Abstract
The hypertriglyceridemic waist (HTGW) and metabolic syndrome (MS) are associated with increased cardiometabolic risk. We evaluated the impact of the HTGW on cardiometabolic risk factors in obese women diagnosed with the MS. Thirty-six abdominally obese women with the MS as defined by the International Diabetes Federation (IDF) [(mean (SD); age 49 (11) y, ht 165 (6) cm, wt 95 (16) kg] participated. The HTGW was defined as follows: a waist circumference ≥80 cm and triglycerides ≥1.7 mM. Unpaired t-tests and Analysis of Covariance (ANCOVA) were employed to detect mean differences between women with MS plus or minus HTGW. Women with the MS plus HTGW had higher total cholesterol (16%, p=0.015), VLDL-cholesterol (97%, p<0.001), non-HDL-cholesterol (16%, p=0.002), insulin (40%, p=0.043), and abdominal visceral fat (24%, p=0.100), and lower total HDL-cholesterol (6%, p=0.024), HDL3 (11%, p=0.031) and Quantitative Insulin Sensitivity Check Index (QUICKI) (5%, p=0.068) compared with women with the MS minus HTGW. Thus, the presence of the HTGW was accompanied by a worsened cardiometabolic risk factor profile in these obese women with the MS. In particular, women with the MS plus HTGW were more insulin resistant compared to women with the MS minus HTGW. In conclusion, the presence of the HTGW in obese women with the MS exacerbates insulin resistance and cardiometabolic risk factors.
Keywords: Adiposity, cardiovascular disease, diabetes, Syndrome X
INTRODUCTION
Both the hypertriglyceridemic waist (HTGW) and the metabolic syndrome (MS) have been associated with increased cardiometabolic risk, including insulin resistance, atherogenic dyslipidemia, hypertension, endothelial dysfunction, low-grade inflammation, and impaired hemostasis (1–5). The HTGW is defined as the simultaneous presence of abdominal adiposity and hypertriglyceridemia (6). Lemieux et al. (6) were the first group to recognize that the HTGW phenotype was associated with increased cardiometabolic risk in men. In particular, the HTGW was associated with the atherogenic triad of hyperinsulinemia, elevated concentrations of apolipoprotein B and small, dense low density lipoprotein cholesterol (LDL-cholesterol) particles. In corroboration, recent data suggest that the HTGW is associated with increased cardiometabolic risk in women (4) and in Canadian Aboriginals where the HTGW was associated with a five-fold increased risk for the development of Type 2 diabetes mellitus (T2DM) (7). The effects of the HTGW in these studies were examined independent of the MS.
Tanko et al. (5) have recently suggested that the combined presence of an elevated waist circumference and elevated triglyceride (TG) concentrations may be the best indicator of cardiovascular disease risk in postmenopausal women independent of the presence of the MS. The HTGW appears to increase the risk associated with T2DM, cardiovascular disease, and all-cause mortality in men and women with and without the MS. However, few data exist (3, 5) that examine the combined impact of the MS and HTGW on both traditional and nontraditional risk factors, and, to our knowledge no data exist that examine the effects of lowering the waist circumference and TG thresholds to match the International Diabetes Federation (IDF) criteria related with the MS on the association between the HTGW and traditional and nontraditional risk factors. Thus, the purpose of the present investigation was to determine whether the HTGW in women with the MS exacerbates the deleterious impact on body composition, cardiorespiratory fitness, lipids and lipoproteins, measures of glucose metabolism, and inflammation using the IDF criteria for the MS and the HTGW.
METHODS
Participants
Thirty-six sedentary-to-lightly active (≤2 days per week of structured exercise), women who met the IDF criteria for the MS (8) participated in the present study. Participants were recruited in an ongoing exercise training investigation at the University of Virginia’s General Clinical Research Center (GCRC). The Institutional Review Board, Human Investigation Committee of the University of Virginia’s Health System approved this study, and each participant provided written informed consent. Exclusion criteria included a history of ischemic heart disease, type 1 diabetes, pulmonary or musculoskeletal limitations to exercise, uncontrolled hypertension (>160/105 untreated or >145/95 treated) or use of vasoactive medications, oral hypoglycemics, insulin, glucocorticoids, anti-psychotics, hormone replacement or birth control, and if pregnant, breast feeding or unwilling to provide written informed consent.
