Abstract
The removal of edema from the air spaces is a critical function of the alveolar barrier requiring intact tight junctions. Alveolar fluid clearance contributes to graft function after transplantation and is associated with survival in patients with acute lung injury. Claudin-4 concentrations are known to increase during lung injury and the loss of claudin-4 decreases alveolar fluid clearance in mice. This study was therefore undertaken to evaluate whether differences in lung expression of the tight junction protein claudin-4 are associated with alveolar fluid clearance or clinical measures of lung function. Alveolar fluid clearance rates were measured in ex vivo perfused human lungs not used for transplantation and were compared with histological lung injury and clinical measures of lung injury in the donors. Claudin-4 staining demonstrated a positive correlation with alveolar fluid clearance (Spearman rank correlation [rs] = 0.71; P < 0.003); however, claudin-4 staining was not strongly associated with histological measures of lung injury. The expression of other tight junction proteins (including ZO-1) was not associated with alveolar fluid clearance or claudin-4 levels. Claudin-4 staining was lower in lungs from donors with greater impairment in respiratory physiology. These data suggest that claudin-4 may promote alveolar fluid clearance and demonstrate that the amount of claudin-4 expressed may provide specific information regarding alveolar epithelial barrier function that strengthens the link between histological changes and physiological impairment.
A crucial function of the alveolar barrier is alveolar fluid clearance (AFC), the vectorial transport of ions, and water across the epithelium (reviewed in Matthay et al1). Impaired AFC rates after transplant are associated with primary graft dysfunction.2 In patients with acute lung injury, impaired fluid clearance is associated with higher mortality.3 Despite the association between AFC and these important clinical outcomes, prediction of graft function or survival for patients with acute lung injury remains imperfect because routine clinical measures (such as oxygenation and radiographical abnormalities) are of limited value.4
A functional measure of alveolar barrier integrity, AFC depends on a sodium concentration gradient across the epithelium. The sodium concentration gradient is established by sodium/potassium adenosine 5′-triphosphatase and is modulated by changes in apical sodium channels and chloride channels. Although Intact tight junctions are required for AFC, differential expression of transmembrane tight junction proteins modulates epithelial paracellular permeability to ions and other solutes.5–11 Foremost among these tight junction proteins is the claudin family.12–14 In both types 1 and 2 pneumocytes, claudin-4 is expressed at high levels.15 Previously reported loss of function studies showed that claudin 4 was required for maximal epithelial barrier function, including alveolar fluid clearance in mice.16 Claudin-4 is of particular interest because it confers low-ion conductance and relative chloride selectivity to the paracellular pathway in Madin-Darby Canine Kidney cells.5 Concomitant transport of chloride is necessary to minimize the energy requirement for sodium transport, and a third of chloride transport in the alveolar epithelium occurs via the paracellular pathway.1,17 Therefore, changes in paracellular sodium and chloride transport would be expected to directly impact alveolar fluid clearance. We hypothesized that increased claudin-4 expression enhances AFC by increasing the transepithelial sodium concentration gradient, but whether differences in claudin-4 expression are of clinical importance remains unknown. Therefore, this study was undertaken to determine whether claudin-4 protein levels are associated with intact AFC or other clinical measures of lung injury in human lungs rejected for transplantation. Although evaluation of donor lungs pre-transplant with a functional measure (such as AFC) might have value, our intention was to examine the potential contribution of claudin-4 to AFC, but not to evaluate claudin-4 as a biomarker of lung injury. In ex vivo perfused human lungs, we found that claudin-4 expression levels were positively correlated with the rate of alveolar fluid clearance.
