Abstract
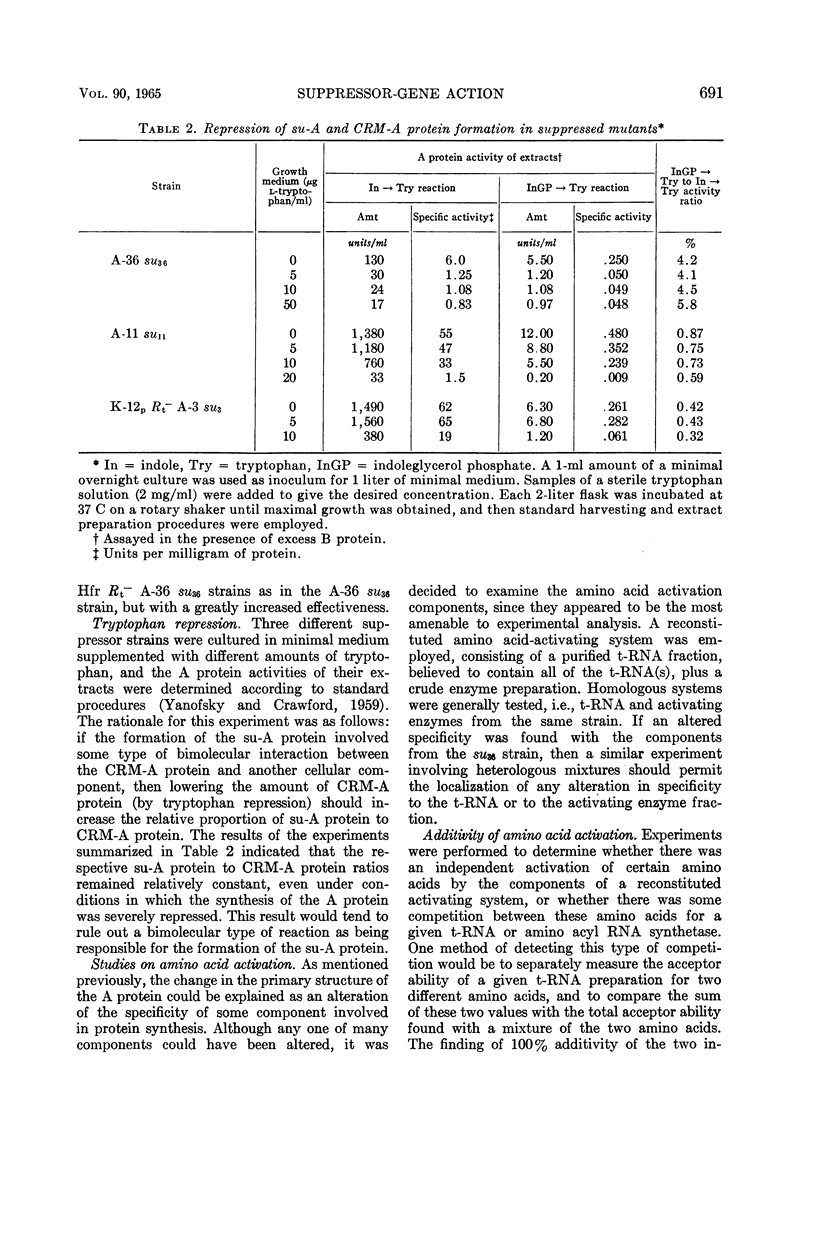
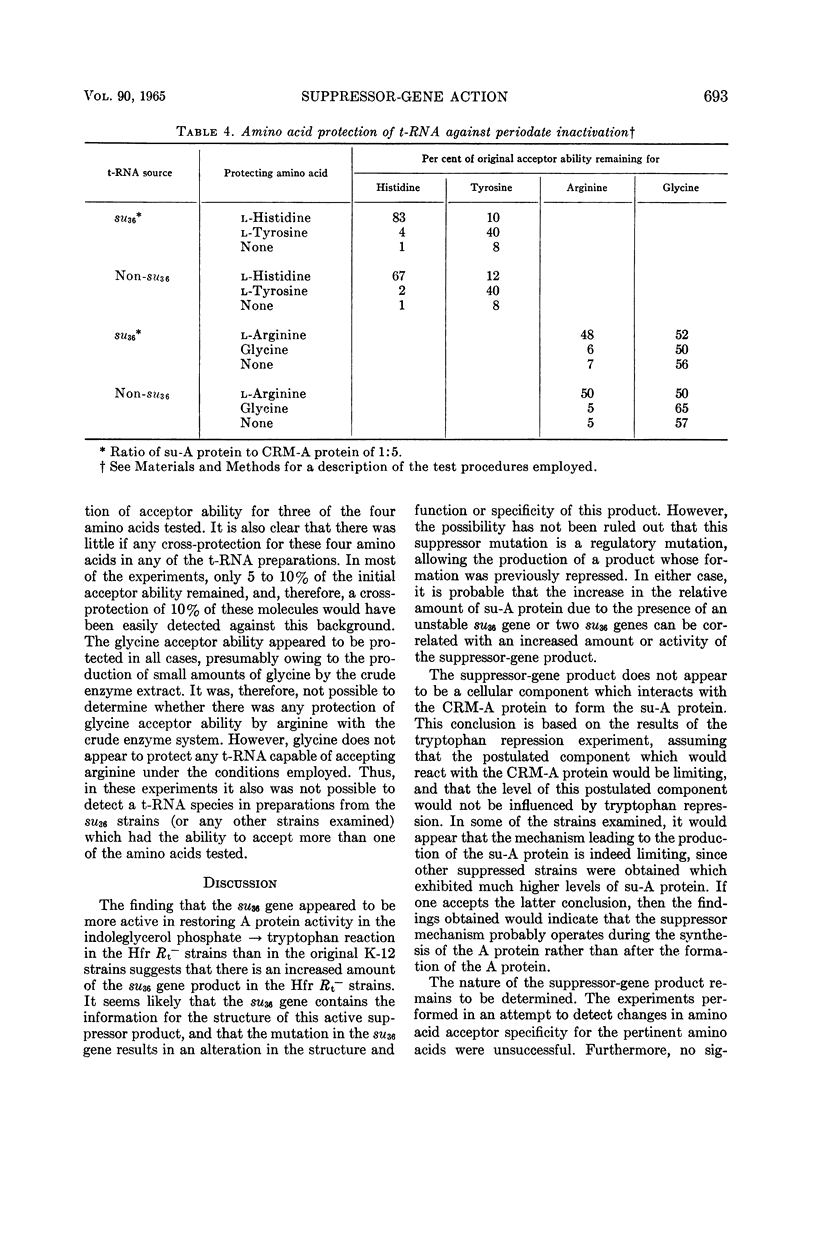
Brody, Stuart (Stanford University, Stanford, Calif.), and Charles Yanofsky. Mechanism studies of suppressor-gene action. J. Bacteriol. 90:687–695. 1965.—Mutations which change the primary structure of the A protein of the tryptophan synthetase of Escherichia coli can be reversed by allele-specific suppressor mutations. Normally, the suppressors of a particular A mutant lead to the appearance of small amounts of a wild-type-like A protein (su-A protein), in addition to the cross-reacting material antigenically similar to the normal A protein (CRM-A protein). In some cases, the particular ratio of su-A protein to CRM-A protein, indicative of a given suppressor gene, was increased when that suppressor gene was transduced into a different strain, such as a K-12 Hfr stock of E. coli. In these cases, there was a general correlation between an increased ratio and a marked instability of the suppressor gene. However, stable suppressed stocks were isolated in the Hfr strain, which also produced a high proportion of su-A protein. The ratios of su-A protein to CRM-A protein remained relatively constant under conditions of tryptophan repression in three different suppressor stocks, suggesting that the formation of each of the su-A proteins does not involve the interaction of a CRM-A protein with any other cellular constituent. It would appear, then, that the changes in the primary structure of the A protein which lead to the formation of the su-A proteins are determined before or during, but not after, the synthesis of the polypeptide chain. The specificity of amino acid activation was investigated in strains bearing one of the suppressor genes. These studies failed to reveal any significant alteration in the amino acyl ribonucleic acid (RNA) synthetases or the transfer RNA molecules for arginine, glycine, histidine, and tyrosine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZER S., CHAMPE S. P. A change from nonsense to sense in the genetic code. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1114–1121. doi: 10.1073/pnas.48.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODY S., YANOFSKY C. Suppressor gene alteration of protein primary structure. Proc Natl Acad Sci U S A. 1963 Jul;50:9–16. doi: 10.1073/pnas.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD I. P. Identification of the triose phosphate formed in the tryptophan synthetase reaction. Biochim Biophys Acta. 1960 Dec 4;45:405–407. doi: 10.1016/0006-3002(60)91474-8. