 m, 18.XI.2006, fl. & fr., Razafitsalama, Torze & Toninjama 1041 (holo-: MO!; iso-: G!, OWU!, P!, TAN!).
Haec species X. capuronii Cavaco & Keraudren ramulis pedicellisque dense pubescentibus, petalis latis et monocarpiis clavatis estipitatis similis, sed ab ea trichomatibus patentibus, foliis chartaceis et petalis externis longioribus angustioribusque (ca. 13 mm × 4.5 mm) differt.
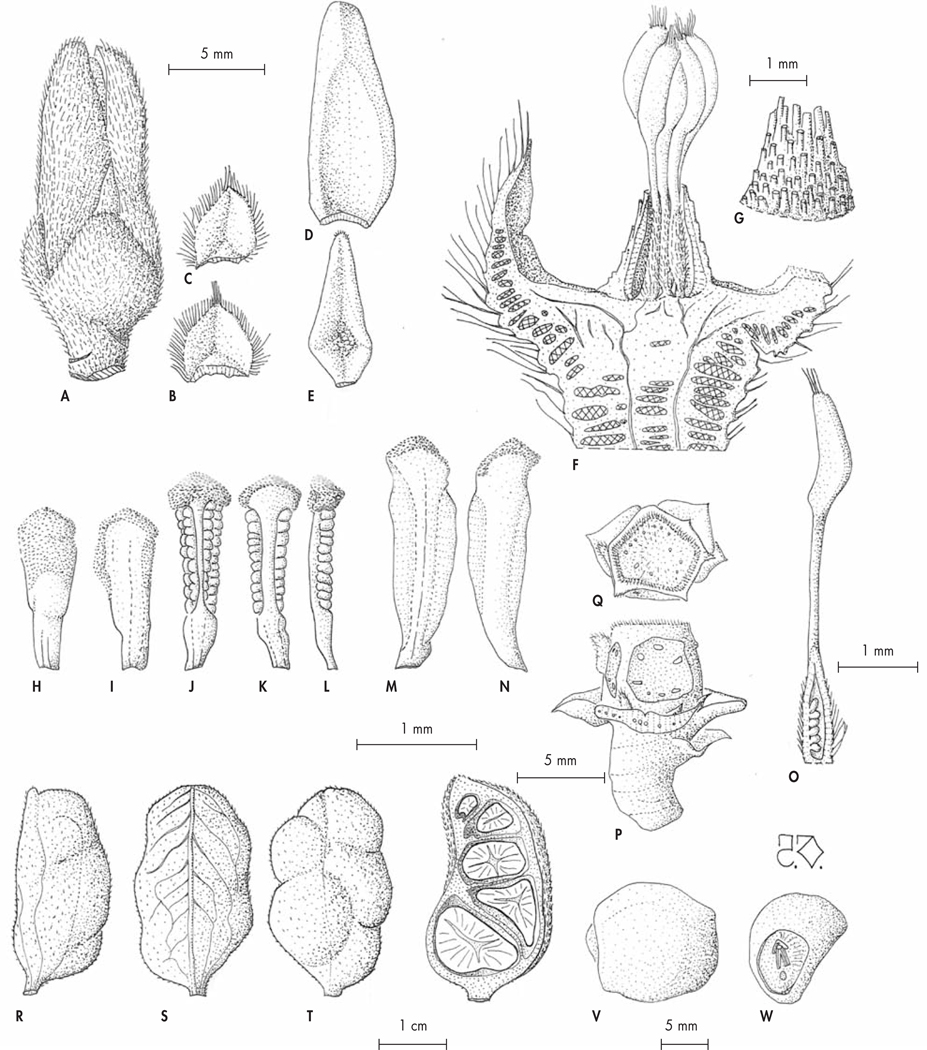
Fig. 2.
Fruiting branch of Xylopia kalabenonensis D. M. Johnson, Deroin & Callm. [Photo: J. Razafitsalama]
Fig. 3.
Flowering branch of Xylopia kalabenonensis D. M. Johnson, Deroin & Callm. [Photo: J. Razafitsalama]
Fig. 4.
Xylopia kalabenonensis D. M. Johnson, Deroin & Callm. A. Floral bud just before anthesis, bracts removed; B–C. Lower and upper bracts; D–E. Outer and inner petals (adaxial surface); F. Receptacle and gynoecium in lengthwise section; G. Detail of the outer surface of the androecial sleeve; H–I. Inner staminode (abaxial and adaxial surfaces); J–L. Stamen (abaxial, adaxial and lateral sides); M–N. Outer staminode (abaxial and adaxial surfaces); O. Carpel, with a lengthwise section of ovary; P–Q. Fruit axis, with remnant upper bract and two sepals (side and top views); R–T. Monocarp (lateral, abaxial, and adaxial surface); U. Lengthwise section of another monocarp; V–W. Seed (lateral side, detail of the hilum).
[Razafitsalama, Torze & Toninjama 1041, P] [Drawings: T. Deroin]
Shrubby tree 6 m tall, dbh 8 cm. Twigs soon lenticellate, initially densely brown-hispid, the trichomes 1.2–2 mm long and persistent on older dark brown twigs; double-branching occasional. Leaves lamina oblong, (larger ones) 8.4–9 × 2.8–2.9 cm, subcoriaceous, apex short-acuminate, the acumen 6–8 mm long, margins slightly revolute, base rounded, glabrous above except for dense erect trichomes on the midrib, trichomes erect below (simple, 4–8-celled, very attenuate, whitish in the basal one-fourth); midrib strongly impressed above, raised and conspicuous below; secondary veins 12–13 per side, irregularly brochidodromous, at midpoint of leaf diverging at nearly 90° from the midrib; secondary and higher order veins indistinct above, raised and conspicuous below; petiole 3–4 mm long, flattened but not canaliculate above, densely hispid. Inflorescences 1-flowered, axillary; pedicels 3 mm long, densely pubescent, 1–2-bracted, the bract circa 3.5 × 3 mm. Buds oblong, obtuse. Sepals half-connate at base, 2.6–4.5 mm long, 4 mm wide, ovate, acute, hispid externally, glabrous internally. Petals silvery sericeous externally; outer petals spreading at anthesis, 13 mm long, 4.5 mm wide at the base, 4 mm wide at midpoint, lanceolate-oblong, apically obtuse; inner petals erect at anthesis, circa 8 mm long, lanceolate, triangular in apical 1/3, keeled externally, apex bluntly acute, base concave and somewhat corrugated, pubescent externally down to point of maximum width (circa 3.5 mm). Stamens circa 300 (about 15% staminodes), 2 mm long, clavate to narrowly oblong, apex of connective hemispheric; filament rudimentary, circa 0.5 mm long; outer staminodes subequal in length to fertile stamens, clavate, flattened and subsessile; inner staminodes shorter than fertile stamens, flattened, oblong; staminal cone 2.7–2.8 mm in diam., 2 mm high, narrowly conical. Carpels 8, circa 3 mm high; stigmas trullate, blunt and firmly intertwined at the apex by long trichomes; ovules circa 6 per carpel, interdigitated to form a single row; torus 3.5–3.7 mm in diam. Fruit a cluster of monocarps, presumably dehiscent (not observed) ; pedicel 5–7 mm long, 3.5 mm thick at midpoint, pubescent, wrinkled; bracts and calyx more or less persistent ; torus 5 mm in diameter, 5 mm high, pubescent. Monocarps 3–5, shortly stipitate (circa 2 mm), 3–3.5 cm long, 1.5–2 cm wide, oblong, abaxially somewhat flattened, gibbous, longitudinally wrinkled, apex rounded or with a short beak, velutinous ; pericarp 0.4 mm thick. Seeds 3–5 per monocarp, oblique to long axis of monocarp, 7–7.6 mm long, 4.3–4.8 mm wide, 3.8–4.1 mm thick, ellipsoid, elliptic in cross-section, rounded at chalazal end and sometimes flattened from contact with adjacent seed, obliquely truncate across the hilum/micropyle, smooth, light brown, not shining; raphe indistinct, flush with surface of seed; micropylar scar sunken or plane on one side and raised and keeled on the other, scar 1.7–1.8 × 0.8–1 mm, oblong or elliptic.
Distribution and ecology
Xylopia kalabenonensis is only known on the sandstone rocky areas of the Kalabenono massif in montane evergreen humid forests (800–900 m).
Conservation status
With just one collection from a restricted and threatened forest, an AOO of 9 km2, and a single subpopulation constituting a single location not encompassed within a protected area, X. kalabenonensis is assigned a preliminary status of “Critically Endangered” (CR A3c; B2ab(iii)).
Notes
The species described here most closely resembles X. capuronii Cavaco & Keraudren from the northeastern coast of Madagascar in its densely pubescent twigs and pedicels, as well as broad petals, but differs in its stiff spreading brown indument, chartaceous rather than coriaceous leaves, more slender outer petals, and monocarps shortly ellipsoidal but distinctly stipitate. The petal orientation at anthesis, with the outer parts widely spreading and the inner ones erect, is similar to that found in the east African species X. mwasumbii D. M. Johnson (Johnson & al., 1999) and different from that observed in most other Madagascar species, in which all six petals are erect with the tips of the inner petals squarrose and emerging through gaps between the outer petals.
Another specimen from Kalabenono (Razafitsalama & al. 1055) differs in several ways from the material assigned here to the new species: the indument is shorter and more lax, the leaf blade is lanceolate, cuneate at the base, slightly longer, and punctate above, the inflorescences are sometimes 2-flowered, the pedicel is slightly longer (5 mm), and the staminal cone is smaller (1.7 mm in diameter). This collection could represent another new species but the material currently available is not sufficient to describe it.