Materials and Methods
Ex Vivo Perfused Human Lung Preparation
All studies were conducted in accordance with the institutional review board with approval from the UCSF and VA Committees on Human Research. Human lungs from brain-dead organ donors not used for transplantation were obtained from the Northern California Transplant Donor Network, who obtained consent from the next of kin. All lungs studied were rejected for concern for lung injury or dysfunction, although not all donors met the criteria for the standardized definition of acute lung injury. Lungs were removed en bloc, inflated, transported on ice to the research laboratory, and perfused, as previously described.18–20 De-identified clinical data were obtained for each donor. On arrival, the pulmonary artery and mainstem bronchus were cannulated and one lung was perfused with leukocyte-poor blood. Pulmonary veins were not cannulated and venous drainage was passive. The lung preparation was suspended in a container surrounded by a heated (38°C) water jacket. Perfusate (Dulbecco's modified Eagle (DME)-H21 medium containing 5% albumin) was collected in the inner container and its temperature was continuously monitored. When the temperature of the perfusate reached 36°C, the lungs were inflated with continuous positive airway pressure of 10-cm H2O, with 95% O2 and 5% CO2. Alveolar fluid clearance was determined by measuring changes in the concentration of intra-airway instilled tracer solution, as previously described.18,20 Briefly, 20 mL of warmed saline containing 5% bovine albumin were instilled into the lung via a 16-gauge catheter. The catheter remained in position and fluid samples were obtained from the catheter at 5 minutes and after 1 hour. The difference in protein concentration between the samples was used to calculate the amount of fluid removed from the air spaces, with increased protein concentration indicating the removal of fluid.
Immunohistochemistry
Tissue samples were collected from the lungs and were formalin-fixed and paraffin-embedded. Epitope retrieval was performed at 95°C with 1.0 mmol/L EDTA at pH 8.0 (for claudin-4), citrate buffer at pH 6.0 (for CD163), Tris-EDTA buffer (for ZO-1), or at 37°C with 0.1% trypsin (for cytokeratin). Sections were incubated overnight at 4°C in mouse anti-claudin-4 (Invitrogen, Carlsbad, CA), rabbit anti ZO-1 (Invitrogen), CD163 (Biocare Medical, Concord, CA), or cytokeratin (Abcam, Cambridge, MA) at a dilution of 1:100 in 2.5% normal horse serum. Endogenous peroxidase activity was quenched with 3% aqueous hydrogen peroxide at room temperature for 30 minutes and sections were subsequently incubated with anti-mouse or anti-rabbit peroxidase conjugated polymer (Vector Labs, Burlingame, CA) for 30 minutes at room temperature. Staining was visualized using diaminobenzydine or Vector VIP Substrate (Vector Labs) and sections were counterstained with hematoxylin and eosin. Sections incubated with 5 μg/mL mouse IgG (Invitrogen) or rabbit IgG (Santa Cruz Biotechnologies, Santa Cruz, CA) instead of the primary antibody were used as isotype controls. Additional normal lung tissue specimens (n = 3) were also obtained (US Biomax, Rockville, MD; Folio Biosciences, Columbus, OH) and immunostained for claudin-4, as previously described.
Histology Scoring
The extent of histological lung injury was determined with blinding to the clinical and immunohistochemistry scores. For each lung, five 10X fields from each of three sections were scored. Morphological evaluation was performed using well-established criteria for lung injury and diffuse alveolar damage, including septal thickening, alveolar inflammatory cell infiltration, and architectural disruption with increased interstitial cellularity.21,22 Each criterion was scored from 0 to 3, and an average score was calculated for each sample for statistical analysis.
Scoring of Immunostained Sections
Five random fields were imaged for each of three sections from every individual lung. To prevent bias, control slides treated with an isotype control antibody were imaged first, and the corresponding field on the claudin-4 immunostained slide was subsequently identified and imaged. All images were acquired using the Plan 10X/0.25 objective (Nikon Eclipse E600, Nikon USA, Melville NY). Unmodified images were analyzed with blinding to the clinical and histological scoring data using the Framework for Image Dataset Analysis (FrIDA) software image analysis tool from the Johns Hopkins University (FrIDA Software, http://bui2.win.ad.jhu.edu/frida, last accessed April 1, 2010), as previously described.23,24 Briefly, color masks were generated to identify brown, blue, and all color pixels. A meta mask created to include all brown pixels and exclude all blue pixels was used to analyze each image. Total surface area of cells occupying the field was estimated using the all color mask. Scores (relative claudin-4 staining) were calculated using the following formula: positive staining on claudin-4 immunostained slide minus positive staining on corresponding isotype control divided by total area of cells in the field. A total of 780 images were analyzed in this manner. Therefore, staining intensity and the proportion of cells staining positive are incorporated into one score. The same protocol was applied to ZO-1 staining of the same samples. Alveolar macrophages were stained with anti-CD163 and the number of macrophages on sections with both high and low claudin-4 staining was determined using mean linear intercept. Briefly, two perpendicular lines were drawn through the images and the number of macrophages intersecting a line was counted. For each lung examined, 10 fields were evaluated in this way with blinding to the claudin-4 level in the sample. The mean number of macrophages among the high claudin-4 and low claudin-4 groups was then compared. The presence or absence of epithelial cells within a section was determined by cytokeratin staining. Cytokeratin positive staining was then compared with claudin-4 staining on the next adjacent section.
