Abstract
Tryptophan metabolism by the kynurenine pathway (KP) is important to the pathogenesis of inflammatory, infectious, and degenerative diseases. The 3-hydroxykynurenine (3-HK) branch of the KP is activated in macrophages and microglia, leading to the generation of 3-HK, 3-hydroxyanthranilic acid (3-HAA), and quinolinic acid, which are considered neurotoxic owing to their free radical–generating and N-methyl-d-aspartic acid receptor agonist activities. We investigated the role of 3-HAA in inflammatory and antioxidant gene expression and neurotoxicity in primary human fetal central nervous system cultures treated with cytokines (IL-1 with or without interferon-γ) or with Toll-like receptor ligands mimicking the proinflammatory central nervous system environment. Results were analyzed by microarray, Western blot, immunostain, enzyme-linked immunosorbent assay, and neurotoxicity assays. 3-HAA suppressed glial cytokine and chemokine expression and reduced cytokine-induced neuronal death. 3-HK also suppressed cytokine-induced neuronal death. Unexpectedly, 3-HAA was highly effective in inducing in astrocytes the expression of hemeoxygenase-1 (HO-1), an antioxidant enzyme with anti-inflammatory and cytoprotective properties. Optimal induction of HO-1 required 3-HAA and cytokines. In human microglia, 3-HAA weakly induced HO-1 and lipopolysaccharide suppressed microglial HO-1 expression. 3-HAA–mediated HO-1 expression was confirmed in cultured adult human astrocytes and in vivo after 3-HAA injection to mouse brains. Together, our results demonstrate the novel neuroprotective activity of the tryptophan metabolite 3-HAA and have implications for future therapeutic approaches for neuroinflammatory disorders.
Indoleamine-2,3-dioxygenase (IDO) is an interferon (IFN)-γ–inducible, rate-limiting enzyme in the kynurenine pathway (KP) of tryptophan metabolism generating various downstream metabolites collectively termed “kynurenines”1 (Figure 1). This process is compartmentalized due to cell-specific expression of the KP enzymes. For example, kynurenine monooxygenase (KMO) is expressed in macrophages and microglia,2–4 whereas kynurenine aminotransferase II (KAT II) is present in astrocytes.5 A well-appreciated biological activity of IDO is T-cell suppression. IDO expressed in antigen-presenting cells (dendritic cells, macrophages, and microglia) can suppress T-cell immunity against viruses, tumors, or transplanted tissues by suppressing T-cell proliferation.1,6,7 IDO induces immune tolerance through tryptophan depletion from T cells but also through generation of KP metabolites, such as 3-hydroxyanthranilic acid (3-HAA).8,9 In line with these findings, administration of 3-hydroxykynurenine (3-HK), 3-HAA, or its synthetic analog in mice has been shown to induce a T-cell phenotype change from TH1 to TH2 and to ameliorate experimental autoimmune encephalomyelitis in an animal model of multiple sclerosis.10
Figure 1.
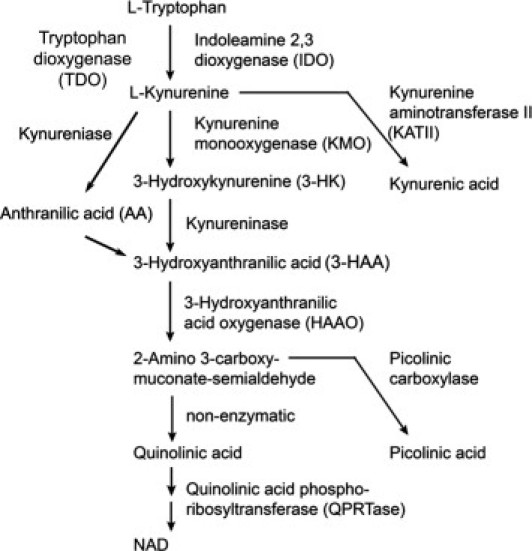
The KP of tryptophan metabolism. A simplified version of the KP demonstrating the major enzymes and intermediates.
In contrast to its well-defined roles in T-cell immunity, the role of IDO and the KP metabolites in innate immunity is less well understood. Microglia are the main cell type engaged in the innate immune response in the central nervous system (CNS), in part due to their abundant immune receptor expression.11–14 Microglial kynurenines, such as 3-HK, 3-HAA, and quinolinic acid (Figure 1), have been implicated as neurotoxins in a number of neurologic diseases due to their free radical–generating and N-methyl-d-aspartic acid (NMDA) agonist activities.15–19 Because of the reported neurotoxic properties of microglial kynurenines, KP enzymes such as KMO are currently being targeted for drug development for neurodegenerative diseases, such as Huntington's disease.17,20
Microglial response to a number of brain insults could also promote injury by establishing a cytokine cascade in the CNS through positive feedback mechanisms (IL-1 induces itself) and activation of astrocytes.21–25 IL-1 is required for human astrocyte inducible nitric oxide synthase (iNOS) and tumor necrosis factor (TNF)-α release26 and is critical for neurotoxicity.27–29 The prolonged presence of proinflammatory cytokines, such as IL-1 or TNF-α, could comprise a common mechanism underlying neurodegeneration. In the current study, we examined the role of 3-HAA, a redox regulator, in cytokine production and neurotoxicity in primary human brain cell cultures to define its potential role during neuroinflammatory processes. We found that 3-HAA suppresses cytokine and chemokine production and neurotoxicity induced by IL-1/IFN-γ and Toll-like receptor (TLR) ligands. We also found that this effect is in part mediated by the unique and potent ability of 3-HAA to induce hemeoxygenase-1 (HO-1) in human glial cells. HO-1 is an inducible enzyme with proven anti-inflammatory and cytoprotective activities, but little is known about its regulation of expression in primary human brain cells.30 Thus, our results have important implications for human CNS diseases.
Materials and Methods
Human Fetal Brain Cell Culture
Human CNS cell cultures were prepared from 16 to 22 weeks of human fetal abortuses as described with minor modifications.31 All tissue collection was approved by the Albert Einstein College of Medicine Institutional Review Board. Primary mixed CNS cultures were prepared by enzymatic and mechanical dissociation of the cerebral tissue followed by filtration through nylon meshes of 230- and 130-μ pore sizes. Single-cell suspension was plated at 1 to 10 × 106 cells/mL in Dulbecco's modified Eagle's medium (DMEM) (Cellgro) supplemented with 5% fetal calf serum (FCS) (Gemini Bio-products, Woodland, CA), penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL) (Gibco, Carlsbad, CA) for 2 weeks, and then microglial cells were collected by aspiration of the culture medium. Monolayers of microglia were prepared in 60-mm tissue culture dishes at 1 × 106 cells per 5 mL of medium or in 96-well tissue culture plates at 4 × 104 per 0.1 mL of medium. Four to 16 hours later, cultures were washed to remove nonadherent cells (neurons and astrocytes). Microglial cultures were highly pure, consisting of more than 98% CD68+ cells. Highly enriched human astrocyte cultures were generated by repeated passage of the mixed CNS cultures, as described previously.26 Mixed neuronal and glial cultures (mixed cultures) were generated by replating the initial CNS cell cultures once in 60-mm or 96-well tissue culture plates after collecting microglia.27 Mixed cultures consisted primarily of neurons and astrocytes at a ratio of approximately 1:2 or 1:3 and a minor population (1% to 2%) of microglia. All cultures were kept as monolayers in DMEM with 5% FCS and antibiotics (complete medium).
Fetal Culture Treatment
Lipopolysaccharide (LPS) and poly(I:C) (PIC) were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human IFN-γ (specific activity, 1 ng = 20 U), IL-1β, and IL-10 were purchased from Peprotech (Rocky Hill, NJ). All cultures were treated in complete medium with PIC at 10 μg/mL, LPS at 100 ng/mL, or cytokines at 10 ng/mL. 3-HAA, 3-HK, hemin, and cobalt protoporphyrin IX (CoPP) were purchased from Sigma-Aldrich. Stock solutions of 3-HAA were prepared in HCl, then further dilutions were made in complete medium. Cultures were treated with 3-HAA at 100 μmol/L, unless otherwise specified.
Adult Human Glial Cell Culture
Adult human brain cell cultures were prepared as previously described.32,33 Brain tissues were obtained from adults undergoing surgical resections performed as treatment for non–tumor-related intractable epilepsy in accordance with the guidelines set by the Biomedical Ethics Unit of McGill University. The material came predominantly from temporal lobe white matter and did not include subependymal regions. Tissue specimens were enzymatically digested and separated on a linear 30% Percoll density gradient (Pharmacia Biotech, Piscataway, NJ). Floating cells were collected after two sequential 24-hour periods during which microglia selectively adhere to culture vessels. The floating cells were grown on glass chamber slides coated with poly-l-lysine (5 × 104 cells per well) in DMEM/F12 supplemented with N1 (Sigma, Oakville, Canada), 0.01% bovine serum albumin, 1% penicillin-streptomycin, and B27 supplement (Invitrogen, Burlington, Canada). The derived cell population consists of 90% to 95% oligodendroglial cells and approximately 5% astrocytes [glial fibrillary acidic protein (GFAP)–positive cells]. The initial adherent cell population served as a source of microglia.