Araliaceae
The paleotropical genus Polyscias J. R. Forst. & G. Forst. comprises circa 210 species (Plunkett & al., in press) and has major centres of diversity in the islands of the Pacific and the western Indian Ocean (Plunkett & al., 2001; Lowry & al., 2004). Based on the results of a recent molecular phylogenetic study (Plunkett & Lowry, in press), the circumscription of the genus is now being expanded to include members of several segregate genera (Lowry & Plunkett, in press), including two present in Madagascar, Cuphocarpus Decne. & Planch. and Gastonia Baill., both of which were recognized in the most recent treatments by Bernardi (1971, 1979). About 115 species of Polyscias are currently known from Madagascar, all but two endemic; more than 80 of these species have not yet been described (Lowry & Plunkett, in press).
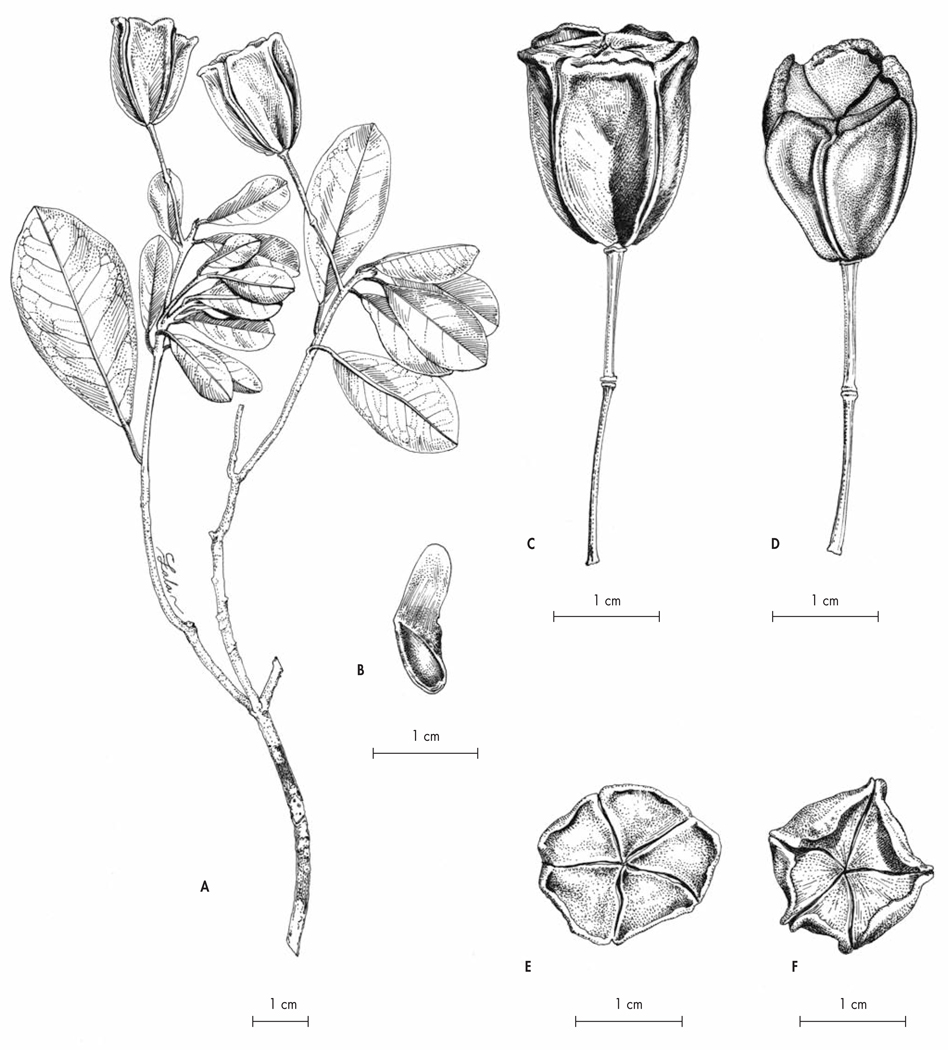
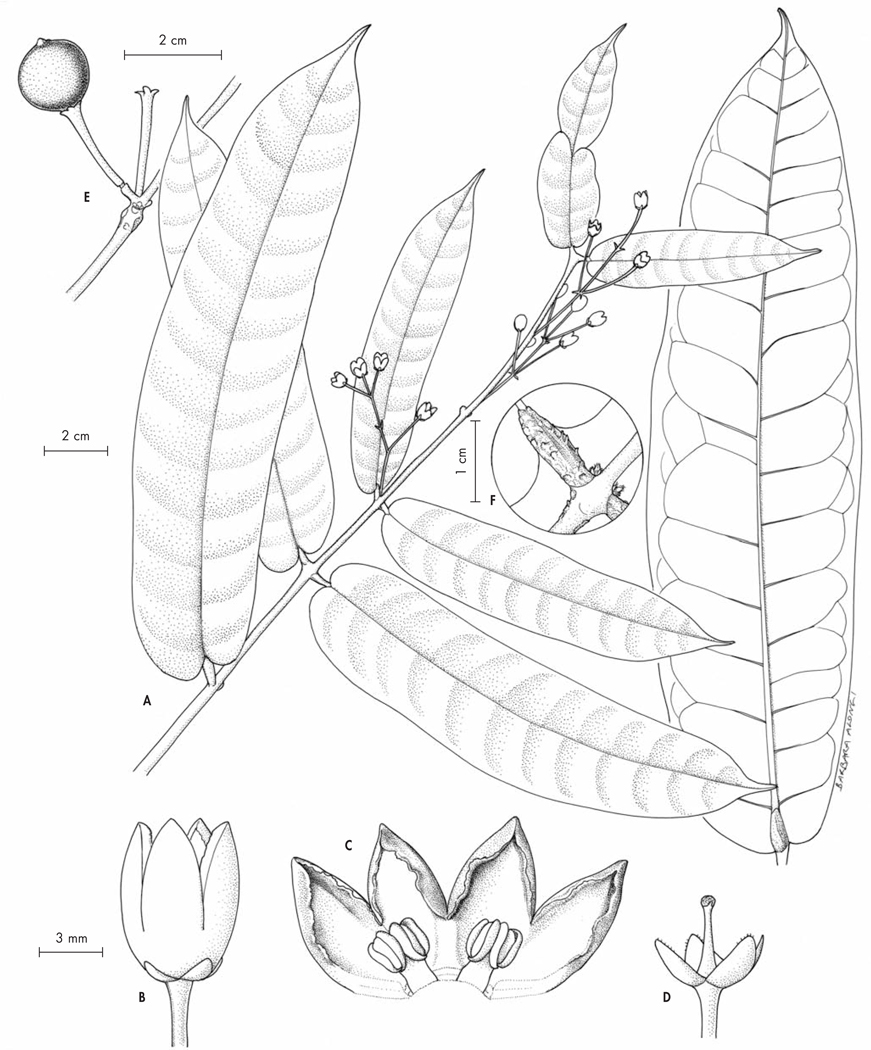
Polyscias kalabenonensis Lowry & Callm., spec. nova (Fig. 5)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambilobe, commune de Beramanja, Anketrabe, forêt du Kalabenono, 13°38’45”S 48°40’45”E, 934 m, fr., 27.XI. 2006, Callmander, Jo Vasaha & Malaza 634 (holo-: MO!; iso-: G!, K!, P!, TAN!).
Haec species a congeneris madagascariensibus foliolis coriaceis, inflorescentiae axe primario pendulo, axibus secundariis 8–10 quoque umbellulas 4–8-floras per longitudinem aequaliter ferente atque fructu immaturo (maturo ignoto) ellipsoideo usque subgloboso stylis brevibus basi crassis apicem versus attenuatis coronato distinguitur.
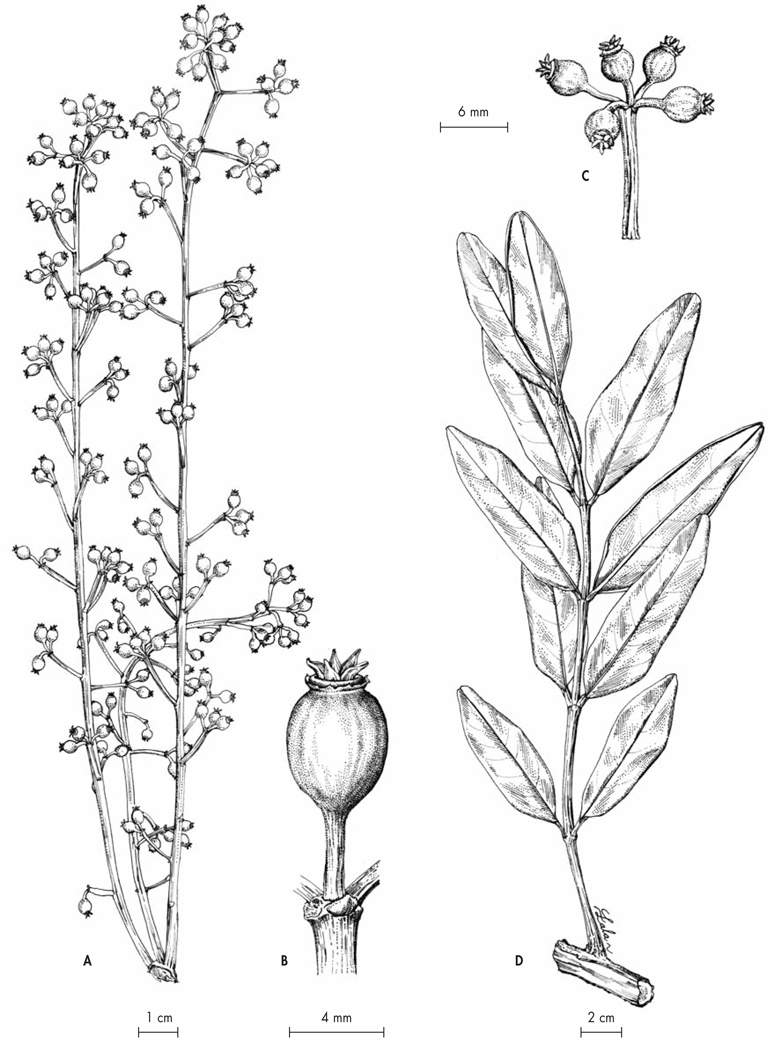
Fig. 5.
