Abstract
Herbal medicines have a long therapeutic history and are still serving many of the health needs of a large population of the world. However, the quality control and quality assurance still remains a challenge because of the high variability of chemical components involved. Herbal drugs, singularly and in combinations, contain numerous compounds in complex matrices in which no single active constituent is responsible for the overall efficacy. This creates a challenge in establishing quality control standards and standardization of finished herbal drugs. Many preparations have been mentioned in Ayurvedic text books for the treatment of Urdhwaga Amlapitta (non-ulcer dyspepsia). Dashanga Kwatha is one such known formulation. In this study, Dashanga Kwatha was converted into tablet form to increase the shelf life, make it easy to dispense, for dose fixation, etc. The Dashanga Kwatha Ghana tablet was subjected to organoleptic analysis, phytochemical analysis, and qualitative analysis to detect the presence of various functional groups, and to high performance thin layer chromatography (HPTLC) examination by optimizing the solvent systems. The investigation revealed the presence of tannins, mucilage, ascorbic acid, alkaloids, saponins, glycosides, flavonoids and carbohydrates mainly.
Keywords: Dashanga Kwatha, decoction, quality control, standardization, tablet
INTRODUCTION
Ancient Indian literature comprises a remarkably broad definition of medicinal plants and considers all plant parts to be potential sources of medicinal substances.[1] However, a key obstacle which has hindered the acceptance of the alternative medicines in the developed countries is the lack of documentation and rigorous quality control. There is a need for documentation of research work carried out on traditional medicines. With this backdrop, it becomes extremely important to make an effort toward standardization of the plant-based medicines.
Administration of drug in various dosage forms provides an opportunity to the physician to choose better options. Various dosage forms have been described in the Ayurvedic texts. One among them is Kwatha Kalpana, i.e. decoction.
Kwatha preparations are one among the Panchavidha Kashaya Kalpana[2] which are highly effective, but they are to be used when freshly prepared and they are often overlooked due to the preparation method and palatability.
In the present study, Dashanga Kwatha,[3] a known formulation used in Urdhwaga Amlapitta (non-ulcer dyspepsia), was converted into tablet form by Rasakriya[4] method to increase the shelf life, make it palatable, easy to dispense, for dose fixation, etc.
With the intention of standardization and quality control of the plant-based drugs, Dashanga Kwatha Ghana was converted into tablet form and analyzed by various analytical parameters.
MATERIALS AND METHODS
Procurement and preparation of plant material
The crude drugs mentioned in Chakradutta in Amlapitta Rogadhikara[5] for the preparation of Dashanga Kwatha were taken from the pharmacy of IPGT and RA, Gujarat Ayurved University, Jamnagar, after proper authentication by the Department of Pharmacognosy of the Institute. Then, the physical impurities were removed and the drugs were washed with water, sorted and sun dried below 45°C. Dried drugs were stored in tightly closed containers. The crude drugs used in Dashanga Kwatha with their botanical identities and parts used are given in Table 1.
Table 1.
Crude drugs of Dashanga Kwatha

Preparation of Dashanga Kwatha Ghana tablet
The authenticated crude drugs were crushed to a coarse powder separately and then mixed thoroughly with 8 parts of water in a stainless steel container and then continuous mild heat was applied until it was reduced to one-fourth of its initial quantity. During the heating process, continuous stirring was done to facilitate the evaporation and avoid any deterioration due to burning of materials. After a desirable reduction in volume was achieved, the Kwatha was filtered through single folded cotton cloth and collected in a separate vessel.
Then, the Kwatha was boiled again over slow fire on a gas stove, maintaining the temperature between 90°C and 95°C till a semisolid consistency is obtained. As the water evaporates, the viscosity of the extract increases, resulting in Ghana[6] form. Then, the Ghana was mixed with the Churna of Dashanga (up to 10% of extract) further forming a solid mass.
The solid mass (Ghana) was forced through a no. 16 sieve and granules were prepared and then dried at 50°C in a hot air oven for 10 hours. The dried granules were passed again through a no. 20 sieve. The formulation was then compressed in a single-punch tablet press with a target weight of 250 mg.
Dashanga Kwatha and Dashanga Kwatha Ghana tablet were subjected to various analytical parameters as follows.