Metabolic syndrome and medical screening protocol
Participants reported to the GCRC for screening after a 10 to 12 hour fast at approximately 09:00 h. Participants provided a detailed medical history and underwent a physical examination, which included an assessment of the five risk factors associated with the MS (8). In brief, waist circumference measurements were taken in triplicate to the nearest 0.1 cm using a non-elastic measuring tape, midway between the iliac crest and the lowest rib, and the mean of the waist circumference measurements was used in accordance with the guidelines established in Lohman et al (9). Blood pressure was assessed in a seated position with legs uncrossed using an automated sphygmomanometer (Dynamap 100, General Electric, Tampa, FL) after participants sat quietly for 10 to 15 minutes. The mean of two measurements of systolic and diastolic pressures were used in accordance to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the Joint National Committee 7 report (10). Fasting blood samples were then drawn and serum was separated by centrifugation. Glucose, total cholesterol, TG, and HDL-cholesterol concentrations were assessed in serum. Glucose concentrations were determined by using an automated glucose analyzer (YSI Instruments 2300 STAT Plus, Yellow Springs, OH). Total cholesterol, TG and HDL-cholesterol concentrations were determined using an Olympus AU640 automatic analyzer (Olympus, Melville, NY). All participants were asked to refrain from caffeine, alcohol, nicotine and vigorous physical activity for 24 hours prior to testing.
Cardiorespiratory fitness assessment
Participants completed a continuous VO2 Peak treadmill protocol. The initial treadmill (Quinton Q65, Seattle, WA) velocity was 60 m·min−1 and the velocity was increased by 10 m·min−1 every 3 minutes until volitional fatigue. Metabolic data were collected during the protocol using standard open-circuit spirometric techniques (Viasys Vmax 229, Yorba Linda, CA) and heart rate was assessed electrocardiographically (Marquette Max-1 electrocardiograph, Marquette, WI). VO2 Peak was chosen as the highest oxygen uptake attained during the exercise protocol. Respiratory exchange ratio, heart rate and blood lactate responses were monitored to insure that participants attained maximal values at the point of volitional exhaustion.
Physical activity assessment
The time spent in physical activity at different intensities was assessed using the Aerobic Center Longitudinal Study’s Physical Activity Questionnaire (11). The questionnaire was administered using an interview technique to increase accuracy (12). Total physical activity was calculated as MET·H·Week−1 (1 MET=3.5 ml·kg·min−1) using the Compendium of Physical Activities (13).
Clinical research center admission
Participants were admitted to the GCRC for a 2-day admission during which the following evaluations were performed (see below). To control for the effects of menstrual cycle on outcome variables, premenopausal women (n=11) were admitted between days 2–8 of their menstrual cycle. All participants were asked to refrain from caffeine, alcohol, nicotine and vigorous physical activity for 72 hours prior to testing.
Abdominal visceral fat and body composition
Body density was measured using air displacement plethysmography (Bod-Pod, Life Measurement Instruments, Concord, CA) corrected for thoracic gas volume as described previously (14). The Siri equation (15) was used to determine percent body fat from body density measurements.
Single-slice (DICOM) CT images were obtained at the level of L4-L5 for subsequent analysis. In brief, participants were tested wearing only a loose fitting hospital gown. Participants were placed in a supine position with their arms extended above their head during the duration of the measurement. An axial image of the abdomen at the level of the L4-L5 inter-vertebral disc space was performed with no angulation, using a lateral pilot for location. All scans were performed using a General Electric Lightspeed CT (GE Medical Systems, Milwaukee, WI) scanner and saved as DICOM images for analysis. Standard CT procedures of 120 kV, 5 mm thickness and a 512 × 512 matrix were used for all participants.