Polyscias kalabenonensis Lowry & Callm. A. Infrutescence; B. Detail of a fruit; C. Detail of an infrutescence; D. Leaf. [A–D: Callmander, Jo Vasaha & Malaza 634, P] [Drawings: R. L. Andriamiarisoa]
Shrub to small tree, 2–6 m tall, hermaphroditic, evergreen, unarmed, trunk unbranched below, 2–5 cm dbh, sparsely branched toward apex. Leaves imparipinnately compound, 35–40 cm long. Leaflets 7 or 9, lateral ones opposite, blades dark green above, usually shiny, paler beneath, coriaceous, narrowly ovate, sometimes narrowly elliptic, 8.5–21 × 3–7 cm (proximal ones slightly smaller), glabrous, secondary veins 10–15 per side, very weakly raised on both surfaces, nearly obscure, base cuneate to attenuate, margins entire, thickened and minutely to moderately revolute, apex rounded-acute to narrowly acute; petiolules 10–15 mm long; rachis weakly articulated at petiolule bases; petiole 5–9 cm long, 4–4.5 mm in diam., the clasping base with a scarious margin 0.5–1.5 mm wide. Inflorescence terminal, a panicle of umbellules, glabrous, primary axis pendant, circa 30–50 cm long; secondary axes circa 8–10, 23–30 cm long, each with 15–30 umbellules borne evenly throughout their length; peduncles 5–15 mm long in flower, 12–23 mm in fruit; bracts caducous (not seen), leaving an evident scar; umbellules with 4–8 flowers; involucre usually inconspicuous or caducous; pedicels 2–3.5 mm long in flower, 2–10 mm in fruit and thickening to circa 1 mm in diam., medium green, glabrous, with an obscure articulation below the ovary. Calyx a low scarious rim, entire or occasionally with a few small teeth or irregularly undulate. Corolla ovoid in bud, circa 2 mm in diam. prior to anthesis; petals 5, narrowly ovate, circa 2–2.5 mm long, light green, reflexed at anthesis. Stamens 5, erect at anthesis; anthers light yellow. Ovary 4–5-carpellate. Fruit a drupe, ellipsoid to subglobose when young (mature fruits unknown), 3–4 mm tall, 3–4 mm wide, medium green mottled with light green marks, glabrous, base and apex rounded, calyx a low rim 0.2–0.5 mm high, tinged dark red, disc depressed hemispheric, styles spreading, circa 0.8 mm long, circa 0.3 mm thick at base, tapering toward apex, ribs not evident; pyrenes (4−)5, each 1-seeded.
Distribution and ecology
Polyscias kalabenonensis is only known on the rocky sandstone summit areas of the Kalabenono massif in mid-elevation, evergreen, subhumid forest (900–1000 m).
Conservation status
With only 4 collections known from this highly threatened area, an EOO of no more than 100 km2, an AOO of 18 km2 and only one subpopulation constituting a single location not encompassed within a protected area, P. kalabenonensis is assigned a preliminary status of “Critically Endangered” (CR A3c; B1ab(iii)).
Notes
Polyscias kalabenonensis can be distinguished from other members of the genus in Madagascar by its coriaceous leaflets, pendant inflorescence with 8–10 secondary axes each of with 4–8-flowered umbellules borne throughout its length, and young fruits that are ellipsoid to subglobose (mature fruits are not known) surmounted by short, thick, tapering styles.
Paratypi
Madagascar. Prov. Antsiranana: Massif du Kalabenono, au N de Belinta, forêt de Kalabenono, sommet rocheux, 13°38’51”S 48°40’32”E, 818 m, buds, 23.XI.2006, Callmander, Jao Vazaha & Malaza 564 (G, K, MO, P, TAN); Préfecture d’Ambilobe, commune de Beramanja, Anketrabe, massif du Kalabenono, forêt de basse et moyenne altitude, 13°39’04”S 48°40’07”E, 803 m, fr., 2.X.2007, Rakotovao & Callmander 3881 (G, P, TAN, MO) ; Forêt de Kalabenono, chaîne de Galoka, 7 km au SE d’Anketrabe, 13°38’59”S 48°40’ 41”E, 785 m, imm. fr., 24.XI.2006, Razafitsalama & Torze 1125 (G, MO, TAN).
Polyscias pachypedicellata Lowry&Callm., spec. nova (Fig. 6)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambilobe, commune de Beramanja, chaîne du Galoka, massif du Kalabenono, 13°38’40”S 48°40’18”E, 632 m, fr., 23.XI.2007, Callmander, Rakotovao & Jo Vasaha 708 (holo-: MO!; iso-: G!, K!, P!, TAN!).
Haec species a congeneris madagascariensibus systemate sexuali ut videtur andromonoico atque pedicellis in fructu usque ad 1.2 mm diam. incrassatis distinguitur.
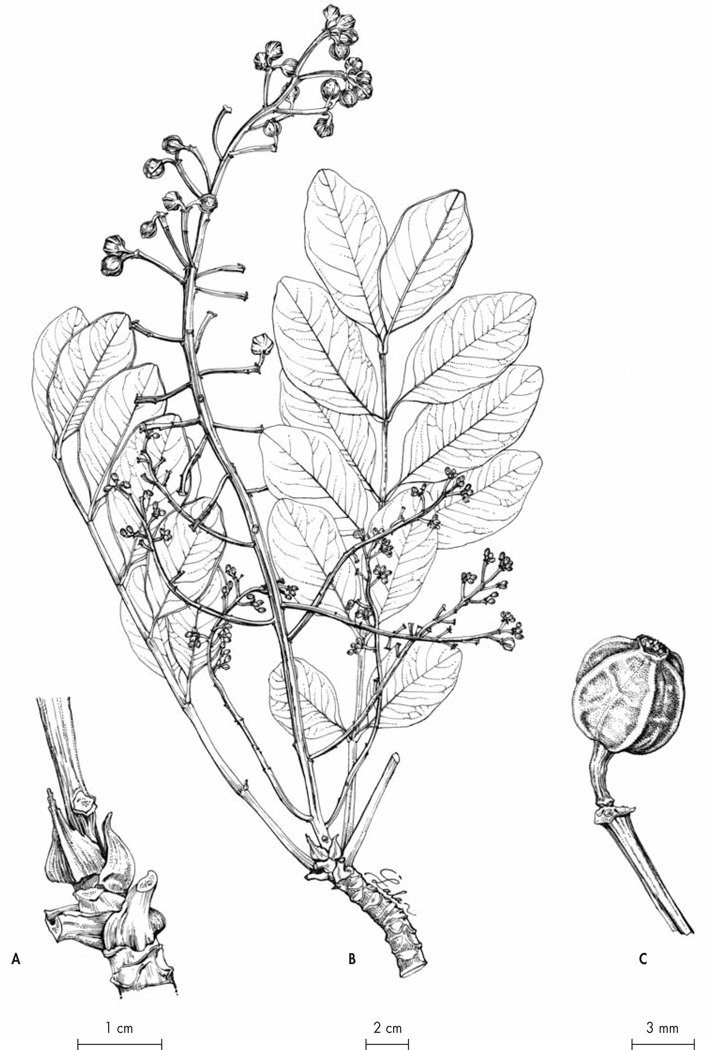
Fig. 6.