Organoleptic parameters: Rupa (color), Rasa (taste), Gandha (odor) and Sparsha (touch)
Physico-chemical parameters: pH of 5% aqueous soln.[7], loss on drying at 110°C[8], ash value[9], acid insoluble ash[10], water soluble extractive[11], methanol soluble extractive[12]
Quantitative tests for tablet: Weight variation test[13], tablet hardness test[14], tablet disintegration time[15], friability[16]
-
Qualitative tests for various functional groups:[17,18]
Tannins, mucilages, sterols/terpenoids, ascorbic acid, alkaloids, saponins, starch, flavonoids, glycosides and carbohydrates
Chromatographic analysis: High Performance Thin Layer Chromatographic (HPTLC)[19]
Test solution: Methanol extract of Dashanga Kwatha Ghana tablets.
-
Stationary phase: Silica gel GF254
Toluene:ethyl acetate (8:1.5 v/v) was selected as the solvent system through trial and error method. The developed plate was visualized under visible day light, short UV (254 nm), long UV (366 nm). The Rf values were recorded.
Toxicological: Heavy metal analysis[20], pesticide residue value[21]
Microbial overload[22] : Bacterial and fungal growth study was carried out
Reagents and chemicals: All the reagents and chemicals used for the study were of analytical grade.
RESULTS
Comparative organoleptic characters were the following: Rupa (color) was dark brown, Rasa (taste) was bitter, Gandha (odor) was characteristic due to the specific properties of the various ingredients and Sparsha (consistency/texture) of Dashanga Kwatha was liquid and Dashanga Kwatha Ghana Tablet was smooth as given in Table 2.
Table 2.
Organoleptic parameters of Dashanga Kwatha and tablet

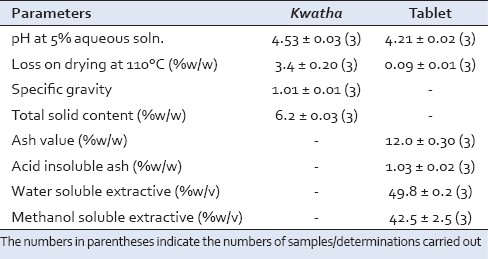
pH of the Kwatha was 4.53 ± 0.03 and that of tablet was 4.21 ± 0.02, loss on drying in Kwatha was 3.4 ± 0.20% w/w and in tablet was 0.09 ± 0.01% w/w as given in Table 3.
Table 3.
Physico-chemical parameters of Dashanga Kwatha and tablet
The average weight of the tablet was 251.23 mg, hardness of tablet was 2.74 ± 0.079 and disintegration time was 33 min and friability was 0.92% as given in Table 4.
Table 4.
Quantitative parameters of Dashanga Kwatha Ghana tablet

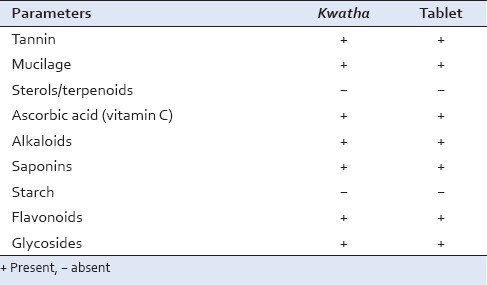
Qualitative analysis reveals the presence of tannins, mucilage, ascorbic acid, alkaloids, saponins, glycosides, flavonoids and carbohydrates in the formulation, whereas sterols/terpenoids and starch were absent as given in Table 5.
Table 5.
Qualitative parameters of Dashanga Kwatha and Dashanga Kwatha Ghana tablet
Findings of the analysis of HPTLC, heavy metals, pesticide residual value, microbial count and pathogens of Dashanga Kwatha Ghana tablet are shown in Tables 6–10, respectively.
Table 6.
Rf values of HPTLC analysis of methanolic extract at long UV (366 nm)
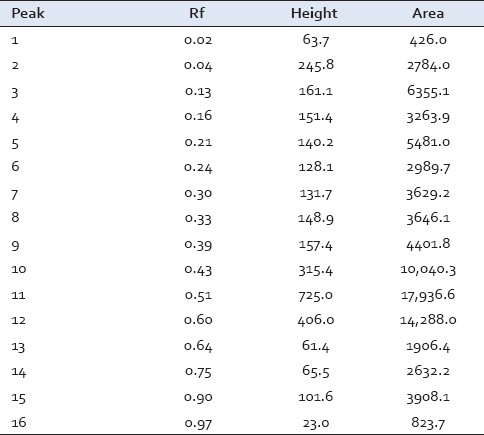
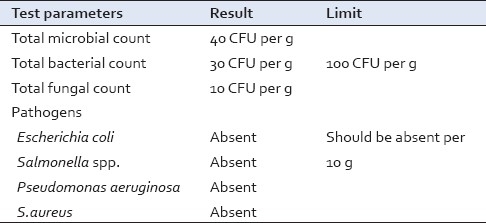
Table 10.
