Abstract
The purposes of this retrospective study were to assess the prevalence of gallbladder sludge (GBS) in a population of cats presented for abdominal ultrasound in a teaching hospital and to determine its association with increased serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin (TB). Gallbladder sludge was detected in 152 (14%) of the cats undergoing abdominal ultrasound between 2004 and 2008. This population was compared to a control group of 32 cats without GBS. Alanine aminotransferase, ALP, and TB mean values were significantly higher in cats with GBS than in controls (P ≤ 0.0005) and odds for increased values in cats with GBS were 4.2 [95% confidence interval (CI): 1.6 to 11.0], 9.5 (95% CI: 2.2 to 41.7), and 4.1 (95% CI: 1.5 to 11.5), respectively (P ≤ 0.007). In conclusion, GBS is an uncommon ultrasonographic finding in cats that is predictive of increased liver enzymes and TB. More studies are needed to establish potential links between GBS and hepatobiliary disease in cats.
Résumé
La boue de la vésicule biliaire sur une échographie est prédictive d’enzymes hépatiques et de bilirubine totale chez les chats. Les buts de cette étude rétrospective étaient d’évaluer la prévalence de boue de vésicule biliaire (BVB) chez une population de chats présentés pour une échographie abdominale dans un hôpital d’enseignement et pour déterminer son association avec un taux sérique accru d’alanine aminotransférase (ALT), d’alkaline phosphatase (ALP) et de bilirubine totale (TB). La boue de la vésicule biliaire a été détectée chez 152 (14 %) des chats subissant une échographie abdominale entre 2004 et 2008. Cette population a été comparée à un groupe témoin de 32 chats sans BVB. Les valeurs moyennes d’alanine aminotransférase, d’ALP et de TB étaient significativement supérieures chez les chats sans BVB que dans le groupe témoin (P ≤ 0,0005) et les probabilités de valeurs supérieures chez les chats avec de la BVB étaient de 4,2 [intervalle de confiance (IC) de 95 % : 1,6 à 11,0], 9,5 (IC de 95 % : 2,2 à 41,7) et 4,1 (IC de 95 % : 1,5 à 11,5), respectivement (P ≤ 0,007). En conclusion, la BVB est une constatation échographique rare chez les chats qui est prédictive de taux accrus d’enzymes hépatiques et de TB chez les chats. De nouvelles études sont requises pour établir les liens potentiels entre la BVB et la maladie hépatobiliaire chez les chats.
(Traduit par Isabelle Vallières)
Introduction
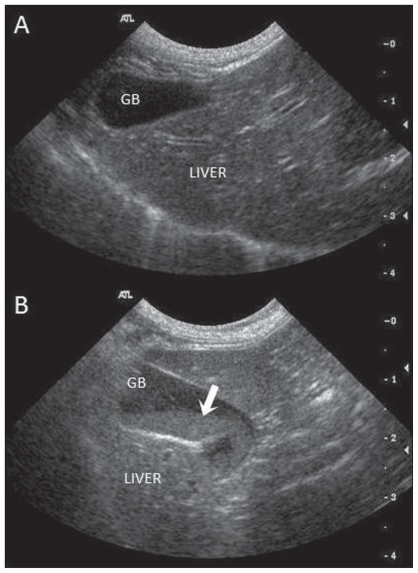
Gallbladder sludge (GBS) is defined as precipitated particulate matter dispersed in a viscous liquid phase within bile (1). It is occasionally identified in cats during abdominal ultrasound examinations and appears sonographically as mobile echoes of variable amplitude without acoustic shadowing that tend to accumulate in the dependent portion of the gallbladder (2) (Figure 1). In humans, GBS is composed of cholesterol monohydrate crystals or calcium bilirubinate granules and other calcium salts embedded in mucus in variable proportions (3). While similar components as well as lipid droplets may contribute to GBS in dogs and cats (4), its exact composition remains unknown in small animals.
Figure 1.