A single trained investigator analyzed each CT image in duplicate using Slice-O-Matic version 4.3 (Tomovision) using a modified protocol previously described (16) for the delineation and quantification of cross-sectional areas of adipose tissue. Adipose tissue cross-sectional areas (cm2) were calculated using standard Hounsfield unit ranges [Adipose tissue: −190 to −30 (17)] by delineating regions of interest with a mouse computer interface, with the thresholding function initially used to set the appropriate Hounsfield unit ranges. Compartmental segmentation was computed once the appropriate threshold Hounsfield unit ranges were set. Subsequently, the region-growing function was used to identify region of interest and to differentiate and quantify adipose tissue depots. The measurement boundary for abdominal visceral fat was defined as the innermost aspect of the abdominal and oblique muscle walls and the posterior aspect of the vertebral body, as described previously (18).
Blood sampling procedures
To obviate nutritional confounds, participants ingested a standardized snack at 20:00 h the evening before, comprising 500 kcal (60% CHO, 20% protein, and 20% fat), and then remained fasting in the GCRC overnight. A forearm venous catheter was inserted at 06:00 h the next morning. At 08:00 h a fasting blood sample was obtained for lipid and lipoproteins, free fatty acids (FFA), HbA1c, glucose, insulin, and markers of inflammation using the following analytical methods. Serum samples for analysis of insulin and markers of inflammation were stored at −70°C until subsequent analysis in the University of Virginia’s GCRC Core Laboratory.
Total cholesterol, LDL-cholesterol, HDL-cholesterol, HDL2, HDL3, intermediate density lipoprotein (IDL), Lp(a), very low density lipoprotein cholesterol (VLDL-cholesterol), and TG concentrations were measured in fresh serum using the vertical autoprofile technique (Atherotech, Birmingham, AL) described previously (19–21). Serum Free Fatty Acid concentrations were measured using an enzymatic colorimetric assay (Mayo Medical Laboratories, Rochester, MN). HbA1c was measured in fresh whole blood using high performance liquid chromatography (HPLC) in the University of Virginia’s Clinical Pathology Laboratory. Serum glucose concentrations were measured in duplicate using a glucose oxidase method (YSI Instruments 2300 STAT Plus, Yellow Springs, OH).
Serum insulin concentrations were measured using a solid-phase chemiluminescent immunometric assay (Diagnostic Products Corporation, Immulite 2000, Los Angeles, CA); the assay sensitivity was 2 ∝U·ml−1 and the mean intra- and interassay coefficients of variation were 4.1% and 5.1% for insulin. Insulin sensitivity was estimated using the Quantitative Insulin Sensitivity Check Index {QUICKI = 1/[log10 glucose (mg·dl−1) + log10 insulin (∝U·ml−1)]} (22), which has been shown to be an accurate predictor of insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp (22, 23).
TNF-alpha and IL-6 concentrations were measured in serum using commercially available high-sensitivity ELISA assays (R&D System, Inc., Minneapolis, MN); the assay sensitivities were 0.5 pg·ml−1 for TNF-α and 0.3 ng·ml−1 for IL-6; the mean intra- and interassay coefficients of variation were 6.7% and 13.4% for TNF-α, and 7.4% and 7.8% for hsIL-6. Serum homocysteine concentrations were measured using a two-phase competitive immunoassay (Diagnostic Products Corporation, Immulite 2000, Los Angeles, CA); the assay sensitivity was 0.5 ∝mol·l−1 and the mean intra- and interassay coefficients of variation were 4.9% and 6.8%. High-sensitivity (hs) CRP concentrations were measured by a commercially available solid-phase chemiluminescent assay (Diagnostic Products Corporation, Immulite 2000, Los Angeles, CA), the assay sensitivity was 0.1 mg·l−1 and the mean intra- and interassay coefficients of variation were 6.7%. Serum adiponectin concentrations were measured using a commercially available ELISA method (Linco, St. Charles, MO); the assay sensitivity was 780 ng·ml−1 and the mean intra- and interassay coefficients of variation were 6.7% and 13.4%.
