There are reportedly more than 1,00,000 drug formulations in the market in India. I have not seen a complete list of all the formulations nor been able to verify this figure which has remained unchanged since my days as a postgraduate (which was a very long time ago). However, anyone who has had the patience to look at the branded formulations in any one of the indexing drug formularies will be convinced that this figure could be correct. One of the recent issues of Indian Drug Review (IDR) had approximately 1980 medicines listed under the alphabet ‘A’.[1] Some other alphabets had much longer lists. So what is wrong with a country having such a large number of medicines, in different dosage formulations of varying strengths?
The case report described in page “189” is one such example of the many dangers of having too many choices. Paracetamol is one of the most commonly used analgesic and antipyretic in children. The oral liquid formulation is used mainly in small children. Leaving aside the fact that the parents could not read the label as it was in Spanish and presumed the strength to be similar to what is commonly prescribed used in the United Kingdom, why should there be a formulation with a dosage strength of 500 mg/5 ml? Any child requiring this dose (500 mg) should, in the normal course of events, be able to swallow a tablet of the same strength. There may be a small group of elderly patients who are not able to swallow tablets who will perhaps need this formulation. But this group will be extremely small, so small that it would not be financially beneficial for these companies to produce this strength solely for this small group of potential users. However, the potential for harm, as illustrated in this report, is much bigger.
Take the example of paracetamol liquid formulations given in Table 1. The table highlights the many strengths available in India for a single formulation such as suspension. It is easy for doctors and pharmacists to get confused as to the quantity of paracetamol in 1 ml when calculating the dosage for small children.
Table 1.
Oral liquid formulations of paracetamol in varying strengths marketed in India[1]
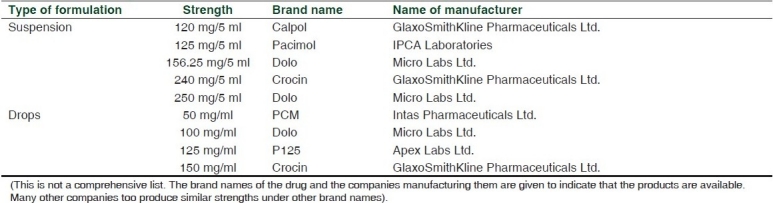
Economically disadvantaged parents of children often buy one medicine and use it in two or more children of varying ages. The dangers of an infant with fever receiving the paracetamol prescribed for a 5-year-old child is all too common. The highest strength of suspension is 50 mg/ml whereas the highest strength in the drops is 150 mg/ml, nearly thrice the strength found in the syrup. It is easy for parents to confuse the drops and the syrup and end up giving a child a very high dose(s) of paracetamol.[2] While in the developed countries paracetamol poisoning is common and drugs like n-acetylcysteine to treat the poisoning will be available all over the country, the same cannot be said of India and other resource-limited countries. In the case report published in this issue, the cause for the child's condition was picked up by an alert medical student. In India there are many primary health centers without qualified doctors visiting on a regular basis. Will a community health worker have the requisite knowledge to be able to suspect such an adverse effect and prevent a clinically adverse outcome?
Some pharmaceutical companies have two oral liquid formulations of different strengths e.g. Febrex (manufactured by Indoco Remedies Ltd),[1] the suspension is 250 mg/5 ml and the syrup is 125 mg/5 ml both of 60 ml in volume. A chemist can easily overlook the difference in strength when dispensing and sell the suspension instead of the syrup and the child will end up getting twice the required dose. Doctors too may easily confuse the strengths when prescribing. In India, these medicines are sold without oral syringes or dosing spoons, and hence the spoons used by parents to medicate their children will range in volume from a teaspoon to a dessertspoon. This makes the dosing even more dangerous (or not) depending on the size of the spoon.
If a child weighed 10 kg, a single dose of paracetamol would be calculated to be 150 mg at 15 mg/kg per dose. If the child were to be given this dose from a suspension of 120mg/5ml strength, the volume would be 6.25 ml. If given the same dose from the 125 mg/5 ml formulation, the volume would be 6 ml. It is obvious that with the kind of inaccurate dosing that parents give children even in the developed countries,[3] the difference in volume received by the child between a formulation of 120 mg/5 ml and 125 mg/5 ml, which in this case is only quarter of a milliliter, will be negligible. Therefore, there is no sound rationale for having two strengths which vary very little.
Why then do we have so many formulations in the market? Pharmaceutical companies try to sell their brand to doctors. The more aggressive the marketing, the chances are that a doctor will prescribe only that company's brand. They also get chemist shops to stock only their brand, and therefore, chemists substitute prescriptions at their whim and fancy, explaining to the patient that the medicine being dispensed by him/her is as good as the one written by the doctor. But a more disturbing reason is that companies often promote their medicines to officers responsible for hospital drug procurement.[4] When the technical specifications for pooled procurement are made, these companies see to it that “their” specifications are included. This means that they may very well be the single company to quote their product in the tender.