Adult Culture Treatment
After 1 week in culture, individual wells were either left untreated or treated with 100 μmol/L 3-HAA or a combination of IL-1β and IFN-γ, 10 ng/mL each, for 24 hours. Individual coverslips were then fixed with 4% paraformaldehyde and stained with monoclonal anti-GFAP antibody conjugated with Alexa 488 and rabbit anti-rat HO-1 IgG (Assay Designs, formerly Stressgen, Plymouth Meeting, PA) followed by goat anti-rabbit Cy3 (also see below for HO-1 immunostain).
ELISA
Cell culture supernatants were harvested and subjected to protein assay by enzyme-linked immunosorbent assay (ELISA) using commercially available antibody pairs (R&D Systems, Minneapolis, MN). Standard curves were generated with known concentrations of recombinant cytokines and chemokines, and the samples were diluted until the optical reading (OD) values fell within the range of the ELISA detection.
Neurotoxicity Assay
Primary human fetal neuronal and glial cultures (mixed cultures) at an in vitro age of approximately 3 to 4 weeks were plated in 96-well tissue culture plates and treated with cytokines or TLR ligands in low serum medium (DMEM and 0.5% FCS). Seventy-two hours later, neuronal death was assayed by vital dye exclusion test, as previously described.27 Both propidium iodide and trypan blue exclusion tests were performed without appreciable difference in the results. The results were scored by counting the number of dead (trypan blue positive or propidium iodide positive) neurons in 4 different ×200 microscopic fields per well in 4- to 6-replicate wells. The selective neuronal death (MAP2 positive) in these cultures, the kinetics of cell death, the roles of TNF-α, nitric oxide, and NMDA receptor antagonist, and the comparison of several different neurotoxicity assays [vital dye exclusion, lactate dehydrogenase (LDH) efflux, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), etc] have all been published.27
HO-1 Knockdown by siRNA
Mixed neuronal glial cultures were transfected with 10 nmol/L control nontargeting small-interfering RNA (siRNA) or human HO-1–specific siRNA (Dharmacon, Chicago, IL) with TransIT-TKO transfection reagents from Mirus (Madison, WI), following the manufacturer's instructions. After incubation with siRNA for 2 to 5 days, cells were washed with fresh medium and then treated with cytokines with or without 3-HAA for an additional 3 days. Immunocytochemistry or commercial ELISA from Stressgen were used to confirm suppression of HO-1 expression by siRNA.
Western Blot Analysis
Western blot analysis was performed as previously described.34 Briefly, cell cultures in 60-mm dishes were scraped into lysis buffer [10 mmol/L Tris-HCl (pH 8.8), 50 mmol/L NaCl, 0.5 mmol/L Na3VO4, 30 mmol/L Na4P2O7, 50 mmol/L NaF, 2 mmol/L EDTA, and 1% Triton X-100] at various time points. Thirty to 70 μg of protein was separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride membrane. The blots were blocked in Tris-buffered saline and 0.1% Tween-20 containing 5% nonfat milk and then incubated with antibodies at 4°C for 16 hours.34 Primary antibodies were HO-1 [Abcam (Cambridge, MA) 1:2000 or Stressgen 1:1000], HO-2 [Santa Cruz (Santa Cruz, CA), 1:300], iNOS (Santa Cruz, 1:1000), and IDO (gift of Osamu Takikawa, mouse IgG1, 1:3000).34 The secondary antibody was either horseradish peroxidase–conjugated anti-mouse or anti-rabbit IgG (Pierce Biotechnology, Rockford, IL) and was used at 1:2000 to 1:10,000 for 1 hour at room temperature. Signals were developed using enhanced chemiluminescence (Pierce Biotechnology). All blots were reprobed with vinculin (Santa Cruz) to control for protein loading. Densitometric analysis was performed using Scion National Institutes of Health Image software (Scion, Frederick, MD).
Real-Time PCR
Quantitative real-time RT-PCR was performed as described previously,34,35 using porphobilinogen deaminase (PBDA; cell culture) or β-actin (mouse brain tissue) as an internal control. Primer sequences for human HO-1 were as follows: forward: 5′-ATGACACCAAGGACCAGAGC-3′ and reverse: 5′-GTGTAAGGACCCATCGGAGA-3′. Primer sequences for mouse HO-1 were as follows: forward: 5′-CTTTCAGAAGGGTCAGGTGTCC-3′ and reverse: 5′-GTGGAGACGCTTTACATAGTGC-3′. Briefly, total RNA was extracted with TRIzol (Invitrogen Life Technologies, Carlsbad, CA), following the manufacturer's instructions. PCR was performed using a SYBR Green PCR mix and conducted with the ABI Prism 7900HT (Applied Biosystems, Carlsbad, CA). All values were expressed relatively to PBDA or β-actin. The median value of the replicates for each sample was calculated and expressed as the CT (cycle number at which each PCR reaches a predetermined fluorescence threshold, set within the linear range of all reactions). ΔCT was calculated by subtracting the CT of the target gene from the CT of the endogenous control gene (PBDA or β-actin) in each sample. The relative amount of target gene expression in each sample was then calculated as 2ΔCT. Fold change was calculated by dividing the 2ΔCT value of test sample by that of control sample (control = 1).
Microarray Analysis
Highly enriched astrocyte cultures were subjected to microarray analysis using the Illumina platform. Briefly, for each total RNA sample, linear amplification and biotin labeling of total RNA (500 ng) were performed using the Illumina TotalPrep RNA Amplification Kit (Ambion Applied Biosystems, Austin, TX). Whole-genome expression analysis was performed by hybridization of amplified RNA to an Illumina HumanHT-12 v3 Expression BeadChip (Illumina Inc., San Diego, CA). With this bead chip, we interrogated >48,000 probes per sample, targeting genes and known alternative splice variants from the RefSeq database release 17 and UniGene build 188. Controls for each RNA sample (>1000 bead types) confirmed sample RNA quality, labeling reaction success, hybridization stringency, and signal generation. All expression data were quantile normalized and background subtracted before analysis using BeadStudio software (Illumina Inc.).
Mouse in Vivo Experiment
C57BL6 mice of approximately 8 weeks of age were manually injected with 3-HAA or vehicle alone (n = 4, each) aiming at the right caudate putamen, using the approximate coordinates (0.5 mm anterior, 2 mm lateral, and 3 mm depth from the bregma) as previously described.36,37 3-HAA was solubilized first in 1N HCl followed by pH adjustment to 7.5 using 1M Tris-HCl, pH 8.8. The amount of 3-HAA administered in each mouse was 40 μg, determined empirically by adjusting for vehicle toxicity. Brain tissues were harvested at 4 and 24 hours for quantitative PCR. For histochemistry, brains were harvested at 24 and 72 hours after injection and were fixed in formalin for 48 hours. Serial coronal sections of approximately 2-mm thickness were made through the entire brain and embedded in paraffin. Sections (5 μmol/L thick) were examined with H&E stain (four different levels through the entire block), TUNEL staining, and HO-1 immunohistochemistry (IHC; see below).
HO-1 IHC and Immunofluorescence
Serial, 5-μm coronal sections of formalin-fixed, paraffin-embedded mouse brain tissues were subjected to IHC as previously described.38 Briefly, sections were treated with Target Retrieval Solution (Dako, Carpinteria, CA) for antigen retrieval and then with rabbit anti-HO-1 antibody (Stressgen) at 1:200 for 2 hours at room temperature. ImmPRESS anti-rabbit Ig peroxidase kit (Vector Laboratories Ltd., Burlingame, CA) in combination with diaminobenzidine was used to develop a brown reaction product. For immunofluorescence, sections were incubated simultaneously with rabbit anti-HO-1 and rat anti-GFAP IgG2a (Invitrogen, Camarillo, CA) at 1:100 for 2 hours at room temperature followed by 16 hours at 4°C. They were then incubated with a cocktail of goat anti-rabbit IgG-Alexa Fluor 488 and goat anti-rat IgG-Alexa Fluor 568 conjugated secondary antibody at 1:1000 for 1.5 hours at room temperature and then coverslipped in Vectashield Hard Set mounting medium with DAPI (Vector Laboratories). Sections were viewed with an Inverted Olympus IX81 electronically motorized microscope and photographed with a Sensicam QE cooled CCD camera.
Statistical Analysis
For ELISA and neurotoxicity data, results shown are pooled data from multiple independent experiments using different brain cases. Values (protein levels) were normalized to those induced by cell stimuli (IL-1β, IFN-γ, IL-1 plus IFN-γ, LPS, or PIC) alone within an individual experiment, then the percentage of inhibition by 3-HAA was calculated. Data from multiple experiments were pooled and the significance of the 3-HAA effect was examined by a single-sample t-test. When representative results from a single experiment are shown, one-way analysis of variance was performed followed by Bonferroni post test. P < 0.05 was considered significant. These results were representative of two to four separate experiments with similar results using cells from different donor brains. All statistics were run using the GraphPad Prism 4.0 software (GraphPad Software, La Jolla, CA).