Hypertriglyceridemic waist
As stated previously, the HTGW is defined as the simultaneous presence of abdominal adiposity and hypertriglyceridemia, specifically, the HTGW has been defined as the simultaneous elevation in both waist circumference and fasting triglycerides (6). In the present study we evaluated the impact of the HTGW on cardiometabolic risk profile in obese women who were also diagnosed with the MS [HTGW: a waist circumference ≥80 cm and TG ≥1.7 mmol/l (lowering waist circumference from 90 cm and TG from 2.0 mmol/l to be consistent with the IDF guidelines (8))].
Statistical analysis
All statistical analyses were conducted using SAS software (SAS Version 9.1.3, Cary, NC). Data are presented as means ± SD. Two-tailed, unpaired t-tests were employed to examine mean differences between groups. In addition, analysis of covariance (ANCOVA) was used and mean differences between groups adjusted for abdominal visceral fat. Non-normally distributed variables were log transformed for analysis.
RESULTS
Table 1 presents the means and standard deviations for measures of cardiorespiratory fitness, physical activity, abdominal visceral fat, body composition, lipid and lipoproteins, measures of glucose metabolism, and markers of inflammation in obese women with MS according to HTGW status.
Table 1.
Measures of cardiorespiratory fitness, physical activity, body composition and cardiometabolic risk factors in obese women with the MS plus or minus HTGW€.
| MS minus HTGW | MS plus HTGW | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | p-value¥ | |
| n | 15 | 21 | ||||
| Age (yr) | 50.4 | 8.1 | 47.4 | 12.3 | 0.413 | - |
| Height (cm) | 166.3 | 6.3 | 164.8 | 5.2 | 0.438 | - |
| Weight (kg) | 97.7 | 19.9 | 93.9 | 17.0 | 0.544 | - |
| BMI (m·kg−2) | 35.4 | 7.4 | 34.6 | 6.1 | 0.735 | - |
| % Body fat | 43.8 | 6.1 | 45.4 | 3.9 | 0.347 | - |
| Waist circumference (cm) | 102.4 | 11.4 | 103.5 | 12.5 | - | - |
| Abdominal visceral fat (cm2) | 137.1 | 44.7 | 169.9 | 64.8 | 0.100 | - |
| VO2 Peak (ml·kg−1·min−1) | 20.4 | 3.1 | 21.7 | 4.0 | 0.303 | - |
| VO2 Peak (ml·kg-FFM−1·min−1) | 36.7 | 4.2 | 39.8 | 7.0 | 0.138 | - |
| MET-H·Week−1 | 157.3 | 71.9 | 114.2 | 53.3 | 0.046 | - |
| Systolic blood pressure (mmHg) | 131.8 | 16.6 | 127.7 | 13.0 | 0.406 | 0.096 |
| Diastolic blood pressure (mmHg) | 77.5 | 11.2 | 79.5 | 7.0 | 0.502 | 0.791 |
| Free fatty acids (mmol·l−1)* | 0.637 | 0.158 | 0.581 | 0.261 | 0.240 | 0.260 |
| HbA1c | 5.7 | 0.5 | 5.6 | 0.4 | 0.374 | 0.211 |
| C-reactive protein (mg·l−1)* | 4.5 | 3.4 | 5.6 | 5.05 | 0.583 | 0.828 |
| Tumor necrosis factor-α (pg·ml−1)* | 3.2 | 4.6 | 2.1 | 2.2 | 0.903 | 0.983 |
| Interleukin-6 (ng·ml−1)* | 3.1 | 2.4 | 3.2 | 4.9 | 0.635 | 0.619 |
| Adiponectin (ng·ml−1)* | 8.3 | 3.4 | 9.1 | 3.6 | 0.375 | 0.535 |
| Homocysteine (μmol·ml−1)* | 7.4 | 1.9 | 7.4 | 1.6 | 0.948 | 0.536 |
Log transformed for analysis;
Adjusted for abdominal visceral fat;
HTGW (waist ≥80 cm and triglycerides ≥1.7 mmol·l−1).