Sagittal ultrasound images of the liver and gallbladder (GB) of 2 cats without (A) and with (B) biliary sludge. (A) The normal GB appears as a teardrop-shaped structure, with anechoic bile surrounded by a thin, poorly visualized wall. (B) Cat with GB sludge (white arrow). The sludge appears as moderately hyperechoic, non-shadowing sediment in the dependent portion of the GB. In this cat, the GB wall is hyperechoic and mildly thickened, presumably the result of cholecystitis.
In humans, GBS is rare in the healthy adult population and uncommon in patients with gastrointestinal disorders, with respective prevalences of 0.0% to 1.7% (5–7) and 5.1% (8). Conversely, in dogs GBS represents a frequent finding with a prevalence of 53% in the healthy population (9).
Many reports in human medicine have shown that GBS can be causally associated with various diseases such as biliary colic, acalculous cholecystitis, and acute pancreatitis (1,10–12). It has been postulated that GBS represents an early stage of cholelithiasis (13,14); however, in dogs, GBS is not significantly associated with hepatobiliary disease (9).
In cats, GBS has been reported with various liver diseases, particularly those affecting the biliary tract (15,16). Also, it has been suggested that GBS may be more significant or more likely associated with disease in cats than in dogs (17–19). However, the prevalence of GBS in cats and its clinical significance have not been specifically investigated.
In small animal medicine, serum biochemistry is commonly used to screen for the presence of hepatobiliary disease, in particular with the measurement of serum liver enzymes [alkaline phosphatise (ALP) and alanine aminotransferase (ALT)], bilirubinemia and bilirubinuria. Indeed, consistent increases in the serum concentration of liver enzymes and bilirubin occur after hepatobiliary injury (20). Furthermore, the pattern of abnormalities in liver enzymes in relation to the signalment, history, physical examination, and serum total bilirubin concentration can indicate a hepatobiliary disorder (21).
The objectives of this study were to determine the prevalence of GBS in the population of cats presented for abdominal ultrasound, to describe the clinical signs and laboratory findings in these cats, and to compare the serum liver enzymes and bilirubinemia in cats with and without GBS. We hypothesized that serum liver parameters are significantly increased in cats with GBS.
Materials and methods
Abdominal ultrasound reports of feline patients presented to the Teaching Hospital of the Faculté de Médecine Vétérinaire of the Université de Montréal (FMVUM) between 2004 and 2008 were searched retrospectively to identify cats in which GBS was recorded. The cats were either referral or first opinion cases and presented for a wide variety of medical or surgical problems. All ultrasound examinations were performed by a board-certified radiologist or a radiology resident using a high-definition ultrasound system (ATL HDI 5000, 5–8 MHz convex transducer; Philips Medical Systems, Bothell, Washington, USA). Archived images of cats in which GBS was reported were reviewed by one of the authors (MAD) to confirm the presence of GBS on high-quality images. Medical records of all cats with GBS were reviewed, and only cats in which measurement of serum ALT, ALP, and total bilirubin (TB) had been performed at the FMVUM laboratory (Beckman Synchron CX5 Delta Clinical System; GMI, Ramsey, Minnesota, USA) and obtained within 1 wk of the ultrasound examination were included in the study. In patients that were followed over time, only the data obtained on initial presentation were considered. Signalment, clinical signs, and urine analysis were recorded where available. Serum liver parameters were considered as increased when the values were above the upper limit of the reference interval determined by the FMVUM laboratory.
A second search was conducted in the same hospital population during the same period of time to randomly identify 32 cats in which GBS was not recorded in the ultrasound report and in which serum ALT, ALP, and TB were measured at the FMVUM laboratory within 1 wk of ultrasound evaluation. A power analysis revealed that such a number of subjects would be sufficient to reveal significant differences at the 0.05 level 80% of the times between the 2 groups. Ultrasound images obtained in these cats were reviewed and considered negative for GBS only if the GB could be well-visualized and appeared fully anechoic on recorded images.