Results
3-HAA Suppresses Cytokine and Chemokine Production
We determined the effect of 3-HAA on cytokine and chemokine production in primary human fetal glial cell cultures. For microglial cultures, the TLR ligands PIC (10 μg/mL) and LPS (100 ng/mL) were used as stimuli.39 For astrocytes, three different combinations of stimuli were applied (IL-1β alone, IL-1 and IFN-γ, or PIC) based on previously determined gene expression patterns.22,27,34 Human astrocytes respond minimally to LPS; therefore, we excluded this stimulus for astrocytes.40 A pilot experiment for dose response showed that 3-HAA at 100 μmol/L maximally inhibited IFN-γ inducible protein 10 (IP-10) production in astrocytes (Figure 2A), and thus all subsequent experiments were performed using 100 μmol/L 3-HAA. High micromolar concentrations have been found in the plasma of mice and humans treated with a 3-HAA analog (supplemental material of Ref. 10); therefore, these concentrations are therapeutically relevant.
Figure 2.
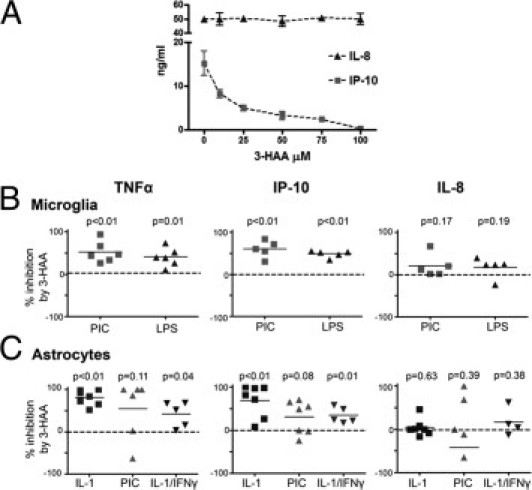
Cytokine suppressive effects of 3-HAA. A: Dose response of 3-HAA. Primary human astrocyte cultures were treated with varying concentrations of 3-HAA (0 to 100 μmol/L) and 10 ng/mL of IL-1β. Culture supernatants were collected 24 hours later, and the levels of the two α-chemokines IP-10 and IL-8 were determined by ELISA. Data shown are mean ± SD from triplicate cultures. IP-10 production was dose dependently suppressed by 3-HAA, but IL-8 was not affected by 3-HAA. B and C: 3-HAA suppression of TNF-α and IP-10 in microglia and astrocytes. Primary human microglia (B) were stimulated with PIC (10 μg/mL) or LPS (100 ng/mL) with or without 3-HAA (100 μmol/L). Astrocytes (C) were stimulated with IL-1 (10 ng/mL), PIC (10 μg/mL), or IL-1/IFN-γ (10 ng/mL) with or without 3-HAA (100 μmol/L). Levels of TNF-α, IP-10, and IL-8 were measured in triplicate wells in each culture, then the percentage of inhibition by 3-HAA was calculated from the mean values. Each symbol represents an independent experiment using different brain case. P values were obtained using the single-sample t-test.
All cultures were pretreated with 3-HAA for 1 hour, and culture supernatants were harvested at 24 hours or 72 hours after stimulation for ELISA for TNF-α, IP-10, and IL-8. Data were expressed as percentage of inhibition by 3-HAA calculated as follows: 100 × (1 − cytokine produced in the presence of 3-HAA/cytokine produced by stimulus alone). The compiled results from multiple5–7 independent experiments are shown in Figure 2, B and C. A single-sample t-test was performed to determine whether the inhibition by 3-HAA was significant.
The results showed that although microglial TNF-α and IP-10 production was significantly inhibited by 3-HAA, IL-8 production was not inhibited. The inhibition was highly significant in both LPS- and PIC-stimulated cultures, with a similar degree of inhibition for both (approximately 50%). In astrocyte cultures, TNF-α and IP-10 production by IL-1 or IL-1/IFN-γ was significantly inhibited, whereas TNF-α and IP-10 production by PIC was variably inhibited. The degree of inhibition was higher in cultures stimulated with IL-1 alone compared with IL-1/IFN-γ, with IL-1–induced TNF-α and IP-10 production showing near-complete inhibition in several cases of astrocytes (Figure 2C). 3-HAA did not inhibit IL-8 production in astrocytes. Together, these results show that 3-HAA suppresses cytokine and chemokine production from microglia and astrocytes but that the suppression is also target protein (and stimulus) specific.
3-HAA and 3-HK Protect Neurons from Cytokine- or TLR Ligand–Induced Death
We have previously shown that in primary human fetal brain cultures composed of neurons and glia cytokine treatment containing IL-1β (with or without IFN-γ) induces highly reproducible neuronal death that is detectable at 72 hours (delayed death) and that the neuronal death is attributable in part to endogenous TNF-α, nitric oxide, and NMDA receptor activity.27 There is evidence that 3-HAA (and related KP intermediates) may have toxic effects on neurons.15–19 However, these experiments were performed in the absence of added cytokines and/or in highly enriched neuronal cultures. In light of the cytokine-suppressive effects that we see in our culture, we next examined whether 3-HAA modulated neuronal survival in mixed CNS cultures treated with cytokines or TLR ligands, which induce neuronal death via glial inflammatory mechanisms.27 Mixed cultures were treated with IL-1 (with or without IFN-γ) or PIC with or without 3-HAA for 72 hours, then neurotoxicity was assessed by vital dye exclusion. Pooled data derived from multiple independent experiments and representative photographs are shown in Figure 3, A and B. The results show that 3-HAA protected human neurons against cytokine- or TLR ligand–induced death. We also tested 3-HK, another microglial KP metabolite upstream of 3-HAA (Figure 1), and these experiments also show that neurons were also protected from cytokine-induced death in the presence of 3-HK in a dose-dependent manner (Figure 3C). We also tested the effect of 3-HAA on cell death in control cultures and found no significant effect on cell death (Figure 3D). In addition, we see no cytologic evidence of astrocyte cytotoxicity by 3-HAA (see Figure 3A, for example). The results together suggest that the role of 3-HAA (and 3-HK) in neurotoxicity is likely dependent on the inflammatory environment of the CNS.
Figure 3.
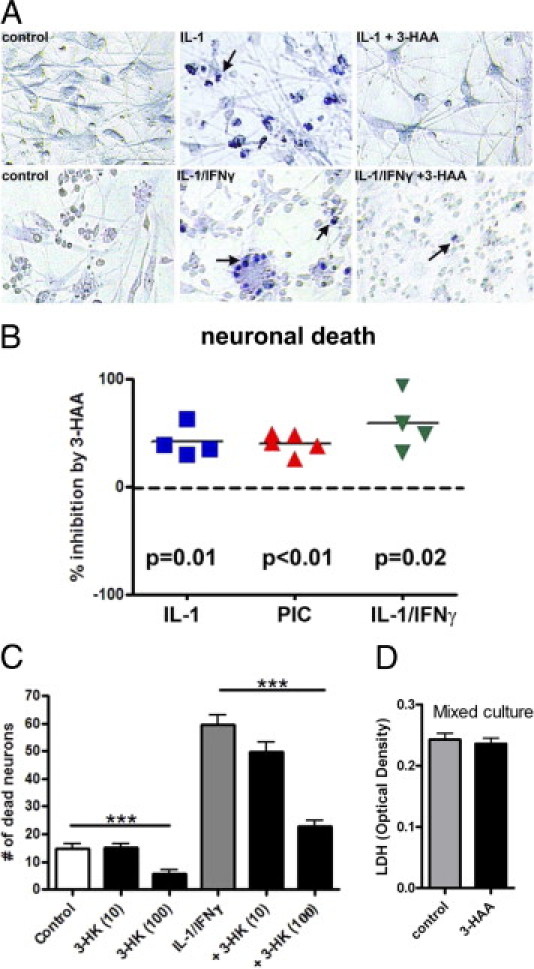
Neuroprotective effects of 3-HAA and 3-HK. Primary human mixed glial and neuronal cultures were stimulated with IL-1 or IL-1/IFN-γ with or without 3-HAA (100 μmol/L) for 72 hours to induce neurotoxicity, as described in Materials and Methods. Control cultures were not treated with cytokines. Culture medium was changed to low serum medium (0.5% FCS) 24 hours before cytokine treatment. A: Representative cultures examined with trypan blue exclusion test show induction of neuronal death with IL-1 or IL-1/IFN-γ (blue nuclei, arrows; cultures derived from two different cases are shown). The number of dead neurons is lower in 3-HAA–treated cultures. B: The effect of 3-HAA on cytokine- or PIC-induced neuronal death was determined in mixed cultures derived from several different brain cases, and the results show significant neuroprotection by 3-HAA in all three conditions (single-sample t-test). C: The effect of 3-HK on IL-1/IFN-γ–induced neurotoxicity was also determined. 3-HK dose dependently (100 μmol/L >10 μmol/L) inhibited neuronal death. 3-HK at 1 μmol/L had no effect (not shown). Data are presented as mean ± SD from quadruple values, and statistics were performed by analysis of variance with Bonferroni posttest (***P < 0.001). Shown is one of three experiments with similar results. D: The effect of 3-HAA on cell survival or death in control mixed cultures. No difference was noted by LDH measurements in culture supernatants collected at 72 hours. Data are OD values pooled from three different cases.