The dangers of this kind of numerous formulations of many strengths have special significance in countries like India since laws meant to protect consumers are not implemented. Under Section 42 of the Pharmacy Act of 1948, no person other than a registered pharmacist can dispense any medicine prescribed by a registered medical practitioner.[5] However, it is common knowledge that many, if not most, registered retail pharmacy shops do not have a pharmacist to dispense medicines. There have been numerous instances where I have been served by shop assistants who can barely read the drug labels in English, leave alone knowing enough to work out the intricacies of drug dosage strengths. Dispensing errors, therefore, can very easily occur, not only in the crowded outpatient department pharmacies of public hospitals but also in wards. A tertiary care hospital in South India reported the incidence of dispensing errors to be 4.8% in a medical ward with more than 40% of them due to wrong medication.[6] But mostly these go unnoticed and unreported due to poor reporting of adverse drug reactions and an unsuspecting, typically gullible public.
The different strengths of insulin available in India are also a cause for concern. Without going into the various formulations of insulin, 100 U/ml and 40 U/ml of various insulin preparations are available in India with syringes specifically manufactured to match these strengths. A person injecting himself or herself insulin of 100 U/ml strength with a 40 U/ml syringe is likely to experience severe hypoglycemic reactions. In a country like ours, where syringes are stored, used and re-used many times over for economic reasons, and where illiteracy is still widespread and quacks are rampant, the possibility of patients and quacks confusing the syringes and the strengths of insulin is very real. In November 2008, the Food and Drugs Administration of the United States of America (USA) recalled syringes from all over USA due to a mix-up in the labeling of 100 U/ml and 40 U/ml insulin syringes.[7] Such an exercise will be nearly impossible here.
Is there a way to safeguard patients from these kinds of accidental mishaps? There are a wide range of patient safety measures which can be instituted by Drugs and Therapeutic Committees of hospitals and other professional bodies. Even the implementation of the Pharmacy Act 1948 will certainly go a long way toward this goal. But all these measures will be easily offset by the sheer numbers of formulations in the market. The only way in which we can save our public is by having a limited number of formulations. We must be aware that drug dispensing is being done in a less than optimal manner all over the country. Having innumerable strengths of formulations is a sure way to invite problems. While it may not be the panacea for all the problems it will certainly limit the quantum. It is time the Drugs Controller General of India took stock of the formulations under each drug and permitted only the strengths necessary to cover the age bands to be marketed in the country. We also need more studies done in public as well as private hospitals and other facilities to document issues such as prescribing and dispensing errors. Lack of evidence does not mean these problems do not exist. Though there are some who believe that the issues related to paracetamol overdosage may be exaggerated,[8] the reverse could be true in our country.[2] Until the drug regulators wake up to this issue, let us hope less children will suffer due to no fault of theirs.
REFERENCES
- 1.Indian Drug Review triple i Drug Compendium. Vol. 16. Bangalore: CMP Medica India Pvt. Limited; 2010. [Google Scholar]
- 2.Kannan R. How overdose of paracetamol hits kids. The Hindu, Chennai. [Last accessed on 2011 May 06]. Available from: http://www.thehindu.com/health/policy-and-issuesarticle553961.ece .
- 3.Li SF, Lacher B, Crain EF. Acetaminophen and ibuprofen dosing by parents. Pediatr Emerg Care. 2000;16:394–7. doi: 10.1097/00006565-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Cortese DA. Managing Conflict of Interest in Clinical Practice. Mayo Clin Proc. 2007;82:607–14. doi: 10.4065/82.5.607. [DOI] [PubMed] [Google Scholar]
- 5.The Pharmacy Act 1948. Enactment date [4th March 1948] [Last accessed on 2011 May 20]. Available from: http://www.dfda.goa.gov.in/uploads/Pharmacy%20Act%201948.pdf .
- 6.Sekhar SM, Mathew MA, Abraham S, Anand A, Sasidharan S. Study on dispensing errors of inpatient prescriptions in a tertiary care hospital. Der Pharmacia Sinica. 2011;2:14–8. [Google Scholar]
- 7.FDA Reports Nationwide Recall of Mislabeled ReliOn Insulin Syringes. 2008. Nov 5, [Last accessed on 2011 May 20]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116976.htm .
- 8.Prescott LF. Therapeutic misadventure with paracetamol: Fact or fiction.? Am J Ther. 2000;7:99–114. doi: 10.1097/00045391-200007020-00007. [DOI] [PubMed] [Google Scholar]