Immunoblotting
Protein extracts were collected from 3 additional lungs in which AFC was measured. Equal amounts of protein were resolved by SDS-PAGE under reducing conditions in triplicate and blotted onto nitrocellulose membranes. The membranes were blocked for 1 hour at room temperature with 5% nonfat milk and probed for claudins-4, -3, -15, occludin, and ZO-1 (all antibodies from Invitrogen) overnight at 4°C and were then probed with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling, Danvers, MA). ß-actin (Cell Signaling) was used as a loading control for densitometry analysis.
Clinical LIS
Murray's clinical lung injury scores (LIS) were computed with blinding to histology and immunohistochemistry scores using the standard criteria.25,26 Briefly, pulmonary dysfunction was graded from 0 to 4 based on oxygenation (PaO2/FIO2), number of quadrants showing alveolar consolidation on a chest X-ray, positive end-expiratory pressure, and respiratory system compliance.
Statistics
Sample distributions were evaluated using Kolmogorov-Smirnov goodness of fit test and found to be non-normally distributed. Differences in claudin-4 immunostaining with respect to lung injury scores were determined by the nonparametric Mann-Whitney U-test. Correlations between two data sets were evaluated using the Spearman rank correlation (rs). Differences among groups in the immunoblot analysis were analyzed with analysis of variance and post hoc Student-Newman-Keuls adjustment. Macrophage counts were compared by an unpaired t-test. P ≤ 0.05 was considered significant.
Results
Claudin-4 Levels Correlate with Intact Alveolar Fluid Clearance
Because alveolar fluid clearance is an important aspect of recovery and survival after transplant or lung injury, we analyzed claudin-4 levels in lung sections with relation to AFC. Claudin-4 levels represented by the score obtained by the de-convolution technique were compared. There was a statistically significant, positive correlation between the rate of AFC and claudin-4 expression by Spearman correlation (rs = 0.713; P = 0.003) (Figure 1A). Representative sections demonstrate the correlation between claudin-4 levels and AFC (Figure 1B). There was no correlation between ZO-1 staining scores and AFC (rs = −0.052; P = 0.84) (Figure 1, C and D). ZO-1 staining did not change with claudin-4 staining (rs = −0.075; P = 0.74). In three additional lung samples, levels of claudin-4, occludin, ZO-1, and claudins-3 and -15 were determined by Western blot analysis and compared with AFC (Figure 1E). Densitometry revealed only claudin-4 levels significantly differed among the samples, showing a significant increase as AFC rates increased (P = 0.001 by analysis of variance) (Figure 1F). Of the tight junction proteins measured by immunohistochemistry or immunoblot analysis, only claudin-4 changed with AFC.
Figure 1.