Microarray Analysis of Astrocyte Gene Modulation by 3-HAA
To search for potential genes responsible for the cytoprotective activities, we next performed microarray analysis of 3-HAA–treated astrocyte cultures. Two different astrocyte cases were examined after treatment with 3-HAA (or IL-1/IFN-γ for comparison) for 6 hours, as described in Materials and Methods. Astrocytes treated with 3-HAA showed few genes that were up- or down-regulated. The genes that were up- or down-regulated by at least 50% (3-HAA to control ratio >1.5 or <0.5) at 6 hours in both astrocyte cultures are listed in Table 1. These included IL-11, a gp130 family neuropoietic cytokine with known neurotrophic activities,41,42 TGF-β1, a pleiotropic immunoregulatory cytokine with neuroprotective properties,28 and tissue inhibitor of matrix metalloproteinase 3 (TIMP3). Three redox enzymes were found to be up-regulated by 3-HAA, and these included phase II detoxifying antioxidant enzyme hemeoxygenase-1 (HMOX-1, HO-1), thioredoxin reductase 1 (TXNRD-1), and astrocytic NADPH oxidase 4 (NOX4). Immediate early response 3 (IER3, IEX-1), a stress-induced gene with regulatory roles in mitochondrial oxidative phosphorylation and reactive oxygen species release,43 was also up-regulated. Also up-regulated by 3-HAA were the transcription factor C/EBPβ and a potassium channel protein KCNK12 (THIK-2). The expression of amyloid precursor protein was reduced by 3-HAA. In addition, IP-10 and IL-8 were decreased and increased, respectively, by 3-HAA.
Table 1.
Effects of 3-HAA on Astrocyte Gene Expression by Microarray Analysis
| Gene symbol | 3-HAA versus control (fold change) |
IL-1/IFNγ versus control (fold change) |
||
|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | |
| IL11 | 3.2 | 2.4 | 14.3 | 10.5 |
| TGFβ1 | 2.8 | 2.6 | 0.9 | 0.5 |
| TIMP3 | 2.0 | 2.1 | 1.5 | 0.7 |
| HMOX1(HO1) | 1.9 | 3.8 | 1.0 | 0.8 |
| TXNRD1 | 2.2 | 1.5 | 1.3 | 0.9 |
| NOX4 | 1.9 | 1.8 | 0.8 | 0.7 |
| IER3 (IEX1) | 6.3 | 2.3 | 34.7 | 14.0 |
| C/EBPβ | 2.3 | 1.8 | 8.8 | 6.2 |
| KCNK12 | 4.4 | 2.2 | 0.5 | 0.2 |
| APP | 0.5 | 0.5 | 0.7 | 0.5 |
| CXCL10 (IP10) | 0.8 | 0.3 | 233.0 | 61.8 |
| IL8 | 3.6 | 1.2 | 109.0 | 57.7 |
Highly enriched human astrocyte cultures were treated with medium alone (control), 3-HAA (100 μmol/L), or cytokines (IL-1β and IFN-γ at 10 ng/mL each) for 6 hours. Microarray analysis was performed with Illumina HumanHT-12 v3 Expression BeadChip as described in Materials and Methods. Data shown are fold change (ratio) of mRNA expression (test sample versus control, untreated sample). 1, no change;>1, increase; <1, decrease. Results from two separate astrocyte cases are shown.
3-HAA Induces HO-1
HO-1 is an inducible antioxidant enzyme with known cytoprotective effects; we therefore asked whether HO-1 might be responsible for the observed neuroprotective activity of 3-HAA. We first validated HO-1 expression further by Western blot, immunostain, and real-time PCR. Astrocyte or mixed cultures were treated with IL-1, IL-1/IFN-γ, or PIC as described for ELISA and neurotoxicity assays in the presence or absence of 3-HAA. Initial analysis showed that astrocytes expressed sustained levels of HO-1 between 16 and 72 hours after 3-HAA treatment; therefore, all subsequent experiments were performed at a 24-hour time point. Figure 4 shows representative Western blots in three different types of cultures. In pure astrocyte cultures (Figure 4A), HO-1 was induced only when 3-HAA was added. Cytokines and PIC synergized with 3-HAA to induce higher levels of HO-1. We also probed the Western blots for two inducible enzymes (iNOS and IDO) that are known to be regulated by immune factors in a redox-sensitive manner. Astrocyte iNOS was induced by IL-1/IFN-γ, and IDO was induced by PIC and IL-1/IFN-γ as reported,26,34 but 3-HAA had no appreciable effects on the expression of either enzyme. The pattern of induction of HO-1 in mixed neuronal glial cultures was similar to that in astrocyte cultures, except for detectable induction of HO-1 by cytokines or PIC alone (Figure 4B).
Figure 4.
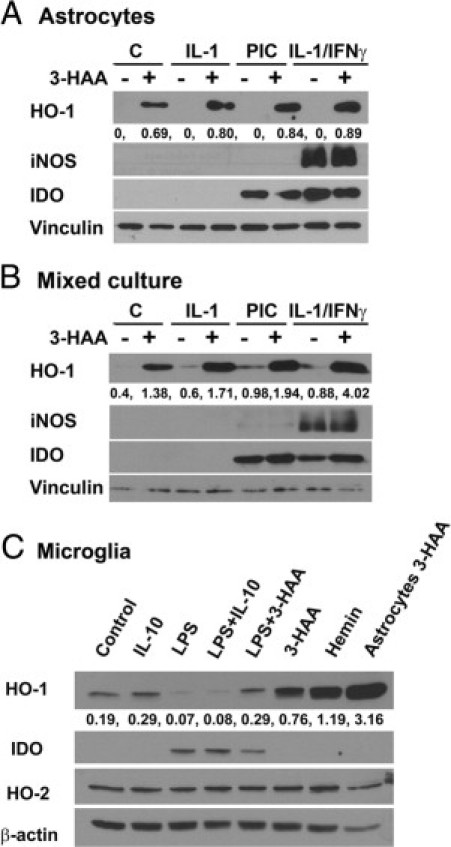
HO-1 expression in human fetal CNS cell cultures. Cultures of astrocytes (A), mixed neurons and glia (B), and microglia (C) were prepared as described in Materials and Methods and treated with 3-HAA (100 μmol/L), recombinant cytokines (all 10 ng/mL), or TLR ligands for 24 hours as described in the Figure 2 legend. Hemin (10 μmol/L) was included as a known inducer of HO-1. Western blotting was performed using a polyclonal rabbit IgG against HO-1 (from Abcam, originally from Stressgen). The blots were stripped and reprobed for iNOS, IDO, HO-2, vinculin, or β-actin (controls for protein loading). The numbers are densitometric ratios of HO-1 to vinculin (A and B) or β-actin (C). The results together indicate that 3-HAA is a strong stimulus for HO-1 induction in human astrocytes and that proinflammatory cytokines and TLR ligands synergize with 3-HAA in the induction of HO-1. However, microglial HO-1 expression was much lower and was regulated differentially by IL-10 and TLR ligands (LPS). The results are representative of five independent experiments for A and B and two for C, with identical results.
We next examined human fetal microglial cultures with stimuli that have previously been shown to induce HO-1 in murine and human monocyte-lineage cells.44–46 As shown in Figure 4C, unlike astrocytes, control microglia showed high basal levels of HO-1, which were further increased and decreased by IL-10 and LPS, respectively. Microglial HO-1 was also induced by 3-HAA, although to a smaller degree than in astrocytes. LPS inhibited microglial HO-1 induced by IL-10 or 3-HAA. Stimulation with hemin was used as a positive control for microglial HO-1 induction. As previously reported, LPS induced microglial IDO expression (Figure 4C) and iNOS was not induced in human microglia by any of the stimuli.26,39 The expression of HO-2 (the constitutive isoform of hemeoxygenase) in microglia paralleled that of β-actin.
Figure 5 shows representative quantitative PCR analysis of HO-1 mRNA expression in mixed neuronal glial cultures and microglial cultures. Cultures were treated as described for Western blot analysis and total RNA harvested at 6 or 16 hours with similar results (16 hours shown). Results are expressed as fold induction over control, and they show that the mRNA induction profile in each culture resembles that of protein very closely. In mixed cultures, HO-1 mRNA was induced by 3-HAA, and 3-HAA showed synergism with cytokines and PIC, with the IL-1/IFN-γ combination being the most effective. Cytokines or PIC alone also induced small amounts of HO-1 mRNA. In microglial cultures, both IL-10 and 3-HAA induced HO-1 and the TLR ligands (LPS or PIC) suppressed HO-1. The amount of HO-1 induction was much higher in mixed cultures compared with microglia.
Figure 5.
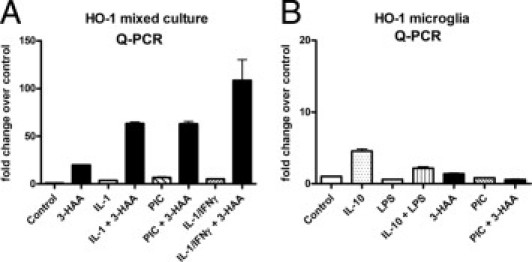
Quantitative PCR analysis of HO-1 mRNA expression. Cultures of mixed neurons and glia (A) or microglia (B) were treated with cytokines or TLR ligands with or without 3-HAA, as described in the Figure 4 legend, then HO-1 mRNA expression was determined by quantitative PCR using PBDA as control. Values are mean ± SD from triplicates. The expression of HO-1 mRNA follows closely that of HO-1 protein shown in Figure 4. Data are representative of two independent experiments with similar results.
