Abstract
Serum antibody titers for canine parvovirus type-2 (CPV-2), canine distemper virus (CDV) and canine adenovirus type-1 (CAV-1) were investigated in 1031 healthy adult household dogs (2 to 18 years old) given an annual inoculation in the previous 11 to 13 months. The number of dogs retaining significant titers of antibodies against CPV-2, CDV, and CAV-1 were 888 (86%), 744 (72%), and 732 (71%), respectively. There were no differences between males and females in antibody titers against the 3 viruses. Antibody titer for CPV-2 was significantly higher in younger dogs than in older dogs, CDV antibody was significantly higher in older dogs than in younger dogs, and CAV titer was not associated with age.
Résumé
Taux d’anticorps anti-parvovirus canin de type 2, anti-virus de la maladie de Carré et anti-adénovirus canin de type 1, chez les chiens de compagnie adultes. Les taux de rétention d’anticorps sériques de la parvovirose canine (CPV-2), de la maladie de Carré (CDV) et de l’encéphalite de Rubarth — type-1 (CAV-1) ont été mesurés chez 1031 chiens de compagnie adultes en bonne santé. Les nombres de chiens ayant un taux conséquent d’anticorps CPV-2, CDV et CAV-1 ont été respectivement de 888 (86 %) de 744 (72 %) et de 732 (71 %). Nous n’avons pas noté de différence sexuelle significative pour les taux d’anticorps CPV-2, CDV et CAV-1. Par groupe d’âge les taux d’anticorps CPV-2 sont plus élevés de façon significative chez les jeunes chiens que chez les chiens plus âgés, et ceux de CDV sont significativement plus élevés chez les chiens âgés que chez les chiens plus jeunes. Les taux de CAV ne sont pas en relation avec l’age.
(Traduit par les auteurs)
Introduction
Canine parvovirus infection caused by canine parvovirus type-2 (CPV-2), canine distemper caused by canine distemper virus (CDV), and infectious canine hepatitis (ICH) caused by canine adenovirus type-1 (CAV-1), are highly contagious, with a high fatality rate. There are no effective medications to treat these diseases, and vaccination is the sole method of protecting individuals from these diseases and preventing the spread of these viruses in the population (1–3).
Canine parvovirus type-2 was first isolated from the diarrheal stool of puppies (4). It is highly resistant to disinfectants and is easily transmitted through both direct and indirect contact. Reports of outbreaks in Japan and countries in Europe and the USA appeared soon after initial isolation of the virus (5,6). Infection follows a fatal course in young dogs, and efforts have focused on developing a live attenuated vaccine that is not easily affected by maternal antibodies (7).
Canine distemper virus is not resistant to disinfectants, but is easily transmitted through aerosols and has a wide host range. Thus, there is concern of infection from outdoor exposure. This virus is thought to have one serotype, but in Finland, where there had not been an outbreak for 16 y, infection occurred in at least 5000 dogs due to differences in the pathogenicity of the vaccine and epidemic strains (8). This indicates the need to understand the immune status against field strains.
Canine adenovirus type-1 causes hepatitis, but effects such as interstitial nephritis and corneal opacity are seen in dogs that have been inoculated with attenuated vaccine (9). Therefore, CAV-2, which is antigenically similar to CAV-1, isolated from dogs with respiratory tract disease (10) is used as a vaccine for ICH. The recommended vaccination protocol in Japan for these infectious diseases, based on the results of earlier experience (11) and challenge infection experiments by vaccine manufacturers, is multiple inoculations in order to avoid interference by maternal antibodies in the first year, followed by annual inoculations from the second year onwards. Very few cases are currently encountered in adult dogs. However, there are recent reports on side effects, including hypersensitivity reactions and immune-mediated hemolytic anemia (IMHA) from vaccination (11–13).
In order to investigate the benefits of additional vaccinations, we determined the retention of antibodies against these viruses in adult dogs brought to our clinic, identified the immune status of the household, and identified trends in antibody titer with age, and differences associated with sex of the dogs.
Materials and methods
Subject dogs
A total of 1031 adult household dogs aged from 2 to 18 y were examined. All dogs had received 2 or 3 vaccinations in their first year and subsequently received annual inoculations. Comparisons were made according to sex and age. Dogs were divided into 4 groups by sex (423 sexually intact males, 108 neutered males, 264 sexually intact females, and 236 neutered females) and into 16 groups by age. The numbers of dogs in each of the ages 2 to 18 y were 450, 120, 108, 76, 57, 48, 44, 46, 24, 18, 19, 8, 6, 3, 3, 0, 1, respectively, for each successive year.