Results are presented as number or percentage of cats for categorical variables and mean ± standard deviation (s) for age and weight. Mean values for age (years), weight (kg), ALT, ALP, and TB were compared between groups of cats using an unequal variance t-test, which is appropriate given the large but unequal sample size between the 2 groups. The ratio between observed values and the upper limit of the reference range provided by our laboratory was calculated for ALT, ALP, and TB. A ratio greater than 1 indicates elevated values with respect to the reference range. A chi-squared test was used to determine the association between groups and sex or breeds. Logistic regression analysis was used to determine the odds of increased ALT, ALP, and TB values (ratio > 1) in cats in which GBS was detected by ultrasound. Significance level was set at P < 0.05 throughout. SAS v. 9.1 (SAS Institute, Cary, North Carolina, USA) was used for statistical analyses.
Results
Gallbladder sludge was detected during ultrasonography in 152 cats (92 males, 60 females), which represented a prevalence of 14% in our population of cats that underwent complete abdominal ultrasound during the period (1100 cats). The signalment of cats with GBS is presented in Table 1. At the time of admission, cats were presented with decreased appetite/anorexia (62%), lethargy (55%), weight loss (51%), dehydration (50%), and vomiting (43%). Other clinical signs are presented in Table 2.
Table 1.
Signalment of cats with and without gallbladder sludge
| Cats with GB sludge (n = 152) | Cats without GB sludge (n = 32) | |
|---|---|---|
| Mean age (years) ± s | 10.3 ± 4.8 | 8.0 ± 4.4 |
| Mean weight (kg) ± s | 4.2 ± 1.2 | 4.9 ± 1.7 |
| Breed | ||
| Domestic cats | 76% | 84% |
| Himalayan | 5% | 0% |
| Persian | 4% | 6% |
| Siamese | 4% | 0% |
| Maine Coon | 2% | 6% |
| Abyssinian | 0% | 4% |
| Sexual status | ||
| Castrated males | 57% | 50% |
| Spayed females | 35% | 41% |
| Intact females | 5% | 3% |
| Intact males | 3% | 6% |
s — standard deviation.
GB — gall bladder.
Table 2.
Frequency of clinical signs in cats with gallbladder sludge
| Clinical signs | Prevalence (n = 152) |
|---|---|
| Decreased appetite/anorexia | 62% |
| Lethargy | 55% |
| Weight loss | 51% |
| Dehydration | 50% |
| Vomiting | 43% |
| Heart murmur | 36% |
| Jaundice | 19% |
| Diarrhea | 18% |
| Abdominal pain | 16% |
| Polyuria — polydipsia | 13% |
Most cats with GBS underwent serum biochemistry at the FMVUM for ALT (106/152), ALP (106/152), TB (102/152), and bilirubinuria (73/152). All cats had serum values measured within 1 wk of the ultrasound examination. Values for ALT, ALP, and TB were above the reference interval in 49%, 38%, and 44% of cats, respectively (Table 3). Also, bilirubinuria was above the reference interval in 23% of cats. The median (range) of ALT, ALP and TB, and the range of ratios with respect to the upper limit are also presented in Table 3.
Table 3.
Liver enzymes and total serum bilirubin in cats with and without gallbladder sludge
| Units | ALT U/L | ALP U/L | TB μmol/L |
|---|---|---|---|
| Cats with GBS | |||
| N | 106 | 106 | 102 |
| Median (range) | 62 (7–1044) | 32 (2–2144) | 9 (4–305) |
| Reference interval | 16–63 | 0–50 | 0–10 |
| Frequency of increased values | 49% | 38% | 44% |
| Range of values in relation to the upper limit of the reference interval | 0.1–16.6 | 0.0–42.9 | 0.4–30.5 |
| Cats without GBS | |||
| N | 32 | 32 | 31 |
| Median (range) | 46 (16–142) | 20 (2–58) | 7 (2–75) |
| Reference interval | 16–63 | 0–50 | 0–10 |
| Frequency of increased values | 19% | 6% | 16% |
| Range of values in relation to the upper limit of the reference interval | 0.3–2.3 | 0–1.2 | 0.2–7.5 |
ALT — alanine aminotransferase; ALP — alkaline phosphatase; TB — total bilirubin; GBS — gallbladder sludge.