3-HAA Induces HO-1 in Adult Human Astrocytes in Culture
In light of the finding that in human fetal cultures HO-1 expression was readily induced in astrocytes, we asked whether human astrocytes of adult origin can also express HO-1. Because adult human astrocytes cannot be obtained in purity, we prepared mixed adult CNS cell cultures according to the established protocol32 and then examined the percentage of GFAP-positive cells that express HO-1 by double-label immunofluorescence microscopy. As shown in Figure 6, a high percentage of adult human astrocytes became HO-1 positive after 3-HAA treatment, whereas in control or cytokine (IL-1/IFN-γ) treated cultures, HO-1–positive astrocytes were rare. These results demonstrate that the ability of astrocytes to respond to 3-HAA to express HO-1 is common in both mature and immature human astrocytes. Adult human microglia had low basal levels of HO-1 expression with significant induction in response to hemin but not 3-HAA treatment (data not shown).
Figure 6.
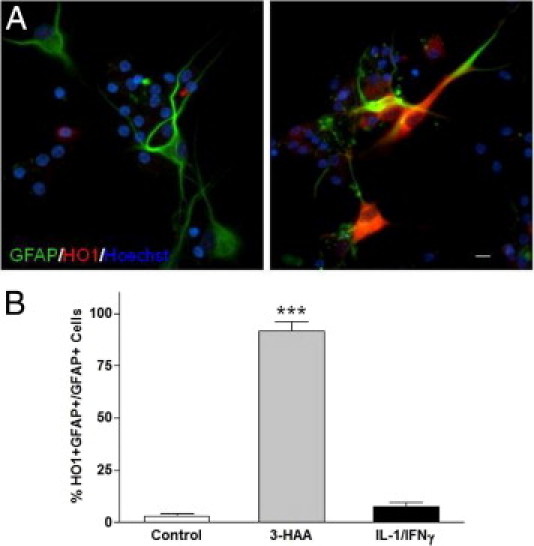
HO-1 expression in adult human astrocytes. Adult human glial cell cultures were prepared from surgical specimens as described in Materials and Methods following standard protocols. After 1 week in culture, individual microwell cultures were either left untreated (A, left) or treated with 3-HAA (A, right) or IL-1/IFN-γ and immunostained for GFAP and HO-1 expression, as described in Materials and Methods. Nuclei were stained with Hoechst (blue). The photographs depict HO-1–positive astrocytes (GFAP+) in adult glial cultures treated with 3-HAA. Scale bar = 10 μmol/L. B: Percentage of HO-1–positive astrocytes were quantified by counting double positive (HO-1+ and GFAP+) or single positive (GFAP+ alone) cells in culture, and this showed that 3-HAA treatment induced HO-1 in most astrocytes, whereas IL-1/IFN-γ had little effect (***P < 0.001). Results are representative of two independent experiments using different donor cells.
Role of HO-1 in Protection of Neurons from Cytokine-Induced Death
To determine whether HO-1 has a role in neuroprotection in our cytokine-treated mixed neuronal glial cultures, we next used siRNA-mediated knockdown of HO-1. Mixed cultures were treated with HO-1 specific siRNA or control siRNA, as described in Materials and Methods, and then stimulated with cytokines (IL-1/IFN-γ) in the presence or absence of 3-HAA. As shown in Figure 7A, the results of these experiments revealed that the neuroprotective activity of 3-HAA was reversed in the presence of HO-1 siRNA. HO-1 immunocytochemistry demonstrated many HO-1–positive astrocytes in cultures stimulated with IL-1/IFN-γ plus 3-HAA, but HO-1–positive cells were virtually absent in HO-1 siRNA-treated cultures (Figure 7B). In parallel experiments, we also examined the effect of a well-known HO-1 inducer, CoPP. CoPP (1 to 10 μmol/L) was a potent inducer of HO-1 in astrocytes (not shown) and when added to cytokine-treated cultures was strongly neuroprotective (Figure 7C). These results together demonstrate a direct role for (astrocyte) HO-1 in protecting neurons from cytokine-induced death.
Figure 7.
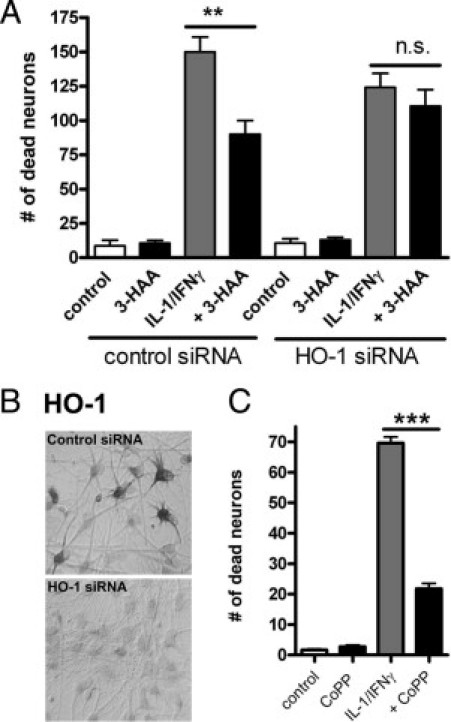
Neuroprotective effects of HO-1. A: HO-1 siRNA was used to knockdown HO-1 expression in mixed neuron and glial cultures. Control siRNA was used to control for nonspecific effects. Briefly, cultures were incubated with siRNA for 2 to 5 days before treatment with a cytokine mixture for an additional 3 days, as described in Materials and Methods. Vital dye exclusion test was used to determine the number of dead neurons as described in the Figure 3 legend. The results show that the neuroprotective effect of 3-HAA is diminished in the presence of HO-1 siRNA. **P < 0.01. NS = nonsignificant. Results are representative of three experiments using different donor cells. B: The effect of HO-1 siRNA on HO-1 protein expression was determined by HO-1 immunocytochemistry. In control siRNA-treated cultures (upper panel), many astrocytes show strong HO-1 immunoreactivity. In HO-1 siRNA-treated cultures, HO-1 immunoreactive cells virtually disappear (lower panel). C: The effect of CoPP, an inducer of HO-1, was examined in the neurotoxicity assay, and the results show that CoPP (1 μmol/L) protected neurons from cytokine-induced death. ***P < 0.001.
3-HAA Induction of HO-1 in Vivo
To determine whether 3-HAA can induce HO-1 in vivo, we injected 3-HAA (or vehicle alone) into the forebrains of approximately 8-week-old C57BL6 mice and then analyzed the brain tissue for HO-1 mRNA and protein expression. As shown in Figure 8A, HO-1 mRNA was induced in the brains of 3-HAA–injected mice relative to vehicle-injected mice. By IHC (Figure 8B), HO-1 expression was noted in both activated microglia and astrocytes, as determined by their characteristic cell shape and their expression of microglial (Iba-1) and astrocyte (GFAP) markers (not shown). There was no gross tissue toxicity in the brains of 3-HAA or vehicle-injected mice as determined by examination of serial interval sections with H&E stain (see Supplemental Figure S1 at http://ajp.amjpathol.org). Rare apoptotic cells, inflammatory cells, and red neurons were detected in both conditions. TUNEL stain was performed to determine the number of apoptotic cells. The results showed that TUNEL-positive cells were indeed rare and the numbers were lower in 3-HAA–injected brains compared with controls (Figure 8C).
Figure 8.
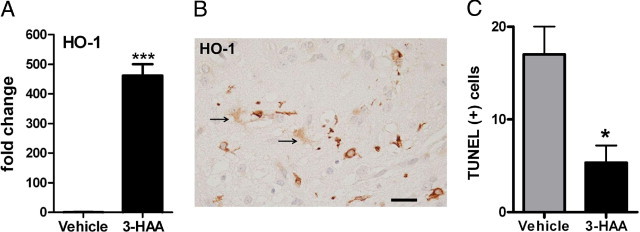
3-HAA induces HO-1 in vivo. Mice were injected intracerebrally with 3-HAA or vehicle only as described in Materials and Methods. A: Quantitative PCR analysis shows that HO-1 mRNA is increased in 3-HAA–injected mouse brains compared with control. Data are expressed as fold change in HO-1 mRNA over control (vehicle injection = 1), using β-actin as the endogenous control gene. ***P < 0.001 t-test. Data are representative of two independent experiments with similar results. B: IHC analyses show that HO-1 was induced in both activated microglia and astrocytes (arrows) in the brains injected with 3-HAA. Scale bar = 40 μm. C: TUNEL stain was performed to detect cells undergoing apoptosis. TUNEL-positive cells were very rare but were noted around the injection sites. TUNEL-positive cell counts were significantly lower in 3-HAA–injected than in vehicle-injected brains. Mean ± SD, *P < 0.05 t-test.