Total microbial count and pathogens
DISCUSSION
Ghana Kalpana, a second derivative preparation of Kwatha Kalpana, is one of the extraction methods in which water soluble material is extracted by Kwatha method and reheated till it is converted into concentrated solid form.
The procedure adopted to convert Dashanga Kwatha Ghana into tablet form was wet granulation method of tablet preparation. Gum acacia was added as the binding agent (1%) of the derived Ghana, i.e. 70 g in powder form along with the 10% powder of Dashanga Kwatha. Its addition imparts cohesiveness and ensures that the tablet remains intact after compression as well as imparts desired hardness and size.[23] However, it has the disadvantage of being variable in its composition based on its natural origin, and it is usually fairly contaminated with bacteria. This material has the advantage of being a low-cost adhesive. Drying of granules in hot air oven was carried out at 45–50°C for 6 hours to reduce microbial proliferation. Granules were prepared by passing through sieve no. 16. In the present study, 1% talc was used as the lubricant. Since lubrication is basically a coating process, the finer the particle size of the lubricant, the more effective the lubricant is likely to be. With the help of tablet punching machine, these granules were compressed into tablets with a target weight of 250 mg.
The organoleptic parameters form the basic criteria for selecting a raw drug and also to confirm the finished product. Texture of tablets was smooth indicating the surface uniformity without cracks. This is the primary character to assess the quality of tablets. Color was brownish, taste was bitter and odor was characteristic due to the specific properties of the various ingredients [Table 2].
The pH conventionally represents the acidity and alkalinity; pH of the Kwatha and tablet showed to be weak acidic in nature.
Loss on drying indicates the moisture content; in the present sample, it was 0.1%.
Ash value depends upon the inorganic substances present in the particular drug; this parameter has importance in quality control and standardization of drugs. The higher the inorganic substances present in drugs, more will be the ash value. Here, the ash value was 12.0 ± 0.30% which may be due to the extensive heating process involved in preparation of this formulation [Table 3].
Various components have their solubility in particular media. In this study, soluble principles of the samples were seen in water and methanol; in water, it was 49.6% and in methanol, it was 40%. In both the media, solubility of sample was more as the sample itself which was used to prepare the tablet was derived from water extractive. In the solubility test, increase in water soluble extractive was found, which depicts its more bioavailability in water medium [Table 3].
Average hardness was 2.75 kg/cm2. Disintegration time was 33 min and friability was not more than 0.92% on an average [Table 4]. Physico-chemical analysis of Dashanga Kwatha Ghana tablet was done for the quality control purpose. Qualitative tests are used to detect the presence of functional groups, which play a very important role in the expression of biological activity. The present study reveals the presence of tannins, mucilage, ascorbic acid, alkaloids, saponins, glycosides, flavonoids and carbohydrates in the formulation, whereas sterols/terpenoids and starch were found to be absent [Table 5].
Preliminary HPTLC profile of Dashanga Kwatha Ghana tablet has been developed [Figures 1–3]. This can be considered as the reference standard for validating this formulation in future [Tables 6 and 7].
Figure 1.
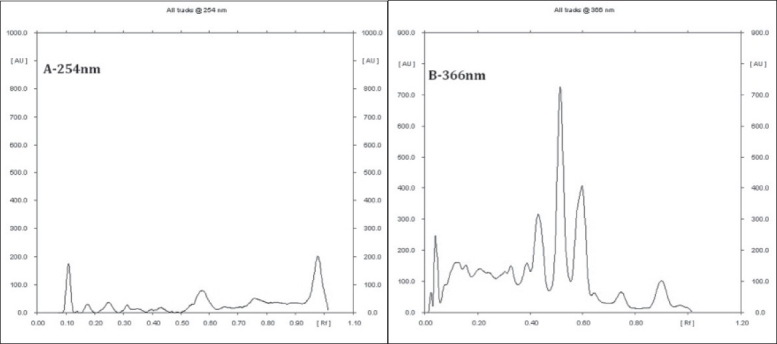
Densitogram of Dashanga Kwatha Ghana tablets (A – 254 nm, B – 366 nm)
Figure 3.