Serologic tests
Serum samples were collected from dogs vaccinated in the previous 11 to 13 mo, and were sent in the frozen state to a commercial veterinary diagnostic laboratory (Marupi Lifetech, Osaka, Japan) for determination of serum antibody titers. Antibody titers for CPV-2 were obtained by a hemagglutination inhibition (HI) test, CDV antibody titers by an immunoperoxidase (IP) test, and CAV-1 antibody titers by a neutralization test (NT).
Antibody titer classification
The antibody titers were classified using the criteria of the commercial veterinary diagnostic laboratory (Marupi Lifetech), based on previous reports (14–20) that indicated the protective titer. The antibody titer that protects against disease as indicated by previous reports was designated as the borderline titer. Titers below the borderline were designated as low. Titers 4-fold higher than borderline were designated as high, based on the previous observation that titers decreased to 1/4 in 1 y.
Many early reports showed 1:80 as a protective titer, but an old type of antigen may have been used. For the present test 1:40 was designated borderline because CPV-2b, a new antigen, was used and a level of 1:40 has been demonstrated to be protective in a challenge infection study (technical information on Rescamune P-ML; Nippon Zennyaku Kougyou). “High” was ≥ 1:160, and “low” was ≤ 1:20.
Other reports have shown that borderline protective titer against CDV was 1:32, as determined by a NT. However in the present study an IP test was used for titration, and 1:160 was determined to be borderline based on regression analysis between the NT and IP tests. “High” was ≥ 1:640, and “low” was fixed at ≤ 1:80.
As an antibody titer of 1:32 to 1:42 against CAV-1 was the most reliable previously reported value, the protective titer for borderline was assigned as 1:40. “High” was ≥ 1:160, and “low” was fixed at ≤ 1:20.
Statistical analysis
To determine whether there were differences in antibody titer by sex, Mann-Whitney U-tests were conducted to compare the reciprocal of the dilution of antibody titers between groups. In order to investigate differences in CPV-2, CDV, and CAV-1 antibody titers by age group, comparisons between the reciprocal of the dilution in each age group were made by a logistic method in the JMP software system (JMP® Statistical Discovery Software; Cary, North Carolina, USA). In all tests P < 0.05 was considered to indicate a significant difference.
Results
“High” status within the population
The antibody retention status in 1031 dogs is shown in Table 1. “High” status against CPV-2 was seen in 888 dogs (86%), against CDV in 744 dogs (72%), and against CAV-1 in 732 dogs (71%).
Table 1.
Retention of antibody against CPV-2, CDV, and CAV-1 in 1031 household dogs (2 to 18 years old) approximately 1 year after the last annual booster inoculation
| Antibody titer | CPV-2 | CDV | CAV-1 |
|---|---|---|---|
| Protective | 888 (86%) | 744 (72%) | 732 (71%) |
| Borderline | 94 (9%) | 186 (18%) | 207 (20%) |
| Susceptible | 49 (5%) | 101 (10%) | 92 (9%) |
CPV-2 — canine parvovirus type-2.
CDV — canine distemper virus.
CAV-1 — canine adenovirus type-1.
Association between sex and antibody titer
Tests for sex differences in CPV-2, CDV, and CAV-1 antibodies revealed no significant differences between sexually intact males, neutered males, sexually intact females, and neutered females.
Association between age and antibody titer
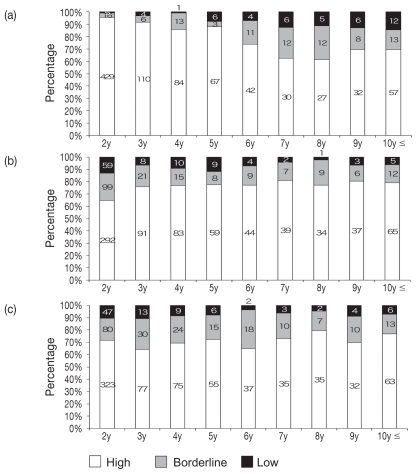
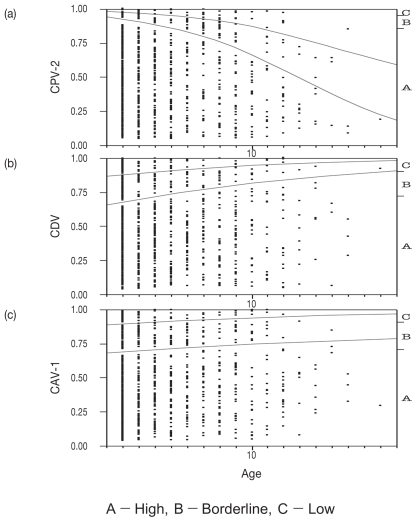
The antibody retention status by age for CPV-2 is shown in Figure 1(a). The proportion of dogs that had antibodies conferring “high” status was 429 of 450 in the 2-year-old group, the highest proportion at 95%. This decreased with age to 30 (62%) of 48 dogs in the 7-year-old group, and 27 (61%) of 44 dogs in the 8-year-old group, which was the lowest proportion. There was a slight increase to 69%, or 32 of 46 dogs, in the 9-year-old group and 69%, or 57 of 82 dogs, in the 10 years and older group. Investigation of the differences of antibody titer for CPV-2 between the reciprocal of the dilution in each age group was done by a logistic method in the JMP software system and is shown in Figure 2(a). This figure revealed that the proportions were significantly higher in the younger groups than in the older groups (P < 0.0001).