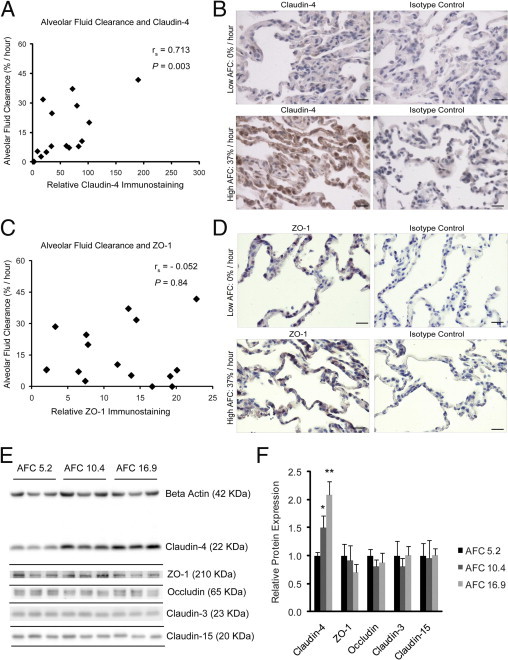
Claudin-4 levels and alveolar fluid clearance (AFC). A: Claudin-4 levels on immunostained distal lung sections were directly correlated with AFC Spearman rank correlation ([rs] = 0.713; P = 0.003; n = 16). B: Representative images demonstrate low claudin-4 staining correlated with low AFC (top left) and high claudin-4 staining correlated with high AFC (bottom left). Isotype controls shown for comparison. C: There was no correlation between ZO-1 staining and AFC (rs = −0.052; P = 0.84; n = 16). D: Representative images demonstrate similar ZO-1 staining in low (top) and high (bottom) AFC samples. Scale bars = 20 μm. E and F: Protein extracts from three additional lungs were resolved by SDS-PAGE in triplicate and levels of claudin-4, ZO-1, occludin, claudin-3, and claudin-15 were determined by immunoblot. The immunoblots were also labeled for β-actin to normalize for total sample protein content. Relative values for each protein were normalized to the lowest AFC sample (AFC = 5.2%/hour). Densitometry revealed that only claudin-4 levels significantly differed among the samples, demonstrating an increase as AFC rates increased (analysis of variance P = 0.001, *P < 0.05 compared with AFC 5.2%/hour, **P < 0.05 compared with both AFC 5.2%/hour and 10.4%/hour). There were no differences in the levels of the other tight junction proteins among the samples. Data are expressed as means ± SD.
Claudin-4 Levels and Histological Lung Injury
There was not a statistically significant correlation between histological lung injury score and claudin-4 levels (Figure 2A; rs = 0.024; P = not significant; n = 20). Representative tissue sections (Figure 2B) demonstrate the variation in claudin-4 staining in samples with both high and low histological lung injury. Claudin-4-positive cells included primarily alveolar epithelial cells (Figure 2B). Furthermore, cytokeratin staining showed that epithelial cells could have either high or low claudin-4 staining and that epithelial cell abundance did not likely account for the difference in claudin-4 staining scores (Figure 2C). Claudin-4-positive cells also included alveolar macrophages. However, there was no difference in the number of macrophages in sections with high or low claudin-4 staining (Figure 2D). In normal lung samples, the claudin-4 staining scores ranged from 4 to 7 (Figure 2F) compared with scores ranging from 2 to 190 in the injured lung samples. Because claudin-4 levels appeared to be providing information related to AFC and were independent of histological lung injury, next we investigated if claudin-4 levels were associated with clinical measures of respiratory physiology impairment.
Figure 2.
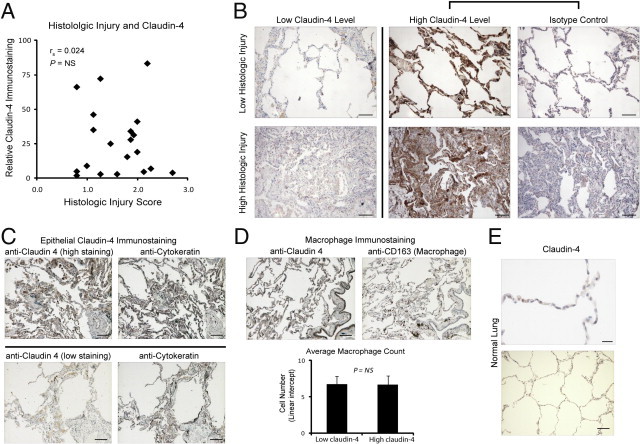
Claudin-4 levels and histological lung injury. A: There was no correlation between histological severity of injury and claudin-4 staining, Spearman rank correlation ([rs] = 0.024; P = not significant [NS]; n = 20). B: Representative distal lung sections demonstrate that low histological lung injury could be associated with either low (top left) or high (top middle) claudin-4 staining. Isotype control for the high claudin-4 staining section is provided (top right). A similar lack of correlation between high histological injury and claudin-4 staining was observed (bottom row). Scale bars = 100 μm. C: Claudin-4 epithelial staining in representative images as demonstrated by overlap of claudin-4 staining with cytokeratin positive cells on consecutive sections from specimens with high (top row) or low (bottom row) claudin-4 staining. Scale bars = 100 μm. D: Representative images demonstrating abundant claudin-4 staining (upper left) with relatively few macrophages identified (anti-CD163, upper right). There was not a statistically significant difference in the mean number of macrophages counted by linear intercept in samples with low (n = 6) and high (n = 6) claudin-4 staining (bottom). Data are expressed as means ± SD. Scale bars = 100 μm. E: Representative claudin-4 staining in a section from normal lung. Claudin-4 scores ranged from 4 to 7 in normal lung samples (n = 3). Scale bar = 20 μm in the upper panel and 100 μm in the lower panel.