Microglial Expression of KP Enzymes Are Differentially Regulated by LPS and IFN-γ
Most KP enzymes are known to be expressed in microglia, but other than IDO, the regulation of the KP enzymes in microglia or macrophages is not well understood. We examined the KP enzyme expression in human microglia activated by the TLR ligand (LPS) or IFN-γ by quantitative PCR (Figure 9). These data show that KP enzymes are differentially regulated by cell activation. Specifically, the enzymes upstream of 3-HAA production [IDO, tryptophan dioxygenase (TDO), KMO, and kynureninase (KYNase)] (Figure 1) were up-regulated, whereas KAT-II, 3-hydroxyanthranilic acid dioxygenase (HAAO), and quinolinic acid phosphoribosyl transferase (QPRTase) were down-regulated by LPS. The same results were obtained by microarray analyses of PIC- or LPS-stimulated microglia (6 and 16 hours) (see supplemental data of Ref. 39). The effect of IFN-γ was different from that of LPS, up-regulating IDO and TDO, down-regulating KAT-II and QPRTase, but having no effect on KMO, KYNase, and HAAO. These results suggest that inflammatory activation of microglia could lead to increased production of KP metabolites but that TLR3/4 activation could preferentially promote production of 3-HAA.
Figure 9.
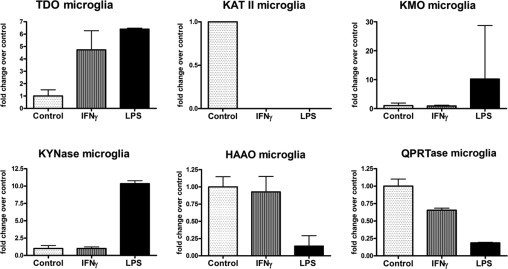
Quantitative PCR analysis of the KP enzymes in microglia. Microglial cultures were stimulated with LPS or IFN-γ for 6 hours, and quantitative PCR was performed for KP enzymes as shown. Data are from triplicate values and represent fold induction over control. LPS and IFN-γ differentially regulate the KP enzymes in human microglia. See Figure 1 for the relative position of each enzyme in the KP.
Summary of the Findings
The results of our study and our hypotheses are summarized in Figure 10. We have shown, in primary human brain cell cultures and in the intact mouse brain in vivo, a unique role of 3-HAA in the induction of the cytoprotective enzyme HO-1 in astrocytes and microglia. 3-HAA can be synthesized in microglia or macrophages downstream of l-kynurenine (Figure 1). l-kynurenine can be generated from l-tryptophan by IDO (or the constitutive enzyme TDO) or can be taken up from the systemic circulation across the intact blood-brain barrier.47 3-HAA uniquely up-regulates human astrocyte expression of HO-1, which is further enhanced in the presence of inflammatory cytokines that are found in many CNS conditions as part of innate and adaptive immune responses. Microglial HO-1 expression appears to be under different regulatory mechanisms because its response to 3-HAA is meager and proinflammatory stimuli down-regulate microglial HO-1 expression. 3-HAA administered to cultures suppresses cytokine production (TNF-α and IP-10, for example) and, furthermore, protects neurons from cytokine-induced death in mixed cultures. The latter appears to be in part mediated by HO-1, as shown by HO siRNA knockdown experiments. Because HO-1 in our cultures is primarily expressed by astrocytes (not neurons), the neuroprotective activity of 3-HAA/HO-1 is most probably mediated by soluble factors downstream of HO-1, such as CO.
Figure 10.
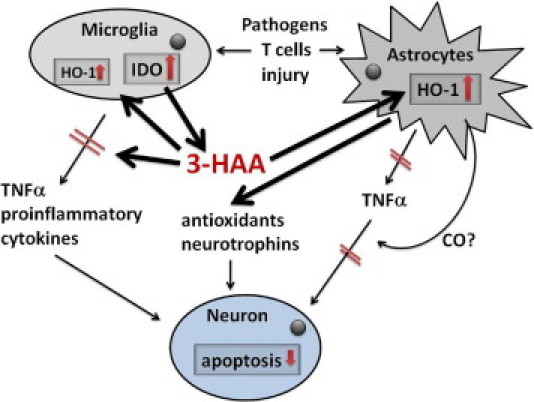
Summary and hypothesis. On the basis of the findings in this study, we hypothesize that KP metabolites such as 3-HAA may have therapeutic value for human CNS diseases through antioxidant, anti-inflammatory, and neuroprotective effects. See text for details.
Discussion
In the current study, we report novel anti-inflammatory, antioxidant, and neuroprotective activities of the tryptophan metabolite 3-HAA. Specifically, in both microglial and astrocyte cultures, 3-HAA inhibited the expression of TNF-α (a proinflammatory cytokine) and IP-10 (a TH1 α-chemokine) induced by various immune stimuli. The lack of effect on IL-8 production in the same culture demonstrated that 3-HAA was not simply inducing cell toxicity and apoptosis, as some studies have suggested.48 The two α-chemokines (IP-10 and IL-8) are often differentially regulated, for example, by extracellular ATP or PI3K/AKT inhibitor,49,50 in addition to 3-HAA as shown in this study. At present, the basis for the differential regulation is not clear. Regardless, suppression by 3-HAA of proinflammatory molecules induced by diverse immunologic stimuli suggests that 3-HAA endogenously produced or administered therapeutically can change the CNS innate immune environment to favor down-modulation of inflammation (Figure 10). Because neuroinflammation is thought to contribute to neurodegeneration in several different types of human CNS diseases, our results have implications for the therapeutic use of 3-HAA or related compounds. Conversely, they also suggest that therapies aimed at blockade of microglial kynurenine production17 could have unintended consequences on inflammation and neuronal survival.
Perhaps the most surprising aspect of our study was that 3-HAA and 3-HK were found to be neuroprotective under the conditions of cytokine-mediated (also glia-mediated) death. In the current study, we show that 3-HAA or 3-HK alleviates neuronal death induced by cytokines in mixed human fetal CNS cultures. Our results contrast with those previously reported in rodent neuronal cultures, many of which reported 3-HK and 3-HAA to be neurotoxins.51–56 However, none of the previous studies examined the effect of these kynurenines in the context of cytokine-induced neurotoxicity. We suspect that several factors, such as species (rodent versus human) and the type of culture (pure versus mixed neuronal glial), in addition to the presence of cytokines, account for these disparate results. For example, in our study, 3-HAA induced the cytoprotectant HO-1 in astrocytes, and cytokines (IL-1/IFN-γ) had a synergistic effect with 3-HAA in inducing HO-1 especially in mixed cultures (Figures 4 and 5). Furthermore, astrocyte HO-1 appears to the readily inducible in human CNS cultures of both fetal and adult origin (Figure 6). In contrast, in murine cultures, microglia rather than astrocytes appear to be the main expressor of HO-1 (see, for example, Ref. 57). These results together suggest that the role of 3-HAA in neurotoxicity is highly context dependent.
It is also interesting to note that recent clinical studies of two unrelated diseases (stroke and osteoporosis) reported that the plasma levels of 3-HAA positively correlated (and those of anthranilic acid negatively correlated) with beneficial clinical outcomes (ie, smaller lesion sizes and positive treatment responses).58,59 These results might be pointing to a possible beneficial role of 3-HAA in humans and the presence of a clinically important alternative KP pathway for 3-HAA generation (Figure 1). The notion that 3-HAA per se is not necessarily toxic is supported by our mouse injection studies, which showed no evidence of cell death beyond what was evident in vehicle-injected brains (Figure 8C; see also Supplemental Figure S1 at http://ajp.amjpathol.org).
Our data show that 3-HAA was a unique inducer of HO-1 because other stimuli (TLR ligands, proinflammatory and anti-inflammatory cytokines) had little or no effect without 3-HAA in inducing astrocyte HO-1. Indeed, the ability of 3-HAA to induce HO-1 has been previously shown in mouse macrophage cell lines.60 In our study, microglia showed significant differences from astrocytes in the expression of HO-1 because microglial HO-1 was suppressed by TLR ligands but enhanced by the anti-inflammatory cytokine IL-10. The findings in microglia are similar to those reported in macrophages44–46 and support the notion that HO-1 may be a marker of “alternatively activated” (M2) macrophage phenotype.61
HO-1 is an inducible enzyme with proven anti-inflammatory and cytoprotective activities.9,30,62–64 HO-1 catalyzes degradation of heme to three main products, CO, biliverdin, and iron, and most studies have found CO to mediate the beneficial biological effects of HO-1.65,66 It is probable that neuroprotection conferred by 3-HAA in our culture is mediated by astrocyte HO-1 through a diffusible metabolite such as CO (Figure 10) because we see HO-1 expression in astrocytes and not in neurons. Most likely HO-1 does not account for all of the anti-inflammatory and neuroprotective activity in our culture because there are a number of additional candidate molecules generated by 3-HAA, each of them deserving further investigation. For example, the immunoregulatory cytokine transforming growth factor-β has been shown to down-modulate inflammatory responses in many systems, and IL-11 has been shown to be a neurotrophic cytokine.42,67 The mechanisms by which 3-HAA inhibited cytokine production are also likely multifactorial because our microglial culture expressed relatively little HO-1 in response to 3-HAA in comparison to astrocytes, yet 3-HAA inhibited TNF-α and IP-10 production more consistently in microglia than in astrocyte cultures. We speculate that 3-HAA is taken up by microglia and degraded further by downstream KP enzymes to quinolinic acid, picolinic acid, and nicotinamide (Figure 1), all of which have proven immunomodulatory activities.10,68,69
Transcription of the HO-1 gene is under the control of antioxidant response element, which is activated by the transcription factor, nuclear factor erythroid-2–related factor 2 (Nrf2). Activation of Nrf2 is typically induced by oxidative stress70,71 and nitrosative stress (nitric oxide).72 In this regard, the low expression of HO-1 in human microglia may in part be due to their inability to express iNOS.26 On the other hand, the unique ability of 3-HAA to induce HO-1 is most certainly related to its free radical–generating ability because free radicals (reactive oxygen species) provide necessary signals for Nrf2 activation.73,74 These results then reveal the two-faced nature of 3-HAA in redox regulation. Indeed, Christen et al75 have previously shown potent antioxidant activities of 3-HAA and 3-HK that were more effective than either ascorbate or vitamin E. They further suggested that the induction of IDO may represent a local antioxidant defense mechanism. It is curious then that the pattern of microglial KP enzyme regulation by LPS (Figure 9) is also suggestive of such a role for IDO/KP metabolites and suggests that enhanced production of 3-HAA might represent an innate immune response to restore the normal homeostatic environment.