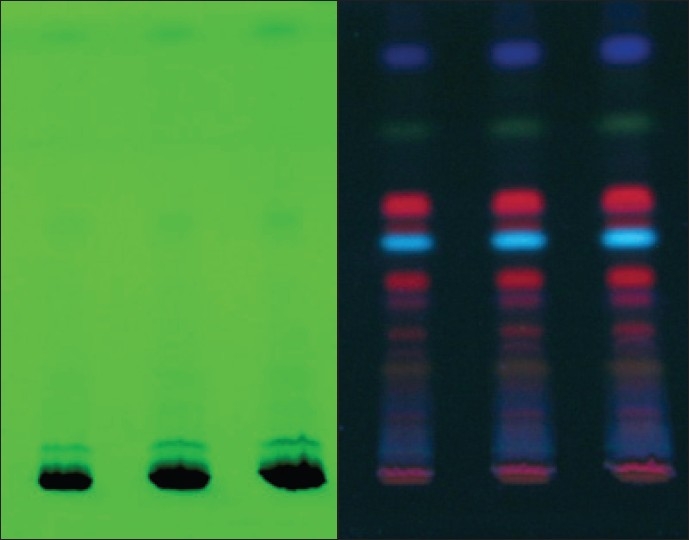
Photograph chromplate (A – 254 nm, B – 366 nm)
Table 7.
Rf values of HPTLC analysis of methanolic extract at short UV (254 nm)

Figure 2.
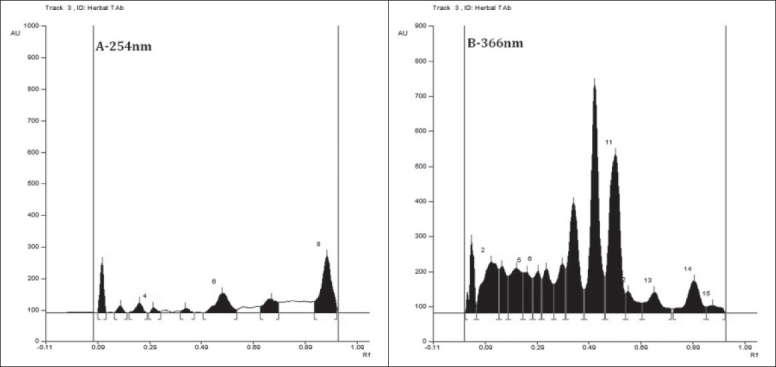
HPTLC profile of Dashanga Kwatha Ghana tablets (A – 254 nm, B – 366 nm)
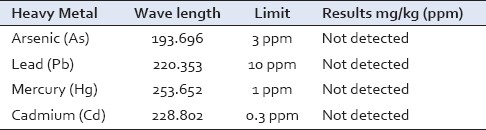
Heavy metals were not detected [Table 8], thus showing the purity of the raw drugs and also the finished product. This also indicates quality control maintenance during pharmaceutical preparation.
Table 8.
Heavy metal analysis
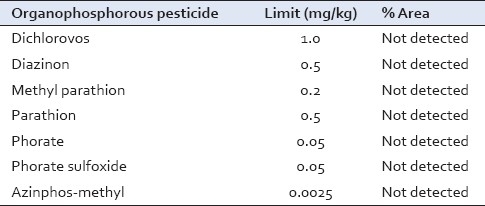
Medicinal plant materials are liable to be affected by pesticide residues which accumulate from agricultural practices of spraying, treating soils during cultivation and through the administration of fumigants during storage. Organophosphorous pesticides were found below the detection limit, which is a clear indication of quality land practices and safe storage of raw drugs [Table 9].
Table 9.
Pesticide residual value
Medicinal plant matters normally carry bacteria and moulds often originating in soil in high numbers. In the present formulation, the microbial count was within permissible limits[24] [Table 10], which indicates the proper hygiene norms followed during the preparation of formulation and packing.
CONCLUSIONS
The study reveals that sufficient quality control parameters were followed during the preparation of formulation. Organoleptic parameters, physicochemical analysis, heavy metal analysis, pesticide residue and microbial overload analysis were carried out as per the norms of WHO guidelines and the absence of heavy metals, pesticides in raw material and microbes in the finished product indicates the genuineness of final product. HPTLC profile generated in this particular study can be considered as a preliminary tool ascertaining the authenticity of Dashanga Kwatha Ghanavati in the tablet form.