High Claudin-4 Levels Are Associated with Lower Clinical LIS
Distal sections from human lung rejected for transplant (n = 20) were obtained, as previously described. Clinical data from the donors were used to compute Murray's clinical LIS as a measure of the level of impairment in respiratory physiology. Donors were grouped into two categories, those with minimal physiological respiratory impairment (LIS < 1; n = 11) and those with greater physiological impairment (LIS ≥ 1; n = 9). Claudin-4 levels were significantly higher in tissue sections from patients with an LIS < 1 (median 34, 25th to 75th percentile = 23 to 56) as compared to those having an LIS ≥ 1 (median 5, 25th to 75th percentile = 3 to 16; P = 0.006) (Figure 3, A and B). ZO-1 staining was not different in patients with high or low LIS (Figure 3C).
Figure 3.
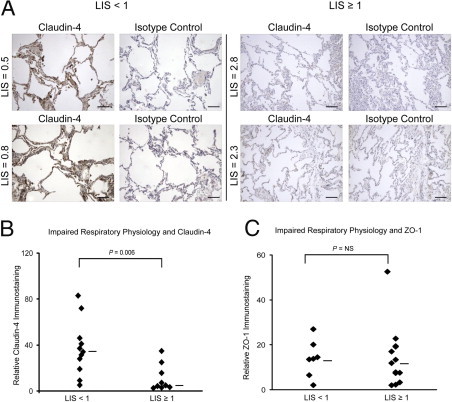
Claudin-4 levels and clinical lung injury scores (LIS). A: Representative sections from two donor lungs with minimal physiological impairment (LIS < 1) demonstrating high claudin-4 staining (left panel) as compared with sections from two donor lungs with more severe physiological impairment (LIS ≥ 1) and low claudin-4 staining (right panel). Isotype controls shown for comparison. Scale bars = 100 μm. B: Distal tissue sections from donor lungs with minimal physiological impairment had significantly higher claudin-4 scores (median 34, 25th to 75th percentile = 23 to 56; n = 11) than donor lungs with more severe physiological impairment (median 5, 25th to 75th percentile = 3 to 16; n = 9) (P = 0.006). C: There was no difference in ZO-1 scores between samples with LIS < 1 and LIS ≥ 1.
Discussion
In this study of human lungs rejected for transplantation, the amount of expressed claudin-4 was positively correlated with intact alveolar fluid clearance. Claudin-4 does not appear to merely be a marker for lung injury severity, as histological injury scores were not associated with claudin-4 levels. Also, cytokeratin staining further confirmed that differences in epithelial cell number did not account for the differences in claudin-4 staining. This is further supported by immunoblot data showing a lack of change in several other tight junction proteins (claudin-3, claudin-15, ZO-1, occludin), whereas claudin-4 levels increased with AFC. Because some alveolar macrophages stained positive for claudin-4, we quantified macrophage numbers in tissue samples and found no difference in macrophage number between samples with high AFC and those with low AFC. Therefore, differences in epithelial cell levels of claudin-4 appear to account for the bulk of the difference in staining. It should be noted that it is uncertain if macrophages express claudin-4 or if positive staining represents phagocytized epithelial cells.