Our study was designed to address the role of the KP metabolite 3-HAA in human brain cells under inflammatory conditions. We believe that these findings may be relevant to human neuroinflammatory and neurodegenerative conditions, in which microglial expression of proinflammatory cytokines is associated with pathology.76,77 We envision that endogenous 3-HAA generated by macrophages and microglia (in concert with proinflammatory cytokines) could induce HO-1 and other cytoprotective molecules in nearby glia. Our animal injection experiments further demonstrated that microglia and astrocytes are capable of expressing HO-1 in vivo in response to 3-HAA. One of the important implications of our study is that macrophage kynurenines do not have uniformly toxic effects and that blockade of upstream enzymes, such as KMO (as is being explored for Huntington's disease), might have unintended deleterious consequences under certain circumstances. Alternatively, our observation that 3-HAA has antioxidant and neuroprotective activity can be exploited therapeutically for relevant human neuroinflammatory and neurodegenerative conditions.
Acknowledgments
We thank Dr. Brad Poulos (Einstein Human Fetal Tissue Repository) for tissue and Drs. Thomas Belbin, Hillel Cohen, and Celia F. Brosnan for helpful discussions.
Footnotes
Supported by National Institutes of Health grants RO1 MH55477, KO1 MH084705, and T32 NS007098, Einstein Center for AIDS Research grant P30 AI051519, and a grant from the German Research Foundation.
D.K. and H.-S.S. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi:10.1016/j.ajpath.2011.05.048.
A guest editor acted as editor-in-chief for this article. No person at Thomas Jefferson University or Albert Einstein College of Medicine was involved in the peer review process or final disposition for this article.
Supplementary data
H&E–stained sections of mouse brains injected with 3-HAA or vehicle (1N HCl). A and B: Frank toxicity occurred only in mice injected with solutions without pH adjustment. Arrows points to an area with tissue necrosis associated with large numbers of dead neurons (inset). B is the low power view of the area in A, demonstrating injection within the caudate putamen. (cc = corpus callosum, s = septum, ac = anterior commissure). C-F: Mice injected with 3-HAA (C and D) or vehicle (E and F) at pH 7.5 showed no gross tissue necrosis, aside from microscopic hemorrhages (arrows) associated with a few apoptotic cells. Approximate original magnifications: ×100 (A), ×25 (B), ×100 (C), ×140 (D), ×100 (E), and ×200 (F).
References
- 1.Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 2.Heyes M.P., Chen C.Y., Major E.O., Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326(pt 2):351–356. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol. 2004;4:12–17. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Guidetti P., Hoffman G.E., Melendez-Ferro M., Albuquerque E.X., Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 6.Munn D.H., Sharma M.D., Hou D., Baban B., Lee J.R., Antonia S.J., Messina J.L., Chandler P., Koni P.A., Mellor A.L. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potula R., Poluektova L., Knipe B., Chrastil J., Heilman D., Dou H., Takikawa O., Munn D.H., Gendelman H.E., Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belladonna M.L., Grohmann U., Guidetti P., Volpi C., Bianchi R., Fioretti M.C., Schwarcz R., Fallarino F., Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 9.Brusko T.M., Wasserfall C.H., Agarwal A., Kapturczak M.H., Atkinson M.A. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 10.Platten M., Ho P.P., Youssef S., Fontoura P., Garren H., Hur E.M., Gupta R., Lee L.Y., Kidd B.A., Robinson W.H., Sobel R.A., Selley M.L., Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 11.Dickson D.W., Lee S.C., Mattiace L.A., Yen S.H., Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 12.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.C. Microglia and innate immunity in neuroAIDS. In: Gendelman H.E., editor. The neurology of AIDS. Oxford University Press; New York: 2010. [Google Scholar]
- 14.Suh H.S., Brosnan C.F., Lee S.C. Toll-like receptors in CNS viral infections. Curr Top Microbiol Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. [DOI] [PubMed] [Google Scholar]
- 15.Heyes M.P., Brew B.J., Martin A., Price R.W., Salazar A.M., Sidtis J.J., Yergey J.A., Mouradian M.M., Sadler A.E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 16.Stone T.W., Mackay G.M., Forrest C.M., Clark C.J., Darlington L.G. Tryptophan metabolites and brain disorders. Clin Chem Lab Med. 2003;41:852–859. doi: 10.1515/CCLM.2003.129. [DOI] [PubMed] [Google Scholar]
- 17.Schwarcz R., Guidetti P., Sathyasaikumar K.V., Muchowski P.J. Of mice, rats and men: revisiting the quinolinic acid hypothesis of Huntington's disease. Prog Neurobiol. 2010;90:230–245. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiarugi A., Meli E., Moroni F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J Neurochem. 2001;77:1310–1318. doi: 10.1046/j.1471-4159.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 19.Guidetti P., Bates G.P., Graham R.K., Hayden M.R., Leavitt B.R., MacDonald M.E., Slow E.J., Wheeler V.C., Woodman B., Schwarcz R. Elevated brain 3-hydroxykynurenine and quinolinate levels in Huntington disease mice. Neurobiol Dis. 2006;23:190–197. doi: 10.1016/j.nbd.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Giorgini F., Guidetti P., Nguyen Q., Bennett S.C., Muchowski P.J. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.C., Dickson D.W., Brosnan C.F. Interleukin-1, nitric oxide and reactive astrocytes. Brain Behav Immun. 1995;9:345–354. doi: 10.1006/brbi.1995.1032. [DOI] [PubMed] [Google Scholar]
- 22.John G.R., Lee S.C., Song X., Rivieccio M., Brosnan C.F. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.C., Cosenza M.A., Si Q., Rivieccio M., Brosnan C.F. The CNS: cells, tissues and reactions to insult. In: Ransohoff R.M., Benveniste E.N., editors. Cytokines and the CNS. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 24.Simi A., Tsakiri N., Wang P., Rothwell N.J. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35:1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- 25.Basu A., Krady J.K., Levison S.W. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Zhao M.-L., Brosnan C.F., Lee S.C. Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1b and IL-1 receptor antagonist. J Immunol. 1996;157:3569–3576. [PubMed] [Google Scholar]
- 27.Downen M., Amaral T.D., Hua L.L., Zhao M.L., Lee S.C. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia. 1999;28:114–127. [PubMed] [Google Scholar]
- 28.Basu A., Krady J.K., Enterline J.R., Levison S.W. Transforming growth factor beta1 prevents IL-1beta-induced microglial activation, whereas TNFalpha- and IL-6-stimulated activation are not antagonized. Glia. 2002;40:109–120. doi: 10.1002/glia.10118. [DOI] [PubMed] [Google Scholar]
- 29.Thornton P., Pinteaux E., Gibson R.M., Allan S.M., Rothwell N.J. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J Neurochem. 2006;98:258–266. doi: 10.1111/j.1471-4159.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 30.Syapin P.J. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.C., Liu W., Brosnan C.F., Dickson D.W. Characterization of human fetal dissociated CNS cultures with an emphasis on microglia. Lab Invest. 1992;67:465–475. [PubMed] [Google Scholar]
- 32.Yong V.W., Yong F.P., Olivier A., Robitaille Y., Antel J.P. Morphologic heterogeneity of human adult astrocytes in culture: correlation with HLA-DR expression. J Neurosci Res. 1990;27:678–688. doi: 10.1002/jnr.490270428. [DOI] [PubMed] [Google Scholar]
- 33.Cui Q.L., Fragoso G., Miron V.E., Darlington P.J., Mushynski W.E., Antel J., Almazan G. Response of human oligodendrocyte progenitors to growth factors and axon signals. J Neuropathol Exp Neurol. 2010;69:930–944. doi: 10.1097/NEN.0b013e3181ef3be4. [DOI] [PubMed] [Google Scholar]