The control group consisted of 32 cats without GBS on ultrasound. The signalment of these cats is presented in Table 1. Alanine aminotransferase, ALP, and TB were measured in 32, 32, and 31 cats, respectively. The median (range) of ALT, ALP and TB, and the range of ratios with respect to the upper limit are presented in Table 3.
Mean age and weight were compared between cats with GBS and controls. The unequal variance t-test indicated that cats with GBS (n = 152) were significantly older (P = 0.01) and lighter (P = 0.03) than controls (n = 32). The chi-squared test showed no association between group and sex (P = 0.76), and between group and breeds (P = 0.36).
The unequal variance t-test indicated that mean values were significantly higher in cats with GBS than in controls for ALT (P < 0.0001), ALP (P = 0.0002), and TB (P = 0.0005). Logistic regression indicated that the odds of observing values above the reference interval were significantly related to the group for ALT (P = 0.004), ALP (P = 0.003), and TB (P = 0.007). Odds for increased values for ALT, ALP, and TB were 4.2 [95% confidence interval (CI): 1.6 to 11.0], 9.5 (95% CI: 2.2 to 41.7), and 4.1 (95% CI: 1.5 to 11.5), respectively, in cats with GBS in comparison with controls.
Discussion
The results of this study indicate that GBS is an uncommon sonographic finding in cats, but when present is significantly associated with increased liver enzymes and total bilirubinemia. The prevalence of GBS in our population of cats (14%) was lower than that reported in dogs (9). In that study, GBS was present in about half of the dogs without hepatobiliary disease (48% to 53%), with a non-significant tendency to be more prevalent (62%) when hepatobiliary diseases were identified. Conversely, GBS is an uncommon ultrasonographic finding in healthy adult humans (≤ 1.7%) (5 to 7), increasing somewhat in prevalence with gastrointestinal disease (5.1%) (8).
In our study, nonspecific clinical signs such as decreased appetite or anorexia, lethargy, dehydration, weight loss, and vomiting were commonly recorded. In dogs, it is generally accepted that GBS is common in anorexic or fasted patients (4,9). Interestingly, the mean body weight of cats with GBS was significantly reduced when compared with the control group. It is possible, therefore, that the GBS in the cats in this study was a secondary effect of poor appetite, rather than specifically related to hepatobiliary disease. Also, another significant finding is that cats with GBS in our study were significantly older when compared to control cats. This is consistent with a previous study that found GBS to be an age-related phenomenon in dogs (9).
In humans, pregnancy, prolonged fasting, major abdominal surgery, total parenteral nutrition, weight loss, bone marrow or solid organ transplantation, octreotide and ceftriaxone treatment have been associated with GBS. Many reports have shown that GBS can be causally associated with hepatobiliary disease such as biliary stasis, abdominal pain due to biliary colic, acalculous cholecystitis, and acute pancreatitis (1,10–12). In almost all of these conditions, impaired GB contractility has been implicated in the pathogenesis of sludge (12).
As in humans, several studies support the hypothesis that GBS is a significant sonographic finding in cats. For instance, the presence of GBS has been associated with cholangiohepatitis complex (CHC), cholecystitis, and extrahepatic biliary obstruction (EHBO) in cats (17–19,22). Gallbladder sludge was also detected in 40% of cats with hepatobiliary or gastrointestinal disease (16) and in 62% of cats with EHBO (15). These studies suggest that GBS could be related to the presence of cholestasis in cats, unlike dogs. Whether cholestasis leads to the inspissation of bile, producing GBS (17,18), or, the opposite, that GBS causes bile flow obstruction (22), remains to be determined. Finally, although GBS and cholelithiasis have been reported concurrently in cats, a causative association has yet to be confirmed (23).