Polyscias pachypedicellata Lowry & Callm. A. Base of the leaves on the branch; B. Fruiting branch with leaves; C. Detail of a fruit. [A–C: Razafitsalama & Torze 1112, P] [Drawings: R. L. Andriamiarisoa]
Tree, unarmed, 6–15 m tall, andromonoecious (or possibly hermaphroditic), evergreen, trunk unbranched below, unarmed, 10–20 cm dbh, sparsely branched toward apex. Leaves imparipinnately compound, 30–60 cm long. Leaflets 9–15 (almost always odd in number), lateral ones opposite, blades medium green above, usually shiny, slightly paler beneath, thick chartaceous, elliptic to obovate, 6–14 × 3–7 cm, (proximal ones slightly smaller), glabrous, secondary veins 10–12 per side, raised on both surfaces, base cuneate to attenuate, margins entire, minutely to moderately revolute, apex rounded or acute to short acuminate; petiolules 8–12 mm long; rachis weakly articulated at petiolule bases; petiole 5.5–9 cm long, 4–4.5 mm in diam., the weakly clasping base with a slightly scarious margin to 1 mm wide. Inflorescence terminal, a panicle of umbellules, glabrous, primary axis erect, circa 40–70 cm long; secondary axes circa 30–40, the basal ones 23–30 cm long, progressively shorter toward the apex, each with 20–30 umbellules borne evenly throughout their length, the basal ones apparently abortive, leaving an evident scar; peduncles 7–20 mm long in flower, to 25 mm in fruit, nearly always with 1 or 2 scars below the apex, each left by a caducous flower and its subtending broadly ovate bract ; umbellules with 5–8 flowers ; involucre caducous, bracts broadly ovate; pedicels 1–2 mm long, 0.7–1 mm in diam. in flower, to 4.5 mm in fruit, thickening to circa 1.2 mm in diam., very pale green, glabrous, with an articulation below the ovary. Calyx a scarious undulate rim, entire or somewhat lacerate. Corolla narrowly ovoid in bud, circa 2–2.5 mm in diam. prior to anthesis; petals 5, narrowly ovate, circa 3–3.5 mm long, greenish white, spreading at anthesis. Stamens 5, erect at anthesis ; anthers whitish. Ovary 5-carpellate, pale green, glabrous, globose in flower. Fruit a drupe, globose, 4.5–7 mm in diam., medium pale green when immature, turning reddish purple at maturity, contrasting with the whitish pedicel, glabrous, base and apex rounded, articulation obscure, forming an indistinct ring near the base of the fruit above the point of insertion of the pedicel, calyx a low rim 0.2 mm high, disc slightly concave, styles short, spreading, not exceeding 0.5 mm, ribs evident; pyrenes 5, each 1-seeded.
Etymology
The species epithet refers to the thickened pedicels of both the flowers and fruits, a feature that distinguishes P. pachypedicellata from other members of the genus in Madagascar.
Distribution and ecology
Polyscias pachypedicellata has only been recorded from the rocky sandstone summit area of the Kalabenono massif, where it occurs in mid-elevation subhumid forest (900–1000 m).
Conservation status
With only 4 collections known from this highly threatened area, an EOO of no more than 100 km2, an AOO of 18 km2 and only 1 subpopulation constituting a single location, which is not encompassed within a protected area, P. kalabenonensis is assigned a preliminary status of “Critically Endangered” (CR A3c; B1ab(iii)).
Notes
In addition to its distinctive thick pedicels, P. pachypedicellata can also be distinguished from other Malagasy members of the genus by its sexual system, which appears to be andromonoecious based on examination of the available material.
Paratypi
Madagascar. Prov. Antsiranana: Chaîne Galoka, Mont Galoka, Fokontany Anketrabe-Belinta, 13°38’ 40”S 48°40’18”E, 632 m, 23.XI.2007, Callmander, Buerki & Manjaribe 709 (G, P, MO, TAN) ; Préfecture d’Ambilobe, commune de Beramanja, Anketrabe, massif du Kalabenono, versant NW du Kalabenono, 13°38’37”S 48°40’21”E, 704 m, 24.XI.2007, Rakotovao, Callmander & Torze 3775 (MO, TAN); Chaîne de Galoka, forêt de Kalabenono, 9 km au SE d’Anketrabe, 13°38’30”S 48°40’53”E, 976 m, 23.XI.2006, Razafitsalama & Torze 1112 (G, MO, P, TAN).
Polyscias wohlhauseri Lowry & Callm., spec. nova (Fig. 7)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambilobe, commune de Beramanja, chaîne du Galoka, Anketrabe-Belinta, Mt. Galoka, Galoka sommet, 13°35’ 36”S 48°43’53”E, 1153 m, fr., 9.XI.2004, Wohlhauser & Patrick 707 (holo-: G!; iso-: K!, MO!, NY!, P!, TAN!).
Haec species quoad pedicellum sub ovario articulo cupuliformi pseudocalycem formante praeditum P. ornatae (Baker) Harms similis, sed ab ea foliolis majoribus atque inflorescentia diffusiore ex axibus floribusque pauciioribus constante distinguitur.
Fig. 7.
Polyscias wohlhauseri Lowry & Callm. A. Detail of a fruit; B. Fruiting branch with leave.
[A–B: Wohlhauser & Patrick 707, P] [Drawings: R. L. Andriamiarisoa]
Shrub to small tree, 3–8 m tall, hermaphroditic(?), evergreen, unarmed, trunk sparsely to moderately branched, 2–8 cm dbh. Leaves imparipinnately compound, 12–24 cm long. Leaflets 3, 5 or 7 (leaves rarely unifoliolate), the lateral ones opposite, blades bright green above, shiny (especially in young foliage), paler olive yellow green beneath, chartaceous to subcoriaceous, obovate or occasionally nearly elliptic, proximal ones 5–7.5 × 1.5–3 cm, distal ones 10–13 × 4–6 cm, glabrous, secondary veins 5–8 per side, weakly raised on both surfaces, tertiary veins obscure, base attenuate, margins entire, minutely thickened and revolute, apex rounded, rarely broadly acute; petiolules to 5 mm long or leaflets sessile; rachis weakly articulated at petiolule bases; petiole 1.5–4 cm long, 2–2.5 mm in diam., the weakly clasping base with a minute scarious margin. Inflorescence terminal, a loose open panicle of umbellules, glabrous, primary axis to circa 10 cm long; secondary axes 2–4, 7–12 cm long, or primary axis highly reduced and secondary axes appearing to be born together from the branch tip, umbellules 4–6 per secondary axis, most or all in a terminal umbel, occasionally 1–2 borne along the axis; peduncles 20–40 mm long; involucral bracts unknown, caducous; umbellules with 3–7 fruits; pedicels 13–15 mm long, 0.6–0.8 mm in diam. in fruit, glabrous, with an evident cupuliform articulation (pseudocalyx) below the ovary. Flowers unknown, ovary 4–5-carpellate. Fruit a drupe, ellipsoid to weakly obovoid or nearly globose, 5–7.5 mm tall, 4–7.5 mm wide, glabrous, base broadly acute to nearly rounded, apex rounded, calyx a low rim < 0.5 mm high, disc flat, styles divergent, circa 0.5 mm long, broadly conical, ribs 4–5, inconspicuous when fresh, evident when dry; pyrenes 4–5, each 1-seeded.
Etymology
The species is named in honour of Sébastien Wohlhauser, who collected the type material and co-organized the first field expedition to Mt Galoka in 2005 along with MWC and SB.
Distribution and ecology
Polyscias wohlhauseri is only known from the rocky sandstone summit area of the Kalabenono massif in mid-elevation subhumid forest (900–1000 m).
Conservation status
With only 4 collections known from this highly threatened area, an EOO of no more than 100 km2, an AOO of 18 km2 and only 2 subpopulations constituting a single location, neither of which is within a protected area, P. wohlhauseri is assigned a preliminary status of “Critically Endangered” (CR A3c; B1ab(iii)).
Notes
Polyscias wohlhauseri resembles the widespread P. ornifolia (Baker) Harms in having a distinctive cupuliform articulation below the ovary forming a pseudocalyx, but differs by its larger leaflets (the largest ones reaching 10–13 × 4–6 cm in P. wohlhauseri vs 6.5–9(−10) × 3.4–4(−4.5) cm in P. ornifolia) and a more open, diffuse inflorescence with fewer axes and flowers.
Paratypi
Madagascar. Prov. Antsiranana: Chaîne Galoka, Mt. Galoka, Fokontany Anketrabe-Belinta, 13°35’20”S 48°43’43”E, 23.II.2005, 930 m, Callmander, Buerki & Manjaribe 365 (G, MO, TAN, P); préfecture d’Ambilobe, commune de Beramanja, Anketrabe, massif du Kalabenono, sur le plateau le plus haut du Kalabenono, 13°39’26”S 48°40’14”E, 1090 m, 4.XII.2007, Rakotovao & al. 3908 (G, K, P, TAN, MO) ; forêt de Kalabenono, chaine de Galoka, 7 km au SE d’Anketrabe, 13°38’59”S 48°40’41”E, 785 m, 24.XI.2006, Razafitsalama & Torze 1114 (G, MO, TAN).
Malvaceae
Nesogordonia Baill. is an Afro-Malagasy genus with 18 species currently recognized, fourteen of which are endemic to Madagascar (Barnett, 1987, 1988), three to Africa and one to the Comores Islands (Labat & al., 2000). Members of the genus can be easily recognized by their woody capsulate fruit bearing winged seeds.
Nesogordonia rakotovaoi Rakotoar., Andriambolol. & Callm., spec. nova (Fig. 8)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambilobe, commune de Beramanja, chaîne du Galoka, versant NW du massif du Kalabenono, 13°39’10”S 48°40’ 29”E, 1007 m, fr., 29.XI.2007, Rakotovao, Jao Vasaha, Ancelme, Torze & Alexi 3837 (holo-: MO!; iso-: G!, K!, P!, TAN!).
Haec species a N. abrahamii Barnett et N. bernieri Baill. lamina folari obovata usque elliptica glabrescente domatiis carentibus atque capsula apice concava trichomatibus stellatis parce vestita lateribus ornamento manifeste notata distinguitur.
Fig. 8.
Nesogordonia rakotovaoi Rakotoar., Andriambolol. & Callm. A. Fruiting branch; B. Seed; C–D. Fruit details; E–F. Fruit from above.