Cardiorespiratory fitness and physical activity
The mean VO2 Peak values classified the subjects as unfit by VO2 Peak standards (i.e., having VO2 Peak values that fall within the lowest 20th percentile for their age and sex) (24), regardless of HTGW status. There were no significant differences in VO2 Peak between women with MS plus or minus HTGW (Table 1). Women with MS plus HTGW reported ~27% (p=0.046) fewer MET-H·week−1 than women MS minus HTGW (Table 1).
Abdominal visceral fat and body composition
There were no significant differences in body weight, % body fat, or BMI. Abdominal visceral fat values were ~24% (p=0.100) higher in women with MS plus HTGW than women with MS minus HTGW (Table 1).
Glucose metabolism
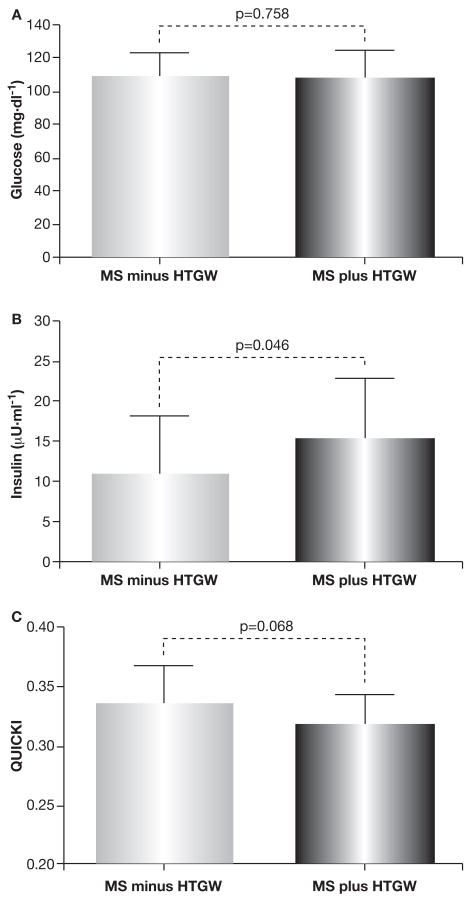
There were no significant differences in fasting FFA or HbA1c between women with the MS plus or minus HTGW (Table 1). Moreover, there were no significant differences in fasting blood glucose (Fig. 1A) Fasting insulin concentrations were ~43% (p=0.043) higher in women with MS plus HTGW, compared to women with MS minus HTGW (Fig. 1B). Insulin sensitivity assessed by the QUICKI model was ~5% lower in women with MS plus HTGW (p=0.068) compared to women with MS minus HTGW (Fig. 1C).
Fig. 1.
Fasting blood glucose, insulin, and insulin sensitivity (Quantitative Insulin Sensitivity Check Index: QUICKI) in obese women with the MS plus or minus HTGW.
QUICKI= 1/[log10 glucose (mg·dl−1) + log10 insulin (∝U·ml−1)]; HTGW (waist ≥80 cm and triglycerides ≥1.7 mmol·l−1).
Lipids and lipoproteins
Total cholesterol, VLDL, TG, non-HDL-cholesterol, Lp(a) concentrations were significantly elevated in the women with the MS plus HTGW compared to women with the MS minus HTGW (Table 2). HDL-cholesterol, HDL2, and HDL3 concentrations were ~6% (p=0.024), ~17% (p=0.067), and ~11% (p=0.031) lower in women with MS plus HTGW compared to women with MS minus HTGW (Table 2).
Table 2.