- 34.Suh H.S., Zhao M.L., Rivieccio M., Choi S., Connolly E., Zhao Y., Takikawa O., Brosnan C.F., Lee S.C. Astrocyte indoleamine 2, 3 dioxygenase (IDO) is induced by the TLR3 ligand poly IC: mechanism of induction and role in anti-viral response. J Virol. 2007;81:9838–9850. doi: 10.1128/JVI.00792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivieccio M.A., Suh H.S., Zhao Y., Zhao M.L., Chin K.C., Lee S.C., Brosnan C.F. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J Immunol. 2006;177:4735–4741. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- 36.Suh H.S., Cosenza-Nashat M., Choi N., Zhao M.L., Li J.F., Pollard J.W., Jirtle R.L., Goldstein H., Lee S.C. Insulin-like growth factor 2 receptor is an IFNγ-inducible microglial protein that facilitates intracellular HIV replication: implications for HIV-induced neurocognitive disorders. Am J Pathol. 2010;177:2446–2458. doi: 10.2353/ajpath.2010.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J., Zheng J.H., Zhao M., Lee S., Goldstein H. Increased in vivo activation of microglia and astrocytes in the brains of mice transgenic for an infectious R5 human immunodeficiency virus type 1 provirus and for CD4-specific expression of human cyclin T1 in response to stimulation by lipopolysaccharides. J Virol. 2008;82:5562–5572. doi: 10.1128/JVI.02618-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosenza-Nashat M., Zhao M.L., Suh H.S., Morgan J., Natividad R., Morgello S., Lee S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–328. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh H.S., Zhao M.L., Choi N., Belbin T.J., Brosnan C.F., Lee S.C. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology. 2009;392:246–259. doi: 10.1016/j.virol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.C., Liu W., Dickson D.W., Brosnan C.F., Berman J.W. Cytokine production by human fetal microglia and astrocytes: differential induction by LPS and IL-1b. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 41.Zhang Y., Taveggia C., Melendez-Vasquez C., Einheber S., Raine C.S., Salzer J.L., Brosnan C.F., John G.R. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci. 2006;26:12174–12185. doi: 10.1523/JNEUROSCI.2289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakashima K., Wiese S., Yanagisawa M., Arakawa H., Kimura N., Hisatsune T., Yoshida K., Kishimoto T., Sendtner M., Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999;19:5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen L., Zhi L., Hu W., Wu M.X. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death Differ. 2009;16:603–612. doi: 10.1038/cdd.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricchetti G.A., Williams L.M., Foxwell B.M. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76:719–726. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- 45.Lee T.S., Chau L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 46.Kirino Y., Takeno M., Watanabe R., Murakami S., Kobayashi M., Ideguchi H., Ihata A., Ohno S., Ueda A., Mizuki N., Ishigatsubo Y. Association of reduced heme oxygenase-1 with excessive Toll-like receptor 4 expression in peripheral blood mononuclear cells in Behcet's disease. Arthritis Res Ther. 2008;10:R16. doi: 10.1186/ar2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amori L., Guidetti P., Pellicciari R., Kajii Y., Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009;109:316–325. doi: 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morita T., Saito K., Takemura M., Maekawa N., Fujigaki S., Fujii H., Wada H., Takeuchi S., Noma A., Seishima M. L-tryptophan-kynurenine pathway metabolite 3-hydroxyanthranilic acid induces apoptosis in macrophage-derived cells under pathophysiological conditions. Adv Exp Med Biol. 1999;467:559–563. doi: 10.1007/978-1-4615-4709-9_69. [DOI] [PubMed] [Google Scholar]
- 49.John G.R., Simpson J.E., Woodroofe M.N., Lee S.C., Brosnan C.F. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J Neurosci. 2001;21:4134–4142. doi: 10.1523/JNEUROSCI.21-12-04134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park C., Lee S., Cho I.H., Lee H.K., Kim D., Choi S.Y., Oh S.B., Park K., Kim J.S., Lee S.J. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:428–456. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 51.Smith A.J., Stone T.W., Smith R.A. Neurotoxicity of tryptophan metabolites. Biochem Soc Trans. 2007;35:1287–1289. doi: 10.1042/BST0351287. [DOI] [PubMed] [Google Scholar]
- 52.Okuda S., Nishiyama N., Saito H., Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 53.Okuda S., Nishiyama N., Saito H., Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci U S A. 1996;93:12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidetti P., Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur J Neurosci. 1999;11:3857–3863. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 55.Nakagami Y., Saito H., Katsuki H. 3-Hydroxykynurenine toxicity on the rat striatum in vivo. Jpn J Pharmacol. 1996;71:183–186. doi: 10.1254/jjp.71.183. [DOI] [PubMed] [Google Scholar]
- 56.Eastman C.L., Guilarte T.R. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989;495:225–231. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 57.Min K.J., Yang M.S., Kim S.U., Jou I., Joe E.H. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forrest C.M., Mackay G.M., Oxford L., Stoy N., Stone T.W., Darlington L.G. Kynurenine pathway metabolism in patients with osteoporosis after 2 years of drug treatment. Clin Exp Pharmacol Physiol. 2006;33:1078–1087. doi: 10.1111/j.1440-1681.2006.04490.x. [DOI] [PubMed] [Google Scholar]
- 59.Darlington L.G., Mackay G.M., Forrest C.M., Stoy N., George C., Stone T.W. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci. 2007;26:2211–2221. doi: 10.1111/j.1460-9568.2007.05838.x. [DOI] [PubMed] [Google Scholar]
- 60.Oh G.S., Pae H.O., Choi B.M., Chae S.C., Lee H.S., Ryu D.G., Chung H.T. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem Biophys Res Commun. 2004;320:1156–1162. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 61.Sierra-Filardi E., Vega M.A., Sanchez-Mateos P., Corbi A.L., Puig-Kroger A. Heme Oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215:788–795. doi: 10.1016/j.imbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Ryter S.W., Choi A.M. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009;41:251–260. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C., Hossieny P., Wu B.J., Qawasmeh A., Beck K., Stocker R. Pharmacologic induction of heme oxygenase-1. Antioxid Redox Signal. 2007;9:2227–2239. doi: 10.1089/ars.2007.1783. [DOI] [PubMed] [Google Scholar]
- 64.Kapturczak M.H., Wasserfall C., Brusko T., Campbell-Thompson M., Ellis T.M., Atkinson M.A., Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao L.H., Wysk M., Davis R.J., Flavell R.A., Choi A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 66.Ryter S.W., Alam J., Choi A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 67.Mehler M.F., Rozental R., Dougherty M., Spray D.C., Kessler J.A. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993;362:62–65. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- 68.Alberati-Giani D., Malherbe P., Ricciardi-Castagnoli P., Kohler C., Denis-Donini S., Cesura A.M. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells. J Immunol. 1997;159:419–426. [PubMed] [Google Scholar]
- 69.Stone T.W., Forrest C.M., Mackay G.M., Stoy N., Darlington L.G. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis. 2007;22:337–352. doi: 10.1007/s11011-007-9064-3. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang K.W., Lee S.J., Kim S.G. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 72.Naughton P., Foresti R., Bains S.K., Hoque M., Green C.J., Motterlini R. Induction of heme oxygenase 1 by nitrosative stress: a role for nitroxyl anion. J Biol Chem. 2002;277:40666–40674. doi: 10.1074/jbc.M203863200. [DOI] [PubMed] [Google Scholar]
- 73.Dykens J.A., Sullivan S.G., Stern A. Oxidative reactivity of the tryptophan metabolites 3-hydroxyanthranilate, cinnabarinate, quinolinate and picolinate. Biochem Pharmacol. 1987;36:211–217. doi: 10.1016/0006-2952(87)90691-5. [DOI] [PubMed] [Google Scholar]
- 74.Opitz C.A., Wick W., Steinman L., Platten M. Tryptophan degradation in autoimmune diseases. Cell Mol Life Sci. 2007;64:2542–2563. doi: 10.1007/s00018-007-7140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christen S., Peterhans E., Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ona V.O., Li M., Vonsattel J.P., Andrews L.J., Khan S.Q., Chung W.M., Frey A.S., Menon A.S., Li X.J., Stieg P.E., Yuan J., Penney J.B., Young A.B., Cha J.H., Friedlander R.M. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 77.Silvestroni A., Faull R.L., Strand A.D., Moller T. Distinct neuroinflammatory profile in post-mortem human Huntington's disease. Neuroreport. 2009;20:1098–1103. doi: 10.1097/WNR.0b013e32832e34ee. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H&E–stained sections of mouse brains injected with 3-HAA or vehicle (1N HCl). A and B: Frank toxicity occurred only in mice injected with solutions without pH adjustment. Arrows points to an area with tissue necrosis associated with large numbers of dead neurons (inset). B is the low power view of the area in A, demonstrating injection within the caudate putamen. (cc = corpus callosum, s = septum, ac = anterior commissure). C-F: Mice injected with 3-HAA (C and D) or vehicle (E and F) at pH 7.5 showed no gross tissue necrosis, aside from microscopic hemorrhages (arrows) associated with a few apoptotic cells. Approximate original magnifications: ×100 (A), ×25 (B), ×100 (C), ×140 (D), ×100 (E), and ×200 (F).