[A–C, E: Rakotovao, Jao Vasaha, Ancelme, Torze & Alexi 3837, TAN; D, F: Callmander, Razafitsalama, Jao Vazaha & Malaza 637, TAN] [Drawings: R. L. Andriamiarisoa]
Small tree to 3–5 m tall; young stems with sparse short stellate trichomes; old stems glabrous, striate, brown to greyish, bearing ± prominent leaf scars; bark fibrous, with many prominent lenticels. Leaves alternate, persistent ; petiole 2–5 mm long; blade obovate to elliptic, 2.5–4.2 × 1–2.2 cm, coriaceous, discolorous, with minute stellate trichomes above, glabrous below, base obtuse to broadly cuneate, margins entire, shallowly undulate and weakly revolute, apex rounded and sometimes mucronulate or rarely retuse; venation conspicuous on both surfaces, but especially below, midrib prominent below, prolonged at the apex of the blade into a very short mucro, secondary veins 5–7 on each side, anastomosing towards the margin, the veinlets forming a dense polygonal network; domatia absent. Flowers unknown. Infrutescence 1-fruited, axillary, axes 2–2.7 cm long, densely tomentose; pedicel articulated 8–12 mm below the fruit. Fruit an obconic capsule, brown, sparsely covered with short stellate indument, ribbed towards the base, 17–22 × 12–15 mm, with prominent ornamentation 2 mm high on the edges; apex concave with a very short central umbon; rim raised, well-defined and angular. Seeds obovoid, winged, circa 7–9 × 3–5 mm; wings narrow, obtuse, 7–8 mm long, 4–5 mm wide.
Etymology
The species is dedicated to Charles Rakotovao, a botanist working for the Missouri Botanical Garden in Madagascar, who collected the type material. He has contributed significantly to improving our knowledge of the northern mountains region, thanks in large part to the more than 1000 plant specimens he has collected there.
Distribution and ecology
Nesogordonia rakotovaoi is known from only two collections from the rocky sandstone summit of the Kalabenono massif in subhumid forest (900–1000 m).
Conservation status
With just two collections known from a restricted and threatened forest, an EOO of no more than 100 km2, an AOO of 18 km2, and only one subpopulation constituting a single location, which is not encompassed within a protected area, N. rakotovaoi is assigned a preliminary status of “Critically Endangered” (CR A3c; B1ab(iii)).
Notes
Among the members of the genus known from Madagascar, N. rakotovaoi most closely resembles N. abrahamii Barnett and N. bernieri Baill. The new species differs, however, in having glabrescent obovate to elliptic leaves (10–22 × 25–42 mm) lacking domatia (vs densely stellate-pubescent and bearing domatia in N. abrahamii, and oblong to lanceolate measuring 15–40 × 40–120 mm in N. bernieri). Neogordonia rakotovaoi is a small tree growing to no more than 5 m tall, whereas both N. abrahamii and N. bernieri can become considerably larger trees 10–15 m high. The capsule of N. rakotovaoi bears a concave apex with prominent ornamentation on the lateral part and is sparsely covered with stellate indument (vs concave and densely stellate in N. abrahamii and with a flat to convex (nearly retuse) apex and densely stellate in N. bernieri).
Paratypus
Madagascar. Prov. Antsiranana: Kalabenono, au N de Belinta, sommet nord-ouest, ouest de la grande falaise, 13°38’45”S 48°40’45”E, 934 m, fr., 27.XI.2006, Callmander, Razafitsalama, Jao Vasaha & Malaza 637 (TAN).
Hibiscus L
The genus Hibiscus s.l. is characterized by a 5-locular ovary, 5 apical teeth on the staminal column and 5 or 10 stylar branches that terminate in globose stigmas. According to the most recent revision (Hochreutiner, 1955), 46 species of Hibiscus are found on Madagascar, 31 of which are endemic to the island. Members of this genus and taxa belonging to several closely related endemic genera appear to have arrived on Madagascar by at least two separate colonization events (Koopman & Baum, 2008).
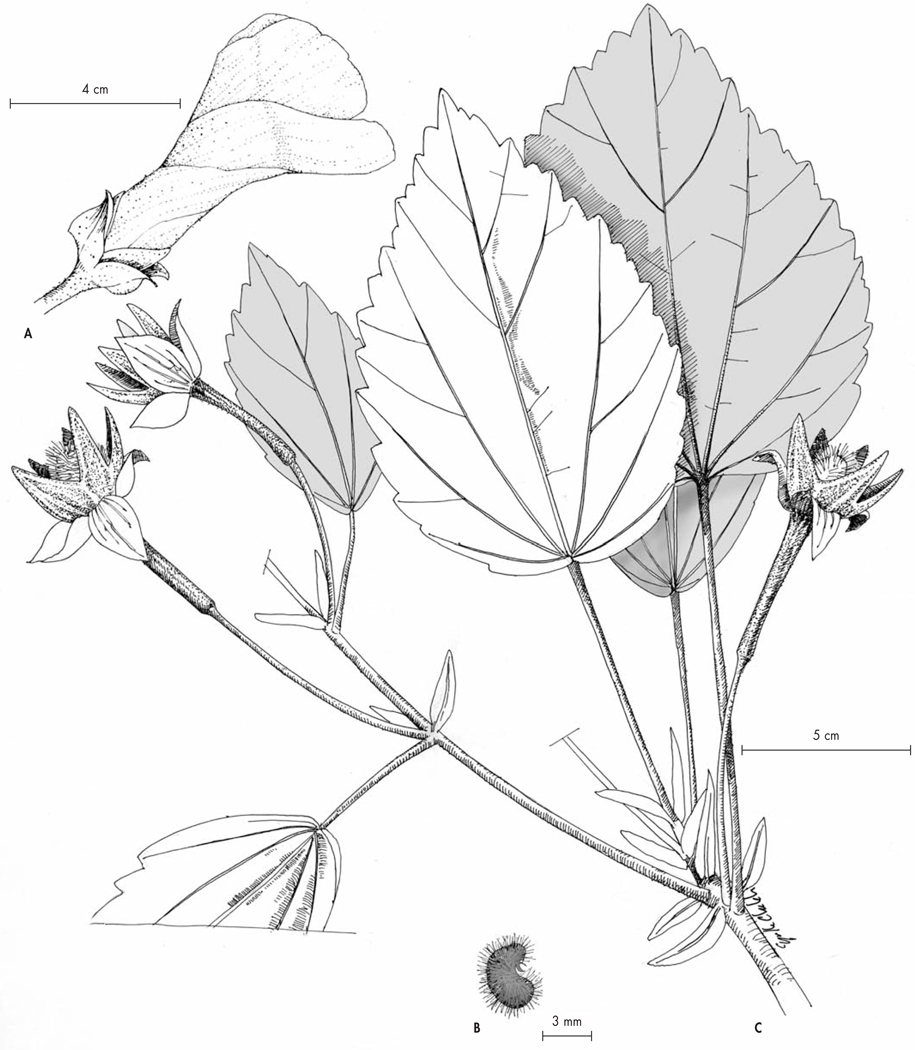
Hibiscus lamalama Callm., Buerki & Koopm., spec. nova (Fig. 9)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambilobe, commune de Beramanja, Anketrabe, forêt du Kalabenono, 13°38’50”S 48°40’34”E, 778 m, fr., 23.XI. 2006, Callmander, Razafitsalma & Torze 580 (holo-: P!; iso-: G!, K!, MO!, TAN!, WIS!).
Haec species a H. grandistipulatus (Hochr.) Hochr. et H. ankaramyensis Hochr. stipulis oblanceolatis marginibus revolutis, lamina foliari ovata scabra glabra vel parce pubescente, pedunculo cum pedicello 10–13 cm longo, epicalycis segmentis 4 ovatis atque petalis flavis distinguitur.
Fig. 9.
Hibiscus lamalama Callm., Buerki & Koopm. A. Flower; B. Seed; C. Fruiting branch.
[A–B: Callmander, Razafitsalma & Torze 580, P; C: Rakotovao & al. 4463, TAN] [Drawings: C. Chatelain]
Tree 15 m tall, trunk 10 cm in diameter. Stipules foliaceous, persistent, oblanceolate, 1.5–3 × 0.5 cm, margin revolute. Leaves 11–15 × 7–12 cm, simple, alternate, ovate, base cuneate to subcordate, margins crenate-dentate, slightly revolute, apex weakly acuminate, blade discolorous, abaxial surface scabrous with sparse stellate trichomes, adaxial surface tomentose with sparsely distributed stellate trichomes, venation palmate, raised on the adaxial surface, covered with minute stellate trichomes, base of veins each with a black auriculate appendage (perhaps domatia) 1–1.5 × 2–2.5 mm; petiole 7–13 cm, sparsely covered with stellate trichomes. Flowers axillary, solitary or geminate, peduncle and pedicel combined 5–6 cm long in flower and 10–13 cm in fruit, peduncle with sparse stellate indument, articulated one-third of distance from base, pedicel with dense stellate indument, enlarged at flower base. Corolla infundibuliform, petals 8–9 cm long, yellow, sparsely pubescent at the base adaxially, staminal column ca. 5 cm long, bearing stamens on the lower two-third of the staminal column in an indiscernable whorl, stigmatic lobes 5. Epicalyx lobes 4, free to base, ovate, acuminate, 1.2–1.5 × 0.8–1 cm, abaxially with stellate indument at base, dark brown; sepals 5, 2.5–3 × 0.8–1.2 cm, ovate, united at the base, coriaceous, with stellate indument abaxially, pubescent with appressed white trichomes on the inner surface, yellowish. Fruit a dry ovoid capsule, 2 × 1.5 cm, densely pubescent with erect brown trichomes, 4 mm long. Seeds circa 8 per locule, baculiform, densely covered with brownish to reddish trichomes, 4–5 × 1.5 mm.