It is not certain if the generally higher levels of claudin-4 in the injured lungs compared with normal tissue represent differences in baseline expression or differences in the level of increase in the protein with injury, however, previously published data might support the latter. One study examined claudin-4 expression in lung tissue from patients with interstitial lung disease.27 Relatively normal appearing alveolar structures in that study showed claudin-4 staining that was similar to staining in the normal lungs included in this study. Another prior study examined claudin-4 expression by immunohistochemistry in developing human lungs and found increased claudin-4 staining in alveolar epithelial cells during the saccular and alveolar stages of development that appears very similar to alveolar epithelial claudin-4 staining in our injured lung samples.28 It is also possible that changes in claudin-4 levels with injury are transient or occur in a time frame different from changes in tissue histology, however we found no association between ZO-1 staining and AFC, further supporting a specific role for claudin-4 in alveolar barrier function.
Claudin-4 levels are known to influence paracellular transport by conferring relative chloride selectivity and decreasing ion conductance, and because of this, it is plausible that claudin-4 plays a role in determining alveolar fluid clearance rates in human lungs. For example, in Madin Darby Canine Kidney cells, overexpression of claudin-4 increased transepithelial electrical resistance and increased chloride selectivity of the paracellular pathway.5 Also, claudin-4 knockdown in primary rat alveolar type 2 cells and human distal lung epithelial cells resulted in a significant decrease in transepithelial electrical resistance.16 As one of the most abundant claudins in the alveolar epithelium,15 differences in claudin-4 expression are likely to influence paracellular permeability. Previous experimental studies point to a role for claudin-4 in AFC regulation, as well.16 These studies showed that knockdown of claudin-4 reduced baseline and β-adrenergic agonist-stimulated alveolar fluid clearance. The decrease in fluid clearance was associated with an increased susceptibility to pulmonary edema and lung injury. It is notable that claudin-4 is specifically induced in experimental lung injury,16 and may therefore represent a protective response in the alveolar epithelium to promote alveolar fluid clearance. The present data from human lungs rejected for transplantation are entirely consistent with the previous experimental data. Although the known function of claudin-4 supports a role for this protein in alveolar barrier paracellular transport regulation, it remains possible that claudin-4 could influence other pathways important for ion transport or barrier integrity.
We also found that claudin-4 levels were associated with lower clinical LIS. Thus claudin-4 levels may provide information on alveolar epithelial function that is reflected in clinical respiratory function variables. LIS consists of oxygenation, compliance, and chest X-ray measures, all of which may be affected to varying degrees by pulmonary edema and lung consolidation. Improvement in AFC or a decrease in paracellular permeability would be predicted to have a favorable affect on all of these clinical variables. It is possible that claudin-4 levels are one determinant of alveolar fluid clearance in lung injury patients.
To our knowledge, this is the first report of a significant association between the level of a claudin and alveolar epithelial barrier function, as measured by net alveolar fluid clearance in the human lungs. The change in claudin-4 expression levels is specific. Levels of other tight junction proteins did not change and ZO-1 levels did not correlate with AFC or claudin-4 levels. These data suggest that a change in a particular tight junction component during injury influences alveolar barrier function. Investigation of claudin-4 was hypothesis-driven, based on prior experimental work. This study did not include pathway analysis to examine all components of tight junctions or ion transport, but it is possible that such an analysis would not reveal a role for a single claudin in this context. The primary finding of this study is particularly important because AFC is a well-described clinical marker of alveolar barrier function associated with patient mortality in acute lung injury and graft function in donor lungs. It remains to be investigated if claudin-4 levels are altered from baseline during injury in human lungs, however, these results suggest the possibility that there are important differences in claudin-4 expression among individuals with injured lungs. Further investigation into the regulation and function of claudin-4 may provide new insights into the pathogenesis of alveolar barrier dysfunction in patients with acute lung injury.
Acknowledgments
We thank the Northern California Transplant Donor Network and donor families for making these studies possible.
Footnotes
Supported by grants from the National Institutes of Health (HL88440 to J.A.F.) and the National Heart, Lung and Blood Institute (HL551856 to J.A.F. and M.A.M.).
D.R. and M.J.L. made equal contributions to this study.