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. THE FORMATION OF A NEW ENZYMATICALLY ACTIVE PROTEIN AS A RESULT OF SUPPRESSION. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1280–1287. doi: 10.1073/pnas.45.8.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON G. W., SMITH-KEARY P. F. Episomic control of mutation in Salmonella typhimurium. Heredity (Edinb) 1963 Feb;18:1–20. doi: 10.1038/hdy.1963.1. [DOI] [PubMed] [Google Scholar]
- GAREN A., SIDDIQI O. Suppression of mutations in the alkaline phosphatase structural cistron of E. coli. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1121–1127. doi: 10.1073/pnas.48.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDERSEN W. B. NEW TYPE OF STREPTOMYCIN RESISTANCE RESULTING FROM ACTION OF THE EPISOMELIKE MUTATOR FACTOR IN ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:510–516. doi: 10.1128/jb.86.3.510-516.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. F. The stability of spontaneous and ultraviolet-induced reversions from auxotrophy in Escherichia coli. J Gen Microbiol. 1963 Feb;30:289–297. doi: 10.1099/00221287-30-2-289. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Preiss J., Berg P., Ofengand E. J., Bergmann F. H., Dieckmann M. THE CHEMICAL NATURE OF THE RNA-AMINO ACID COMPOUND FORMED BY AMINO ACID-ACTIVATING ENZYMES. Proc Natl Acad Sci U S A. 1959 Mar;45(3):319–328. doi: 10.1073/pnas.45.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ N. M. NATURE OF ETHYL METHANESULFONATE INDUCED REVERSIONS OF LAC-MUTANTS OF ESCHERICHIA COLI. Genetics. 1963 Oct;48:1357–1375. doi: 10.1093/genetics/48.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERBIN H., CHAIKOFF I. L., IMADA M. R. Rapid sensitive method for determining H3-water in body fluids by liquid scintillation spectrometry. Proc Soc Exp Biol Med. 1959 Oct;102:8–12. doi: 10.3181/00379727-102-25125. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., HELINSKI D. R., MALING B. D. The effects of mutation on the composition and properties of the A protein of Escherichia coli tryptohan synthetase. Cold Spring Harb Symp Quant Biol. 1961;26:11–24. doi: 10.1101/sqb.1961.026.01.006. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Crawford I. P. THE EFFECTS OF DELETIONS, POINT MUTATIONS, REVERSIONS AND SUPPRESSOR MUTATIONS ON THE TWO COMPONENTS OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Jul;45(7):1016–1026. doi: 10.1073/pnas.45.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]