Etymology
This species is named after a ritual that takes place on Kalabenono mountain, which has spiritual significance for the local Sakalava-Antakarana people and refers to an important local queen named “Ampanjakakalabenono”. As legend has it, upon the queen’s death the mountain took on her shape: Kalabenono means ‘woman with large breasts’, which aptly describes its distinctive form. Today local people continue to celebrate a ritual, called lamalama in their dialect. This specific epithet honours the Royal spirit embodied in the form of the mountain.
Distribution and ecology
Hibiscus lamalama is only known from one collection in the sandstone massif of Kalabenono along a river in mid-elevation subhumid forest (800 m).
Conservation status
With a single collection in a restricted and threatened forest, an AOO of 9 km2, and only one subpopulation constituting a single location, which is not encompassed within a protected area, H. lamalama is assigned a preliminary status of “Critically Endangered” (CR A3c; B2ab(iii)).
Notes
Hibiscus lamalama appears to be closely related to two other species of Malagasy Hibiscus that grow in the Sambirano region and also bear large leaves and flowers: H. ankaramyensis Hochr. and H. grandistipulatus (Hochr.) Hochr. These taxa were formerly assigned to Hibiscus sect. Spatula Hochr. but are probably members of the Euhibiscus clade to which H. lamalama most likely also belongs (Koopman & Baum, 2008). Hibiscus lamalama can be easily distinguished from its two putative relatives by the following characters: stipules oblanceolate, margins revolute, 1.5–3 × 0.5 cm (vs rounded, 8 × 9 mm in H. grandistipulatus, and caducous and minute in H. ankaramyensis) ; leaves sparsely pubescent, scabrous with non pubescent domatia (vs densely pubescent with domatia at the base in H. grandistipulatus, and soft and densely pubescent in H. ankaramyensis, Hochreutiner, 1955: 71: fig. 19-4); flowers with yellow petals, peduncle and pedicel combined 10–13 cm long in fruit, and 4 ovate epicalyx lobes (vs petals tawny, peduncle and pedicel combined 5–6 cm long in fruit, epicalyx segments 7–9, lanceolate-acute in H. grandistipulatus, and petals pink, peduncle plus pedicel combined 4 cm long, epicalyx segments 7–8, ovate-cordate in H. ankaramyensis).
Paratypi
Madagascar. Prov. Antsiranana: Kalabenono, au N de Belinta, versant Sud Ouest du massif rocheux, 13°38’37”S 48°40’03”E, 484 m, fl., 19.III.2009, Rakotovao & al. 4463 (G, MO, P, TAN).
Meliaceae
The pantropical genus Trichilia P. Browne comprises circa 70 species in the Neotropics, 14 in Africa, two in Indo-Malaysia and six in Madagascar (Leroy & Lescot, 1996), two of which have not yet been described. The genus belongs to the monophyletic sub-family Melioideae T. D. Pennington & Styles (Muellner & al., 2003) and differs from other members of this group by having loculicidal capsular fruits and arillate seeds (Pennington & Styles, 1975). Trichilia from Madagascar has never been revised in its entirety, although it was studied by Leroy & Lescot (1996) who described two new species. Leroy and Lescot (pers. comm.) also produced a draft treatment of this genus for the “Flore de Madagascar et des Comores” that remains unpublished. Unfortunately, this draft is incomplete and did not take into account of all available material of the genus. After carefully reviewing all Malagasy material of Trichilia in P and G we were able to match two new collections from Kalabenono with six older gatherings from low elevation Sambirano forest that had not been identified previously, which we describe here as a new species.
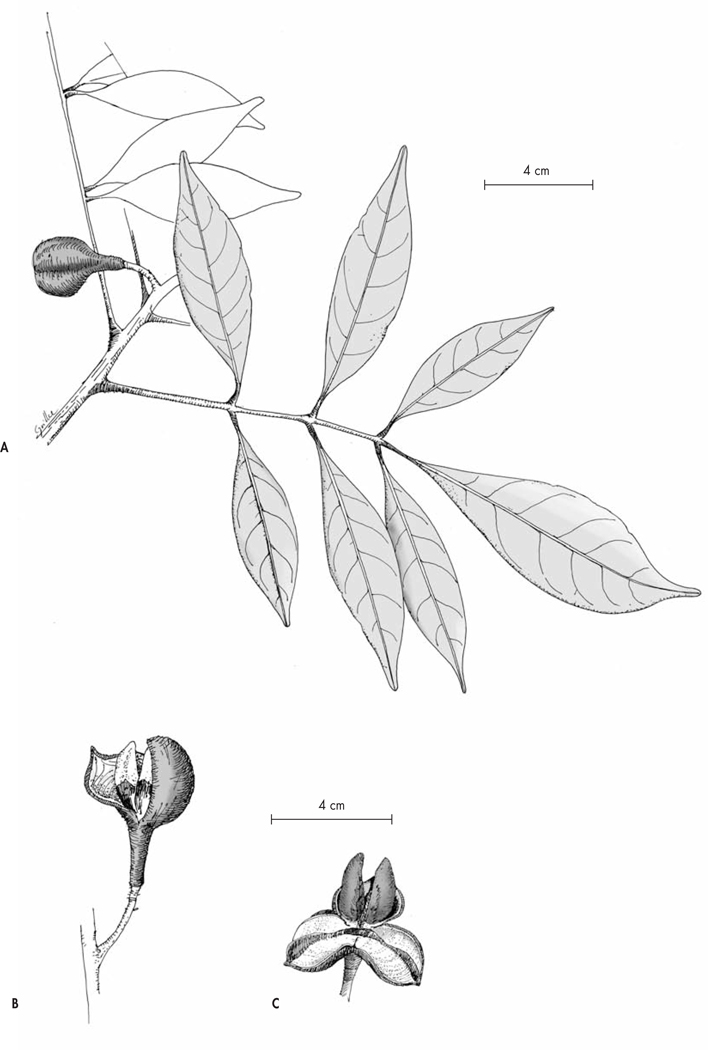
Trichilia sambiranensis Callm. & Phillipson, spec. nova (Fig. 10, 11)
Typus: Madagascar. Prov. Antsiranana: Chaîne Galoka, Mt. Galoka, Fokontany Anketrabe-Belinta, 13°35’08”S 48°42’58”E, 500 m, 13.II.2005, fr., Callmander, Buerki & Manjaribe 298 (holo-: G!; iso-: K!, MO!, P!, TAN!).
Haec species T. mucronatae (Cav.) Harms maxime affinis, sed ab ea foliis 5-foliolatis, foliolis subcoriaceis ellipticis reticulo obscuro atque fructu circa 3 × 3 cm longo inferne longe (circa 1 cm) attenuato differt.
Fig. 10.
Trichilia sambiranensis Callm. & Phillipson. A. Fruiting branch; B–C. Fruit.
[A–B: Callmander, Buerki & Manjaribe 298, G, P; C: Service Forestier 10926, G] [Drawings: C. Chatelain]
Fig. 11.
Fruiting branch of Trichilia sambiranensis Callm. & Phillipson. [Photo: M. Callmander]
Treelet 3–5 m high, stems glabrous. Leaves imparipinnate, 25–35 × 15–25 cm (including petiole); petiole 4–8 cm long, circa 2 cm in diam., glabrous, flattened on the upper surface, thickened at the base and somewhat amplexicaul. Leaflets generally 5, blade elliptic, 8–15(−19) × 2.5–5(−7) cm, sub-coriaceous, glabrous, easily broken when dry, base attenuate, margins entire, coarsely undulate, slightly revolute when dry, apex acuminate, the acumen 1–2 cm long, midrib and secondary veins prominent on the both surfaces, 7–10 pairs of alternate secondary veins, reticulation hardly visible (to the naked eye); petiolules 5–6 mm (lateral leaflets), 10–15 mm (terminal leaflets), thickened at the base, blackish, glabrous. Flowers unknown. Infructescence lateral, fruits usually solitary, rarely 2(−3); peduncle 1.5–2 cm long, thick, woody; pedicel 1–3 mm long, 2–3 mm diam. Fruit a red capsule, trigonous at maturity (subglobose when immature), circa 3 × 3 cm, with an attenuate lower portion circa 1 cm long, dehiscence loculicidal, splitting to circa two-third of its length into 3 woody valves, calyx caducous. Seeds 6 (2 per locule), 1.8–2 × 0.8–1 cm, carinate, brown, with an orange arillode covering the lower half.
Distribution and ecology
Trichilia sambiranensis is known from low elevation forest in the Sambirano region of northwestern Madagascar.
Conservation status
With an EOO of 1780 km2, an AOO of 36 km2, and 4 subpopulations, two of which are situated within two protected areas (Lokobe, Manongarivo), T. sambiranensis is assigned a preliminary status of “Endangered” (EN B1ab(iii)+B2ab(iii)).
Notes
Trichilia sambiranensis most closely resembles T. mucronata (Cav.) Harms, but differs from it by several characters. The new species bears leaves that measure (25−) 30(−35) × (15−)20(−25) cm, comprising two pairs of subcoriaceous leaflets, (vs 15–16 × 13 cm with one pair of papyraceous leaflets); the leaflets are elliptic, with obscure reticulation, 8–15(−19) × 2.5–5(−7) cm (vs ovate to lanceolate, reticulation visible, 10 × 3.5 cm) ; the fruits are circa 3 × 3 cm in size with a long attenuated lower portion measuring circa 1 cm (vs circa. 2 × 2 cm with a short attenuated lower portion circa 0.5 cm long).