ACKNOWLEDGMENT
The author would acknowledge the Dean and Director of IPGT and RA, Gujarat Ayurved University, Jamnagar.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shankar D, Ved D.K. A balanced perspective for management of Indian medicinal plants, The Indian Forester. Vol. 129. Dehradun: Nabu Press; 2003. pp. 275–88. [Google Scholar]
- 2.Charaka, Charaka Samhita, Chakrapaanidatta . In: Ayurved Dipika, Commentary. Reprint ed. Acharya YT, editor. Varanasi: Chaukhambha Surabharati Prakashana; 2005. p. 31. [Google Scholar]
- 3.Sharma PV. In: Chakradutta, editor. Varanasi: Chaukhambha Publishers; 2007. p. 413. [Google Scholar]
- 4.Shastri Parashuram, Sharangadhara Samhita. 4th ed. Varanasi: Chaukhamba Orientalia; 2000. p. 206. [Google Scholar]
- 5.Sharma PV, Chakradutta, editors. Varanasi: Chaukhambha Publishers; 2007. p. 413. [Google Scholar]
- 6.Acharya Yadavavji Trikamji. Siddha Yoga Sangraha. 11th ed. Nagpur: Baidyanath Ayurveda Bhavana; 2000. p. 4. [Google Scholar]
- 7.Government of India, Ministry of Health and Family welfare. Appendix - 8.1:A-95. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 8.Government of India, Ministry of Health and Family welfare. Appendix - 8.7:A-89. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 9.Government of India, Ministry of Health and Family welfare. Appendix - 3.38:A-54. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 10.Government of India, Ministry of Health and Family welfare. Appendix - 3.38:A-54. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 11.Government of India, Ministry of Health and Family welfare. Appendix - 3.38:A-54. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 12.Government of India, Ministry of Health and Family welfare. Appendix - 3.38:A-54. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 13.Government of India, Ministry of Health and Family welfare. Vol. 2. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 736. [Google Scholar]
- 14.Government of India, Ministry of Health and Family welfare. Vol. 2. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 734. [Google Scholar]
- 15.Government of India, Ministry of Health and Family welfare. Appendix - 7.1:A-80. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 16.Government of India, Ministry of Health and Family welfare. Appendix - 7.1:A-80. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 17.The Ayurvedic Pharmacopoeia of India. Part I. 1st ed. Vol. 1. New Delhi: Department of AYUSH, Ministry of Health and Family Welfare; 2001. p. 214. [Google Scholar]
- 18.Baxi AJ, Shukla VJ, Bhatt UB. Methods of qualitative testing of some Ayurvedic formulations. Jamnagar: Gujarat Ayurved University; 2001. pp. 5–12. [Google Scholar]
- 19.Chatwal GR, Anand SK. Industrial Method of Chemical Analysis. 5th revised and Enlarged ed. New Delhi: Himalaya Publishing House; 2005. pp. 2.272–2.503. (2.599- 2.616, 2.673-2.700). [Google Scholar]
- 20.Anonymous, Quality Control Methods for Medicinal Plant Materials (An Authorized publication of World Health Organisation, Geneva) New Delhi: A.I.T.B.S. Publishers & Distributors (Regd.); 2002. pp. 68–70. [Google Scholar]
- 21.Anonymous, Quality Control Methods for Medicinal Plant Materials (An Authorized publication of World Health Organisation, Geneva) New Delhi: A.I.T.B.S. Publishers & Distributors (Regd.); 2002. pp. 53–61. [Google Scholar]
- 22.Hill WE, Datta AR, Feng P, Lampel KA, Payne WL. Identification of Food borne Bacterial Pathogen. In: Merker IR, editor. FDA's Bacteriological Analytical Manual. 8th ed. Washington: AOAC International Publishers; 1995. pp. 353–88. Revision A/1998. [Google Scholar]
- 23.Remington . The Science and Practice of Pharmacy. 3rd Indian Reprint ed. 1 and 2. New Delhi: Wolters Kluwer Publishers; 2009. The Science and Practice of Pharmacy, Vol.1 and 2, 3rd Indian Reprint ed; p. 891. 45. [Google Scholar]
- 24.Feng P. Rapid Methods for Detecting Foodborne Pathogens. In: Merker IR, editor. FDA's Bacteriological Analytical Manual. 8th ed. Washington: AOAC International Publishers; 1995. pp. 548–64. Revision A/1998. [Google Scholar]