References
- 1.Matthay M.A., Folkesson H.G., Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 2.Ware L.B., Golden J.A., Finkbeiner W.E., Matthay M.A. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med. 1999;159:980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 3.Matthay M.A., Wiener-Kronish J.P. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 4.Ware L.B., Matthay M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 5.Van Itallie C., Rahner C., Anderson J.M. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Itallie C.M., Fanning A.S., Anderson J.M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 7.Colegio O.R., Van Itallie C., Rahner C., Anderson J.M. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 8.Alexandre M.D., Jeansonne B.G., Renegar R.H., Tatum R., Chen Y.H. The first extracellular domain of claudin-7 affects paracellular cl- permeability. Biochem Biophys Res Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 9.Himmerkus N., Shan Q., Goerke B., Hou J., Goodenough D.A., Bleich M. Salt and acid-base metabolism in claudin-16 knockdown mice: impact for the pathophysiology of fhhnc patients. Am J Physiol Renal Physiol. 2008;295:F1641–F1647. doi: 10.1152/ajprenal.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes J.L., Van Itallie C.M., Rasmussen J.E., Anderson J.M. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Yu A.S., Enck A.H., Lencer W.I., Schneeberger E.E. Claudin-8 expression in madin-darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–17359. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 12.Furuse M., Sasaki H., Fujimoto K., Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneeberger E.E. Claudins form ion-selective channels in the paracellular pathway: Focus on “Claudin extracellular domains determine paracellular charge selectively and resistance but not tight junction fibril architecture”. Am J Physiol Cell Physiol. 2003;284:C1331–C1333. doi: 10.1152/ajpcell.00037.2003. [DOI] [PubMed] [Google Scholar]
- 14.Mrsny R.J., Brown G.T., Gerner-Smidt K., Buret A.G., Meddings J.B., Quan C., Koval M., Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafemina M.J., Rokkam D., Chandrasena A., Pan J., Bajaj A., Johnson M., Frank J.A. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L724–L734. doi: 10.1152/ajplung.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wray C., Mao Y., Pan J., Chandrasena A., Piasta F., Frank J.A. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–L227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X., Fukuda N., Barbry P., Sartori C., Verkman A.S., Matthay M.A. Novel role for cftr in fluid absorption from the distal airspaces of the lung. J Gen Physiol. 2002;119:199–207. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank J.A., Briot R., Lee J.W., Ishizaka A., Uchida T., Matthay M.A. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L52–L59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briot R., Frank J.A., Uchida T., Lee J.W., Calfee C.S., Matthay M.A. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest. 2009;135:269–275. doi: 10.1378/chest.08-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of e. Coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoelz C., Negri E.M., Lichtenfels A.J., Concecao G.M., Barbas C.S., Saldiva P.H., Capelozzi V.L. Morphometric differences in pulmonary lesions in primary and secondary ARDS: A preliminary study in autopsies. Pathol Res Pract. 2001;197:521–530. [PubMed] [Google Scholar]
- 22.Tomashefski J.F., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 23.Gurel B., Iwata T., Koh C.M., Jenkins R.B., Lan F., Van Dang C., Hicks J.L., Morgan J., Cornish T.C., Sutcliffe S., Issacs W.B., Luo J., De Marzo A.M. Nuclear myc protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol. 2008;21:1156–1167. doi: 10.1038/modpathol.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornish T., Morgan J., Gurel B., De Marzo A.M. FrIDA: An open source framework for image dataset analysis. Arch Pathol Lab Med. 2008;132:856. [Google Scholar]
- 25.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 26.Offner P.J., Moore E.E. Lung injury severity scoring in the era of lung protective mechanical ventilation: the pao2/fio2 ratio. J Trauma. 2003;55:285–289. doi: 10.1097/01.TA.0000078695.35172.79. [DOI] [PubMed] [Google Scholar]
- 27.Kaarteenaho-Wiik R., Soini Y. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009;57:187–195. doi: 10.1369/jhc.2008.951566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaarteenaho R., Merikallio H., Lehtonen S., Harju T., Soini Y. Divergent expression of claudin -1, -3, -4, -5 and -7 in developing human lung. Respir Res. 2010;11:59. doi: 10.1186/1465-9921-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]