Paratypi
Madagascar. Prov. Antsiranana: Lokobe RNI, 13°25’S 48°19’E, 30–100 m, 14.VI.1994, Antilahimena 140 (P, MO, TAN); Nosy Be, forêt de Lokobe, 13°24’30”S 48°18’30”E, VIII.1960, Bosser 14768 (P); Ambanja, commune de Beramanja, Kalabenono, 13°38’38”S 48°40’07”E, 520 m, 24.XI.2007, Callmander, Rakotovao & Malaza 733 (G, MO, P, TAN); Forêt de Lokobe, versant W, rive gauche de l’Andranobe, 13°24’30”S 48°18’30”E, 210 m, 6.XII.1989, Deroin & Badré 196 (P) ; Marovato, Ambanja, 13°57’S 48°33’E, 25.VIII.1954, Réserves Naturelles 6262 (G, P, MO, TAN); Maromiandra, Ambanja, 14°00’S 48°13’E, 7.X.1954, Service Forestier 10926 (G, MO, P, TAN) ; Sambirano, forêt de Lokobe, 13°24’30”S 48°18’30”E, 200 m, 1.XI.1954, Service Forestier 11408 (G, P, TAN, MO) ; Manongarivo, Besinkara, 14°04’S 48°17’E, 7.VI.1996, Totozafy Be 573 (G, K, MO, P, TAN, TEF, WAG).
Oleaceae
The genus Noronhia Thouars is endemic to Madagascar and the Comoro Islands, where it is widely distributed and highly diversified. In the most recent taxonomic treatment, which is more than fifty years old, Perrier de la Bâthie (1949, 1952) recognized one species as endemic to the Comoros and 40 species from Madagascar, all endemic and many with very restricted distributions. More recently Bosser (1973) described two additional species from Madagascar and Labat & al. (1999) a second endemic species from the Comoros. A new taxonomic revision is currently underway (Hong-Wa, unpubl.). Among the material available from the Sambirano region, two collections represent a new species only known from the Kalabenono massif.
Noronhia jeremii Hong-Wa & Callm., spec. nova (Fig. 12)
Typus: Madagascar. Prov. Antsiranana: Préfecture d’Ambanja, Commune de Beramanja, massif du Kalabenono, 13°38’38”S 48°40’07”E, 520 m, fl., 25.XI.2007, Callmander & Razafitsalama 740 (holo-: MO!; iso-: G!, K!, P!, TAN!).
Haec species a N. capuronii Bosser lamina folari lineari longiore, petiolo glabro, fructu ampulliformi usque pyriformi atque pericarpio crasso duro distinguitur.
Fig. 12.
Noronhia jeremii Hong-Wa & Callm. A. Flowering branch; B. Flower; C. Corolla from within; D. Calyx with style; E. Fruit; F. Petiole.
[A–D, F: Callmander & Razafitsalama 740, MO; E: Callmander, Jao Vazaha & Malaza 537, MO] [Drawings: B. Alongi]
Shrubs 3 m tall, entirely glabrous; bark white and suberous on old stems, exfoliating into thin papyraceous flakes on young stems. Leaves opposite, simple, entire; blades dull green above, brownish beneath, broadly linear, (10−)15(−27) × 2–3.5(−7) cm, coriaceous, base subcordate to truncate, margins entire, flat to slightly revolute, apex acuminate to cuspidate, midrib yellowish to reddish, sunken and canaliculate above, prominent beneath, secondary veins brochidodromous, indistinct to slightly distinct above, barely distinct beneath, sometimes looking like dashes, 20–28 per side, looping 0.2–0.5 cm from the margin; petiole white, suberous, canaliculate, 0.5–1.5 cm long, 0.3–0.6 cm in diam., attached on the lower surface of the leaf blade. Inflorescences cymose, axillary, 4–7.5 cm long, with 5–7 flowers; peduncle purple, slender, 0.2–2.1 cm long, pedicel purple, slender, 1–2.5 cm long. Flowers 4–9 mm long; sepals green, 4, ovate, 1.8–2.5 × 1–1.5 mm, acute, united at their base. Corolla yellow, cupulate, fleshy, lobes 4, deltoid, 5–8.5 × 2–4 mm, margin involute in the distal third, corolla tube 3 mm long, thickened at the base to surround the androecium and gynoecium, corona absent; stamens 2, filament adnate to corolla tube, 0.8 mm long, anthers 1.5 × 1.4 mm, thick, opening by a longitudinal slit. Ovary superior, ovoid, 1.3–1.5 × 0.9–1.3 mm, bilocular, ovule 1, pendant, style 2 mm long, stigma capitate, 0.3 mm wide. Fruits ampulliform to subglobose, apiculate, 1.2–1.8 × 1.6–2 cm; pericarp thick, hard; seed brown, 1, subglobose, 1 × 1.3 cm.
Etymology
This species is dedicated to Jeremi (Jimmy) Razafitsalama, a botanist working for the Missouri Botanical Garden in Madagascar and head of their office in Antsiranana, who collected the type material and was a member of the team during the second expedition in 2006.
Distribution and ecology
Noronhia jeremii is only known from two collections made in the sandstone massif of Kalabenono along rivers in low elevation subhumid forest (circa 500 m).
Conservation status
With only two collections from a restricted and threatened forest, an AOO of 18 km2, and only one subpopulation constituting a single location, which is not encompassed in the protected area network, N. jeremii is assigned a preliminary status of “Critically Endangered” (CR A3c; B1ab(iii)+B2ab(iii)).
Notes
Noronhia jeremii can be easily recognized by its long, broadly linear leaf blades, its petiole that is attached to the lower surface of the leaf blade, its flower lacking a corona, and its apiculate fruit. Because of the absence of a corona, this new species is placed within sect. Ecoronulatae H. Perrier (Perrier de la Bâthie, 1952). Noronhia jeremii appears to be related to N. capuronii Bosser, both of which lack a corona, have their petiole attached on the lower surface of the leaf blade, and have apiculate fruit. However, N. jeremii can be distinguished by a number of well-defined characters such as a linear leaf blade (vs lanceolate in N. capuronii) that is ≥ 10 cm long (vs ≤ 10 cm), a glabrous (vs pubescent) petiole, a hard (vs crustaceous) pericarp.
Paratypi
Madagascar. Prov. d’Antsiranana: Ambilobe, Beramanja, Anketrabe, forêt de Kalabenono, plateaux peu vallonnés entre crêtes, 13°38’20”S 48°40’16”E, 374 m, fr., 19.XI.2006, Callmander, Jao Vasaha & Malaza 537 (G, K,MO, P, TAN).
Acknowledgements
The authors thank the Parc Botanique et Zoologique de Tsimbazaza, ANGAP (Association Nationale pour la gestion des Aires Protégées) and the local office of the Missouri Botanical Garden (MBG) in Antananarivo for assistance in Madagascar. We are grateful to Laurent Gautier, Louis Nusbaumer and Nicolas Fumeaux for assistance at the Conservatoire et Jardin botaniques de la Ville de Genève as well as Alyse Rothrock and Zachary Rogers for help with the management of project collections at MBG in St. Louis. We thank Roy Gereau for preparing most of the Latin diagnoses, and Barbara Alongi, Roger Lala Andriamiarisoa and Cyrille Chatelain for the fine illustrations. The authors are also grateful to the curators of the herbaria in Antananarivo, Geneva, St. Louis, Neuchâtel and Paris for access to their collections. They also thank MNHN for the unpublished draft treatment of the “Flore de Madagascar et des Comores” by Leroy and Lescot. Finally, we thank Laurent Gautier and George Schatz for helpful comments on an earlier version of the manuscript. Financial support was provided to MWC by the Swiss Embassy in Antananarivo, Conservation International-Madagascar, and the National Geographic Society (grant no. 8114-06). Additional support was provided through and International Cooperative Biodiversity Group (ICBG) project (MWC Principal Investigator) jointly funded by the U.S. National Institutes of Health, National Science Foundation and Department of Agriculture (Cooperative Agreement U01 TW000313). The participation of MWC, PBP, SA and PPL was also supported by grants from the U.S. National Science Foundation (0743355; PPL Co-PI) and the Andrew W. Mellon Foundation (PBP and PPL Co-PIs).
Contributor Information
Martin W. Callmander, Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri, 63166-0299, U.S.A. and Conservatoire et Jardin botaniques de la Ville de Genève, ch. de l’Impératrice 1, CP 60, 1292 Chambésy, Geneva, Switzerland. martin.callmander@mobot-mg.org
Charles Rakotovao, Missouri Botanical Garden, Madagascar Research and Conservation Program, BP 3391, Antananarivo 101, Madagascar.
Jeremi Razafitsalama, Missouri Botanical Garden, Madagascar Research and Conservation Program, BP 3391, Antananarivo 101, Madagascar.
Peter B. Phillipson, Missouri Botanical Garden, P.O. Box 299, St. Louis, MO, 63166-0299, U.S.A. and Département Systématique et Evolution (UMR 7205), Muséum National d’Histoire Naturelle, case postale 39, rue Cuvier 57, 75231 Paris CEDEX 05, France
Sven Buerki, Institut de Botanique, Université de Neuchâtel, Emile-Argand 11, 2009 Neuchâtel, Switzerland.
Cynthia Hong-Wa, Department of Botany, University of Missouri-St. Louis, One University Blvd, St. Louis, MO 63121-4000, U.S.A. and Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri, 63166-0299, U.S.A..