Lipid and lipoproteins in obese women with the MS plus or minus HTGW€.
| MS minus HTGW | MS plus HTGW | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | p-value¥ | |
| n | 15 | 21 | ||||
| Total Cholesterol (mg·dl−1)* | 193.8 | 31.8 | 224.5 | 35.9 | 0.015 | 0.016 |
| Very Low Density Lipoprotein (mg·dl−1)* | 20.0 | 3.4 | 39.3 | 17.6 | <0.001 | <0.001 |
| Low Density Lipoprotein (mg·dl−1)* | 124.3 | 29.3 | 143.9 | 34.0 | 0.090 | 0.075 |
| Low Density Lipoprotein Class | ||||||
| Large (%) | 40.0 | 23.8 | ||||
| Intermediate (%) | 46.7 | 38.1 | ||||
| Small (%) | 13.3 | 38.1 | ||||
| Triglycerides (mg·dl−1)* | 108.1 | 28.2 | 259.2 | 158.2 | - | - |
| High Density Lipoprotein (mg·dl−1)* | 46.1 | 6.4 | 43.4 | 9.8 | 0.024 | 0.009 |
| High Density Lipoprotein2 (mg·dl−1)* | 11.5 | 3.2 | 9.5 | 3.6 | 0.067 | 0.033 |
| High Density Lipoprotein3 (mg·dl−1)* | 38.1 | 3.8 | 33.9 | 7.1 | 0.031 | 0.013 |
| Non-High Density Lipoprotein (mg·dl−1)* | 144.3 | 29.8 | 166.7 | 36.5 | 0.002 | 0.002 |
| Lp(a) (mg·dl−1)* | 8.1 | 4.1 | 6.7 | 3.8 | 0.317 | 0.336 |
Log transformed for analysis,
Adjusted for abdominal visceral fat;
HTGW (waist ≥80 cm & triglycerides ≥1.7 mmol·l−1).
Markers of inflammation
There were no significant differences in hs-CRP, TNF-alpha, IL-6, homocysteine, or adiponectin between groups (Table 1).
DISCUSSION
Although several clinical and epidemiological studies have examined the independent effects of the HTGW and the MS on cardiometabolic risk (1, 4, 6), few studies (3, 5) have examined the combined effects of the HTGW and the MS on traditional and/or non-traditional cardiometabolic risk factors. The primary finding of the present investigation was that the HTGW exacerbates some of the cardiometabolic risk factors associated with the MS in obese women diagnosed with the MS. In particular, women with the MS plus HTGW were more insulin resistant and had a greater degree of atherogenic dyslipidemia compared to women with the MS minus HTGW.
MS and HTGW
Approximately ~60% (21/36) of the women with the MS also fulfilled the criteria for HTGW. This was not surprising in view of recent data indicating that ~70% of women with the MS as defined by the National Cholesterol Education Program Adult Treatment Panel III guidelines (NCEP ATP III) also fulfilled the elevated waist circumference (≥88 cm) and elevated TG (≥150 mg·dl−1) criteria (4).
Cardiorespiratory fitness and physical activity
In the present investigation no significant differences in VO2 Peak were observed between women with the MS plus or minus HTGW. Similarly, recent data from LaMonte et al. (24) showed no significant differences in VO2 Peak between postmenopausal women with or without the HTGW. It should be realized that the majority of women in the present investigation were very unfit for their age and sex (12), and therefore we were unable to examine an independent effect of cardiorespiratory fitness on outcome measures. However, it is possible that the presence of the HTGW may be related to physical inactivity as women with MS plus HTGW reported ~27% (p=0.046) fewer MET-H·week−1 compared to women with MS minus HTGW (Table 1). These data lend further support for the cardiometabolic protective effect of regular physical activity. Although, the assessment of physical activity is less objective than the assessment of VO2 Peak, which may account for some of the observed differences, the administration of the Aerobic Center Longitudinal Study’s Physical Activity Questionnaire using an interview technique has been shown to improve its accuracy and reproducibility (12).