In our study, an elevation in ALT, ALP activity, and TB was present in nearly half of the population of cats with GBS. Logistic regression showed that these cats are significantly more likely to have an elevation of these values when compared to cats without GBS. Increased ALT activity is a marker of hepatocellular damage and the magnitude of activity seems to correlate with the number of cells involved (20); thus, ALT elevation is generally considered to be specific (80%) for hepatobiliary disease in cats (20). Increased ALP activity generally indicates cholestasis, particularly in cats (21). Moreover, elevation in serum ALP in cats is more specific (87%) for hepatobiliary disease than in dogs (21). Total bilirubin is less sensitive than serum liver enzyme measurement for the detection of hepatobiliary disease; however, its elevation is considered more specific (20). Our results show that GBS is related to increased serum liver enzymes and total bilirubin and indirectly suggest that GBS is most likely to be specifically linked to hepatobiliary disease.
Some limitations of this study result from its retrospective design, in that additional cats which had GBS were not recorded in the ultrasound report, may have lead to an underestimation of the true prevalence of this sonographic finding in our hospital population. Only isolated serum liver enzymes and TB values were considered. It would be preferable to study the course of these parameters over time. Another important limitation is that histological analysis of the liver parenchyma, pancreas, and biliary tract was not performed in most cats, precluding determination of a relationship between the presence of GBS and potential hepatobiliary pathology. Finally, other sonographic findings such as liver changes in echogenicity, echotexture and size, biliary abnormalities such as cholelithiasis and wall thickening, and pancreatic and gastrointestinal changes were not considered in this study, mainly because of the inconsistency of description and grading of these parameters in ultrasound reports, and the difficulty in assessing those features retrospectively using still ultrasound images.
In conclusion, our study showed that GBS in cats is an uncommon ultrasonographic finding and that cats with GBS are more likely to have elevated serum ALT, ALP, and TB. Hence, GBS in cats appears to be a significant sonographic finding that may predict hepatobiliary disease; this contrasts with findings in dogs. These results justify prospective studies correlating GBS with histological diagnosis as well as other sonographic features of hepatobiliary disease to better determine its clinical significance.
Acknowledgments
The authors thank the radiologists and imaging residents of the FMVUM Teaching Hospital and the pathologists and residents of the Service de Diagnostic of the same institution, in particular Dr. Benoit Rannou. CVJ
Footnotes
An abstract of this work was presented at the annual meeting of the American College of Veterinary Radiology, on October 23, 2009 in Memphis, Tennessee, USA.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Shaffer EA. Gallbladder sludge: What is its clinical significance? Curr Gastroenterol Rep. 2001;3:166–173. doi: 10.1007/s11894-001-0015-6. [DOI] [PubMed] [Google Scholar]
- 2.Filly RA, Allen B, Minton MJ, Bernhoft R, Way LW. In vitro investigation of the origin of echoes with biliary sludge. J Clin Ultrasound. 1980;8:193–200. doi: 10.1002/jcu.1870080302. [DOI] [PubMed] [Google Scholar]
- 3.Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology. 1986;90:677–686. doi: 10.1016/0016-5085(86)91123-6. [DOI] [PubMed] [Google Scholar]