Nivo Rakotoarivelo, Département de Biologie et Ecologie Végétale, Faculté des Sciences, Université d’Antananarivo, BP 566, Antananarivo 101, and Madagascar Research and Conservation Program, BP 3391, Antananarivo 101, Madagascar.
Sylvie Andriambololonera, Missouri Botanical Garden, Madagascar Research and Conservation Program, BP 3391, Antananarivo 101, Madagascar.
Margaret M. Koopman, University of Wisconsin, Madison, Wisconsin, 53706, U.S.A.
David M. Johnson, Department of Botany-Microbiology, Ohio Wesleyan University, Delaware, Ohio 43015, U.S.A.
Thierry Deroin, Département Systématique et Evolution (USM 602), Muséum National d’Histoire Naturelle, case postale 39, rue Cuvier 57, 75231 Paris CEDEX 05, France.
Andriamandranto Ravoahangy, Missouri Botanical Garden, Madagascar Research and Conservation Program, BP 3391, Antananarivo 101, Madagascar.
Serge Solo, Missouri Botanical Garden, Madagascar Research and Conservation Program, BP 3391, Antananarivo 101, Madagascar.
Jean-Noël Labat, Département Systématique et Evolution (USM 602), Muséum National d’Histoire Naturelle, case postale 39, rue Cuvier 57, 75231 Paris CEDEX 05, France.
Porter P. Lowry, II, Missouri Botanical Garden, P.O. Box 299, St. Louis, MO, 63166-0299, U.S.A. and Département Systématique et Evolution (UMR 7205), Muséum National d’Histoire Naturelle, case postale 39, rue Cuvier 57, 75231 Paris CEDEX 05, France.
References
- Barnett LC. Two new species of Nesogordonia (Sterculiaceae) from Madagascar. Bull. Mus. Nat. Hist. Nat., B, Adansonia. 1987;9:95–100. [Google Scholar]
- Barnett LC. Ph.D. dissertation. Austin: University of Texas; 1988. Systematics of Nesogordonia Baill. (Sterculiaceae) [Google Scholar]
- Bernardi L. Araliacearum Madagascariae et Comores propositum. 2. Revisio et taxa nova Polysciadim. Candollea. 1971;26:13–89. [Google Scholar]
- Bernardi L. Synopsis Araliacearum Madagascariae et Comorarum Insularum (auxilio methodi “Ferulago”) Candollea. 1979;35:117–132. [Google Scholar]
- Besairie H. Recherches géologiques à Madagascar, première suite; la géologie du nord-ouest. Mém. Acad. Malgache. 1936;21:1–259. [Google Scholar]
- Bosser J. Deux nouvelles espèces de Noronhia Stadm. ex Thouars (Oleaceae) de Madagascar. Adansonia ser. 1973;2(13):461–466. [Google Scholar]
- Callmander MW, Schatz GE, Lowry PP., II IUCN Red List assessment and the Global Strategy for Plant Conservation: taxonomists must act now. Taxon. 2005;54:1047–1050. [Google Scholar]
- Callmander MW, Schatz GE, Lowry PP, II, Laivao MO, Raharimampionona J, Andriambololonera S, Raminosoa T, Consiglio T. Application of IUCN Red List criteria and assessment of Priority Areas for Plant Conservation in Madagascar: rare and threatened Pandanaceae indicate new sites in need of protection. Oryx. 2007;41:168–176. [Google Scholar]
- Callmander MW, Buerki S, Wohlhauser S. A new threatened species of Pandanaceae from northwestern Madagascar: Pandanus sermolliana. Novon. 2008;18:421–424. doi: 10.3417/2007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet C. Les forêts du Sambirano et de Nossi-Bé. II. Les Presqu’îles d’Anorotsanga et d’Ambato, le Kalobenono, le Galoka, Nosy Be et Nosy Komba. Bull. Madagascar. 1958;151:1043–1064. [Google Scholar]
- Gautier L. Liste commentée des phanérogames de la Réserve Spéciale de Manongarivo, Madagascar. In: Gautier L, Goodman SM, editors. Boissiera. Vol. 59. NW Madagascar: Inventaire floristique et faunistique de la Réserve Spéciale de Manongarivo; 2002. pp. 105–239. [Google Scholar]
- Goodman SM, Benstead JP. Updated estimates of biotic diversity and endemism for Madagascar. Oryx. 2005;39:73–77. [Google Scholar]
- Hochreutiner BPG. Malvacées. In: Humbert H, editor. Fl. Madagascar & Comores. Vol. 129. Muséum National d’Histoire Naturelle; 1955. [Google Scholar]
- IUCN. Categories and Criteria (version 3.1) Gland, Switzerland: IUCN; 2009. http://www.redlist.org/info/categories_criteria2001.html. [Google Scholar]
- Johnson DM, Mwasumbi LB, Mbago FM. New species of Xylopia and Uvaria (Annonaceae) from Tanzania. Novon. 1999;9:55–60. [Google Scholar]
- Koopman MM, Baum DA. Phylogeny and biogeography of tribe Hibisceae (Malvaceae) in Madagascar. Syst. Bot. 2008;33:364–374. [Google Scholar]
- Labat J-N, Pignal M, Pascal O. Trois espèces nouvelles d’Oleaceae et note sur la présence d’Olea capensis dans l’archipel des Comores. Novon. 1999;9:66–72. [Google Scholar]
- Labat J-N, Munzinger J, Pascal O. Une nouvelle espèce du genre Nesogordonia Baill. (Sterculiaceae) de Mayotte, Archipel des Comores. Candollea. 2000;52:277–280. [Google Scholar]
- Leroy J-F, Lescot M. Taxons nouveaux de Trichilieae (Meliaceae-Meliodeae) de Madagascar. Bull. Mus. Natl. Hist. Nat., B, Adansonia. 1996;18:3–34. [Google Scholar]
- Lowry PP, II, Plunkett GM. A synoptic infrageneric classification of Polyscias J. R. Forst. & G. Forst. (Araliaceae) Bot. Jahrb. Syst [Google Scholar]
- Lowry PP, II, Plunkett GM, Wen J. Generic relationships in Araliaceae: looking into the crystal ball. S. African J. Bot. 2004;70:382–392. [Google Scholar]
- Moat J, Smith P. Atlas of the vegetation of Madagascar. Royal Botanic Gardens, Kew; 2007. [Google Scholar]
- Muellner AN, Samuel R, Johnson SA, Cheek M, Pennington TD, Chase M. Molecular phylogenetics of Meliaceae (Sapindales) based on nulcear and plastid DNA sequences. Amer. J. Bot. 2003;90:471–480. doi: 10.3732/ajb.90.3.471. [DOI] [PubMed] [Google Scholar]
- Pennington TD, Styles BT. A generic monograph of the Meliaceae. Blumea. 1975;22:419–540. [Google Scholar]
- Perrier de la Bâthie H. Biogéographie des plantes de Madagascar. Société d’éditions géographiques, maritimes et coloniales; 1936. [Google Scholar]
- Perrier de la Bâthie H. Révision des Oléacées de Madagascar et des Comores. Mém. Inst. Sci. Madagascar, Sér. B, Biol. Vég. 1949;2:275–310. [Google Scholar]
- Perrier de la Bâthie H. Oléacées. In: Humbert H, editor. Fl. Madagascar & Comores. Vol. 166. Muséum National d’Histoire Naturelle; 1952. [Google Scholar]
- Phillipson PB, Schatz GE, Lowry PP, II, Labat J-N. A catalogue of the vascular plants of Madagascar. In: Ghazanfar SA, Beentje HJ, editors. Taxonomy and ecology of African plants, their conservation and sustainable use: proceedings of the 17th AETFAT Congress, Addis Ababa, Ethiopia. Kew, UK: Royal Botanic Gardens; 2006. pp. 613–627. [Google Scholar]
- Plunkett GM, Lowry PP., II Paraphyly and polyphyly in Polyscias sensu lato: molecular evidence and the case for recircumscribing the “pinnate genera” of Araliaceae. Bot. Jahrb. Syst. (in press) [Google Scholar]
- Plunkett GM, Lowry PP, II, Burke MK. The phylogenetic status of Polyscias (Araliaceae) based on nuclear ITS sequence data. Ann. Missouri Bot. Gard. 2001;88:213–230. [Google Scholar]
- Plunkett GM, Wen J, Lowry PP, II, Mitchell AD, Hen-wood MJ, Fiaschi P. Araliaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Springer-Verlag; (in press) [Google Scholar]
- Randrianasolo A, Lowry PP., II Four new species and one new combination in the Malagasy endemic genus Micronychia Oliv. (Anacardiaceae) Adansonia ser. 2009;2(31):157–168. [Google Scholar]
- Schatz GE. Generic tree flora of Madagascar. Kew: Missouri Botanical Garden & Royal Botanic Gardens; 2001. [Google Scholar]
- Schatz GE, Lescot M. Gazetteer to Malagasy botanical collecting localities. 2008 [ http://www.mobot.org/MOBOT/Research/madagascar/gazetteer]
- Solo S, Rakotovao C, Callmander MW, Andriamandranto R, Randrianarivony TN, Raharimampionona J. Stratégie de conservation du Kalabenono. Missouri Botanical Garden; 2008. [Google Scholar]
- Steininger MK, Harper GJ, Tucker CJ. Forest cover fragmentation and clearance in Madagascar. Washington: Centre for Applied Biodiversity Science, Conservation International, Arlington & NASA Goddard Space Flight Center; 2003. [Google Scholar]