Abdominal visceral fat and body composition
Overall, the participants in the present investigation would be classified as having type-1 obesity by BMI standards and abdominal obesity by waist circumference standards. We observed no significant differences between the participants with the MS plus or minus HTGW for body weight, % body fat, or BMI. Abdominal visceral fat values were elevated to a greater extent in women with the MS plus HTGW compared to women with the MS minus HTGW, potentially placing them at increased cardiometabolic risk (3, 4, 6, 25). Although it could be hypothesized that the women with the MS plus HTGW presented with a more deleterious cardiometabolic risk profile due in part to their greater degree of visceral adiposity, statistical adjustment for abdominal visceral fat values did not significantly impact the deleterious effect that the HTGW had on the cardiometabolic risk profiles in women with the MS. This novel finding suggests that some of the increased risk associated with the HTGW in women with the MS is independent of elevations in abdominal visceral fat.
Glucose metabolism
In the present investigation women with the MS plus or minus HTGW presented with impaired fasting glucose (i.e., fasting glucose ≥100 mg/dl), normal HbA1c (i.e., <6%), and normal FFA levels. As stated previously, both the HTGW and the MS have been associated with insulin resistance (3, 4, 6, 25). The present results suggest that the presence of the HTGW exacerbates insulin resistance in women with the MS. Specifically, fasting insulin concentrations were substantially elevated in women with the MS plus HTGW compared to women with the MS minus HTGW, with concomitantly lower levels of insulin sensitivity as assessed by the QUICKI model. Perhaps the greater degree of hyperinsulinemia and insulin resistance observed in women with the MS plus HTGW could partially be explained by their lower levels of physical activity compared to women with the MS minus HTGW.
Lipids and lipoproteins
Atherogenic dyslipidemia is a primary clinical outcome associated with both the HTGW and the MS (6). Our results indicate that the HTGW exacerbates the development of atherogenic dyslipidemia in obese women with the MS. We recognize that the unfavorable differences observed in VLDL and HDL-cholesterol concentrations between women with the MS plus HTGW compared to women with the MS minus HTGW may be partially dependent on the HTGW classification strategy. Specifically, elevated TG concentrations are associated with elevations in VLDL-cholesterol and a concomitant reduction in HDL-cholesterol. The present findings suggest that the dyslipidemic risk associated with the HTGW in women with the MS accrue at lower waist circumference and TG concentrations than originally proposed (6).
Women with the MS plus HTGW had higher non-HDL-cholesterol concentrations compared to women with the MS minus HTGW. As non-HDL-cholesterol concentrations serve as a marker of apolipoprotein B concentrations, the present results support previous findings of Lemieux et al. (6) who suggested that the presence of the HTGW is associated with elevations in apolipoprotein B concentrations. In the present study ~38% of the women with the MS plus HTGW had small, dense LDL particles (i.e. subclass B), compared with only 13% of the women with the MS minus HTGW (Table 2). The simultaneous combination of hyperinsulinemia, elevated concentrations of apolipoprotein B (i.e., non-HDL), and elevated concentrations of small, dense LDL-cholesterol (i.e., LDL subclass B) in women with the MS plus HTGW indicates the presence of the non-traditional metabolic triad, which has been shown to increase the risk for ischemic heart disease by ~20-fold in men with the metabolic triad (26).
Markers of inflammation
Over the last several years, the impact of low-grade inflammation on increased cardiometabolic risk has received considerable attention. However, we did not observe any significant differences between women with MS plus or minus HTGW for the remaining markers of inflammation, which may be partially explained by the fact that in these obese women with the MS these markers are already elevated independent of the presence of the HTGW. For example, the mean CRP concentration in women with MS minus HTGW was ~4.5 mg·dl−1, which is above the suggested upper limit of CRP of 3 mg·dl−1.
Strengths and limitations
We present cross-sectional data collected as part of an ongoing exercise training study. The primary strengths of the present investigation are that, to our knowledge, this is the first investigation to comprehensively examine the association between the HTGW and body composition, cardiorespiratory fitness, lipids and lipoproteins, measures of glucose metabolism, and inflammation in obese women with the MS. We recognize that the present study is limited by a relatively small sample size of obese, sedentary women with the MS and by the lack of a healthy control group (e.g., age-matched women without any risk factors for the MS). However, it is well established that the presence of the MS exacerbates cardiometabolic risk in otherwise healthy adults. The primary purpose of the present study was to examine whether these women with the MS plus HTGW had a more deleterious cardiometabolic risk factor profile compared to women with the MS minus HTGW.