Figure 1.
Antibody titer against CPV-2(a), CDV(b) and CAV-1(c) by age. Numbers in graph refer to number of dogs.
Figure 2.
Tendency of antibody titer against CPV-2(a), CDV(b) and CAV-1(c) by age.
The antibody retention status by age for CDV is shown in Figure 1(b). The proportion of dogs that had antibodies conferring “high” status was 292 of 450 in the 2-year-old group. At 65%, this was the lowest proportion; the proportion increased in groups aged 3 y or older. Investigation of the differences between the 9 groups using the same method as for CPV-2, revealed that the proportions were significantly higher in the older group than in the younger group (Figure 2(b); P = 0.0004).
The antibody retention status by age for CAV-1 is shown in Figure 1(c). The “high” antibody retention rate tended to be around 70% through all age groups. No significant differences in antibody retention status were seen between any of the groups [Figure 2(c)].
Discussion
The purpose of vaccination is to protect individuals from infectious diseases by enhancing a specific immune response. The most reliable method for identifying immune status for infectious disease in individual dogs is to conduct a challenge infection. However, for ethical reasons, challenge infections are not desirable in pet animals. The resistance of dogs is therefore estimated by in vitro tests. As these infectious diseases are rare in puppies, which have maternal antibodies, it is thought that the existence of antibodies in the blood protects dogs from infection (14–17). Cellular immunity is also important, but tests are costly and require special equipment, making them difficult in practice. We therefore tested how sex and age affect the retention of serum antibody titers. Moreover, as it is difficult to clearly distinguish between antibody titers that can or cannot provide protection, an intermediate area was incorporated (“Borderline”).
The percentage of dogs retaining significant amounts of antibodies against CPV-2, CDV, and CAV-1 were 86%, 72%, and 71%, respectively. These results are comparable with those in other reports (14,17,21,22) from various countries, indicating that adequate immunization has been achieved for this population and outbreaks of these infectious diseases are unlikely in Japan.
Antibody retention for CPV-2 was higher in the younger group; this was similar to the findings of previous reports (21,22). As CPV-2 proliferates in actively dividing cells (23,24), the higher CPV-2 antibody levels in younger dogs are thought to occur because younger dogs have greater cell proliferation and a stronger immune response to the vaccine virus within the body.
Antibody retention for CDV, however, was higher in the older group and increased with age. This was different from the findings of previous reports (22,23), indicating the possibility that CDV boosting by vaccination or the environment or both has occurred.
No decrease in antibody retention with age was seen for CAV-1, but there was little information for CAV-1 antibody. In Japan, ICH is rare at present, and there are probably few opportunities for exposure outdoors. However, CAV-1 is a virus that does not have an envelope and shows strong resistance outdoors. In addition, infected dogs excrete the virus in their urine for a long time, and it is therefore necessary to be aware of the low antibody retention rate for CAV-1.