- 4.Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39:543–598. doi: 10.1016/j.cvsm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Angelico M, De Santis A, Capocaccia L. Biliary sludge: A critical update. J Clin Gastroenterol. 1990;12:656–662. doi: 10.1097/00004836-199012000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Janowitz P, Kratzer W, Zemmler T, Tudyka J, Wechsler JG. Gallbladder sludge: Spontaneous course and incidence of complications in patients without stones. Hepatology. 1994;20:291–294. [PubMed] [Google Scholar]
- 7.Jorgensen T. Prevalence of gallstones in a Danish population. Am J Epidemiol. 1987;126:912–921. doi: 10.1093/oxfordjournals.aje.a114728. [DOI] [PubMed] [Google Scholar]
- 8.Boscaini M, Magnani G, Mandetta S, Montori A. Morphological appearance of low-level echoes in the gallbladder. Interpretation with microscopic biliary analysis and clinical correlation. Surg Endosc. 1987;1:41–49. doi: 10.1007/BF00703087. [DOI] [PubMed] [Google Scholar]
- 9.Bromel C, Barthez PY, Leveille R, Scrivani PV. Prevalence of gallbladder sludge in dogs as assessed by ultrasonography. Vet Radiol Ultrasound. 1998;39:206–210. doi: 10.1111/j.1740-8261.1998.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 10.Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol. 2006;20:1053–1062. doi: 10.1016/j.bpg.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130:301–311. doi: 10.7326/0003-4819-130-4-199902160-00016. [DOI] [PubMed] [Google Scholar]
- 12.Pazzi P, Gamberini S, Buldrini P, Gullini S. Biliary sludge: The sluggish gallbladder. Dig Liver Dis. 2003;35:39–45. doi: 10.1016/s1590-8658(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 13.de la Porte PL, Lafont H, Domingo N, et al. Composition immunofluorescence studies of biliary «sludge» in patients with cholesterol or mixed gallstones. J Hepatol. 2000;33:352–360. doi: 10.1016/s0168-8278(00)80269-x. [DOI] [PubMed] [Google Scholar]
- 14.Jungst D, Del Pozo R, Christoph S, et al. Sedimentation of biliary sludge: Effect on composition of gallbladder bile from patients with cholesterol, mixed, or pigment stones. Scand J Gastroenterol. 1996;31:273–278. doi: 10.3109/00365529609004878. [DOI] [PubMed] [Google Scholar]
- 15.Gaillot HA, Penninck DG, Webster CR, Crawford S. Ultrasonographic features of extrahepatic biliary obstruction in 30 cats. Vet Radiol Ultrasound. 2007;48:439–447. doi: 10.1111/j.1740-8261.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 16.Hittmair KM, Vielgrader HD, Loupal G. Ultrasonographic evaluation of gallbladder wall thickness in cats. Vet Radiol Ultrasound. 2001;42:149–155. doi: 10.1111/j.1740-8261.2001.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 17.Day DG. Feline cholangiohepatitis complex. Vet Clin North Am Small Anim Pract. 1995;25:375–385. doi: 10.1016/s0195-5616(95)50032-4. [DOI] [PubMed] [Google Scholar]
- 18.Penninck D, Berry C. Liver imaging in the cat. Semin Vet Med Surg (Small Anim) 1997;12:10–21. doi: 10.1016/s1096-2867(97)80039-4. [DOI] [PubMed] [Google Scholar]
- 19.Zawie DA, Garvey MS. Feline hepatic disease. Vet Clin North Am Small Anim Pract. 1984;14:1201–1230. doi: 10.1016/s0195-5616(84)50154-5. [DOI] [PubMed] [Google Scholar]
- 20.Webster CR. Liver and pancreatic diseases — Laboratory evaluation of hepatobilary diseases. In: Ettinger JS, Feldmann EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Philadelphia, Pennsylvania: WB Saunders; 2005. pp. 1421–1430. [Google Scholar]
- 21.Center SA. Interpretation of liver enzymes. Vet Clin North Am Small Anim Pract. 2007;37:297–333. doi: 10.1016/j.cvsm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Gaschen L. Update on hepatobiliary imaging. Vet Clin North Am Small Anim Pract. 2009;39:439–467. doi: 10.1016/j.cvsm.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Mayhew PD, Holt DE, McLear RC, Washabau RJ. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. J Small Anim Pract. 2002;43:247–253. doi: 10.1111/j.1748-5827.2002.tb00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]