SUMMARY AND CONCLUSIONS
The presence of the HTGW exacerbates risk factors associated with the MS in obese women with the MS. In particular, the women with the MS plus HTGW were more insulin resistant and had exacerbated dyslipidemia compared to the women with the MS minus HTGW. Moreover, women with the MS plus HTGW appeared to be affected to a greater extent by the non-traditional metabolic triad compared to women with the MS minus HTGW. The present results suggest that the simple determination of whether women with the MS also have the HTGW provides valuable information with respect to further stratifying cardiometabolic risk (i.e., a more deleterious cardiometabolic risk profile) in obese women with the MS. Finally, the present findings support previous suggestions that clinical intervention should focus on treating individual risk factors in addition to treating the MS itself.
Acknowledgments
Grant Support: This publication was made possible by NIH Grant Numbers RR00847 and T32-AT-00052.
Clinical Trial Number: NCT00350064.
The authors wish to thank the staff of the GCRC at the University of Virginia and all of the subjects who participated enthusiastically in the study. The present study was funded in part by an NIH grant to the General Clinical Research Center RR MO100847 and NIH training grant 5T32AT00052.
References
- 1.DeFronzo RA, Ferrannini E. Insulin resistance: a multi-faceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–9. [PubMed] [Google Scholar]
- 3.Kahn HS, Valdez R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am J Clin Nutr. 2003;78:928–34. doi: 10.1093/ajcn/78.5.928. [DOI] [PubMed] [Google Scholar]
- 4.LaMonte MJ, Ainsworth BE, DuBose KD, et al. The hypertriglyceridemic waist phenotype among women. Atherosclerosis. 2003;171:123–30. doi: 10.1016/j.atherosclerosis.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Tanko LB, Bagger YZ, Qin G, Alexandersen P, Larsen PJ, Christiansen C. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111:1883–90. doi: 10.1161/01.CIR.0000161801.65408.8D. [DOI] [PubMed] [Google Scholar]
- 6.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–84. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Pollex RL, Hanley AJ, Zinman B, Harris SB, Hegele RA. Clinical and genetic associations with hypertriglyceridemic waist in a Canadian aboriginal population. Int J Obes. 2006;30:484–91. doi: 10.1038/sj.ijo.0803152. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome- a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 9.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 11.Kohl HW, Blair SN, Paffenbarger RS, Jr, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–39. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 12.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 14.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–7. [PubMed] [Google Scholar]
- 15.Siri WE. Body composition from fluid spaces and density methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC: National Academy of Sciences, National Diary Council; 1969. pp. 223–44. [Google Scholar]
- 16.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:15–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 17.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 18.Clasey JL, Bouchard C, Wideman L, et al. The influence of anatomical boundaries, age, and sex on the assessment of abdominal visceral fat. Obes Res. 1997;5:395–401. doi: 10.1002/j.1550-8528.1997.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35:159–68. [PubMed] [Google Scholar]
- 20.Kulkarni KR, Marcovina SM, Krauss RM, Garber DW, Glasscock AM, Segrest JP. Quantification of HDL2 and HDL3 cholesterol by the Vertical Auto Profile-II (VAP-II) methodology. J Lipid Res. 1997;38:2353–64. [PubMed] [Google Scholar]
- 21.Kulkarni KR, Garber DW, Jones MK, Segrest JP. Identification and cholesterol quantification of low density lipoprotein subclasses in young adults by VAP-II methodology. J Lipid Res. 1995;36:2291–302. [PubMed] [Google Scholar]
- 22.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–25. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 24.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 25.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 26.Lamarche B, Tchernof A, Mauriege P, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]