Our conclusion from this study is that, on the basis of serum antibody titers, a complete series of vaccinations as puppies is essential for CDV because the titer is low in younger dogs and annual vaccination is essential for CPV-2 because the titer is low in older dogs. Furthermore, even though the proportion of susceptible dogs is low, antibody titers should be measured to confirm the antibody retention status for each individual. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Lamm CG, Rezabek GB. Parvovirus infection in domestic companion animals. Vet Clin North Am Small Anim Pract. 2008;38:837–850. doi: 10.1016/j.cvsm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Martella V, Elia G, Buonavoglia C. Canine distemper virus. Vet Clin North Am Small Anim Pract. 2008;38:787–797. doi: 10.1016/j.cvsm.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Decaro N, Martella V, Buonavoglia C. Canine adenoviruses and herpesvirus. Vet Clin North Am Small Anim Pract. 2008;38:799–814. doi: 10.1016/j.cvsm.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eugster AK, Bendele RA, Jones LP. Parvovirus infection in dogs. J Am Vet Med Assoc. 1978;173:1340–1341. [PubMed] [Google Scholar]
- 5.Baba M, Yasoshima A, Kojima A, et al. Canine parvovirus infection in a group of beagles. Exp Anim. 1981;30:141–144. doi: 10.1538/expanim1978.30.2_141. [DOI] [PubMed] [Google Scholar]
- 6.Appel MJG, Cooper BJ, Greisen H, et al. Canine viral enteritis. 1. Status report on corona-and parvo-like viral enteritides. Cornel Vet. 1979;69:123–133. [PubMed] [Google Scholar]
- 7.Bergman JGHE, Muniz M, Sutton D, et al. Comparative trial of the canine parvovirus, canine distemper virus and canine adenovirus type 2 fractions of two commercially available modified live vaccines. Vet Rec. 2006;159:733–736. doi: 10.1136/vr.159.22.733. [DOI] [PubMed] [Google Scholar]
- 8.Ek-Kommonen C, Sihvonen L, Pekkanen K, et al. Outbreak of canine distemper in vaccinated dogs in Finland. Vet Rec. 1997;141:380–383. doi: 10.1136/vr.141.15.380. [DOI] [PubMed] [Google Scholar]
- 9.Bass EP, Gill MA, Beckenhauer WH. Evaluation of a canine adenovirus type 2 strain as a replacement for infectious canine hepatitis vaccine. J Am Vet Med Assoc. 1980;177:234–242. [PubMed] [Google Scholar]
- 10.Emery JB, House JA, Brown WR. Cross-protective to canine adenovirus type 2 by canine adenovirus type 1 vaccination. Am J Vet Res. 1978;39:1778–1783. [PubMed] [Google Scholar]
- 11.Duval D, Giger U. Vaccine-associated immune-mediated hemolytic anemia in the dog. J Vet Intern Med. 1997;10:290–295. doi: 10.1111/j.1939-1676.1996.tb02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray AK. Cat and dog vaccination: Results from the suspected adverse reaction surveillance scheme. Vet Rec. 1998;143:455. [PubMed] [Google Scholar]
- 13.Moore G, Guptill LF, Ward MP, et al. Adverse events diagnosed within three days of vaccine administration in dogs. J Am Vet Med Assoc. 2005;227:1102–1108. doi: 10.2460/javma.2005.227.1102. [DOI] [PubMed] [Google Scholar]
- 14.Böhm M, Thompson H, Weir A, et al. Serum antibody titers to canine parvovirus, adenovirus and distemper virus in dogs in the UK which had not been vaccinated for at least three years. Vet Rec. 2004;154:457–463. doi: 10.1136/vr.154.15.457. [DOI] [PubMed] [Google Scholar]
- 15.Tizard I, Ni W. Use of serologic testing to assess immune status of companion animals. J Am Vet Med Assoc. 1998;213:54–60. [PubMed] [Google Scholar]
- 16.Coyne MJ, Burr JHH, Yule TD, et al. Duration of immunity in dogs after vaccination or naturally acquired infection. Vet Rec. 2001;149:509–515. doi: 10.1136/vr.149.17.509. [DOI] [PubMed] [Google Scholar]
- 17.Twark L, Dodds WJ. Clinical use of serum parvovirus and distemper virus antibody titers for determining revaccination strategies in healthy dogs. J Am Vet Med Assoc. 2000;217:1021–1024. doi: 10.2460/javma.2000.217.1021. [DOI] [PubMed] [Google Scholar]
- 18.Chalmers WSK, Baxendale W. A comparison of canine distemper vaccine and measles vaccine for the prevention of canine distemper in young puppies. Vet Rec. 1994;135:349–353. doi: 10.1136/vr.135.15.349. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael LE, Joubert JC, Pollock RV. A modified live canine parvo-virus vaccine. II. Immune response. Cornell Vet. 1983;73:13–29. [PubMed] [Google Scholar]
- 20.Soma T. Diagnostic application of immunoperoxidase plaque staining test to antibody testing against canine distemper virus. J Environ Dis. 2002;11:5–12. [Google Scholar]
- 21.McCaw DL, Thompson M, Tate D, et al. Serum distemper virus and parvovirus antibody titers among dogs brought to a veterinary hospital for revaccination. J Am Vet Med Assoc. 1998;213:72–75. [PubMed] [Google Scholar]
- 22.Ottiger HP, Neimeier-Förster M, Stärk KD, et al. Serological responses of adult dogs to revaccination against distemper, parvovirus and rabies. Vet Rec. 2006;159:7–12. doi: 10.1136/vr.159.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Hueffer K, Parrish CR. Parvovirus host range, cell tropism and evolution. Curr Opin Microbiol. 2003;6:392–398. doi: 10.1016/s1369-5274(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 24.Parrish CR. Pathogenesis of feline panleukopenia virus and canine parvovirus. Baillière’s Clin Haematol. 1995;8:57–71. doi: 10.1016/S0950-3536(05)80232-X. [DOI] [PMC free article] [PubMed] [Google Scholar]