Abstract
The oviduct is a dynamic structure whose function relies upon cyclic changes in the morphology of both ciliated and secretory luminal epithelial cells. Unfortunately, infection of these epithelial cells by sexually transmitted pathogens can lead to pelvic inflammatory disease, ectopic pregnancies and infertility. The disruption of normal, cyclic apoptosis in the oviductal epithelium appears to be a causal factor of sexually-transmitted oviductal pathology and therefore, these pathways represent a potential target for diagnosis and/or therapeutic intervention. The objective of this study was to determine the normal, cyclic pattern of expression for apoptotic genes in the oviduct of the naturally cycling mouse, generating fundamental information that can be applied to the development of animal models for research and/or the identification of targets for disease intervention. Whole oviducts were collected from regular cycling mice killed at 1 pm on each day of the estrous cycle and the expression of 84 key apoptotic genes determined by targeted PCR super-array. Intact and cleaved caspases were then evaluated by western blotting. The expression of mRNA for genes classified as pro-apoptotic (Bad, Bak1 and Bok) and anti-apoptotic (Bag3, Bnip2 and Xiap) was regulated by day of the estrous cycle (P < 0.05). Differences in the temporal expression of several p53-related genes (Trp53bp2, Trp53inp1 and Trp73), those specific to the TNF superfamily (Tnfrsf10 and Tnfsf10b) and one caspase (Casp14) were also observed (P < 0.05). The cleaved forms of Capases-3, −6 and −12 were all detected throughout the estrous cycle. These results represent the first pathway wide analysis of apoptotic gene expression in the murine oviduct.
INTRODUCTION
Proper function of the oviduct is essential for the establishment of an unassisted pregnancy: the oviduct provides the necessary micro-environment for gamete survival, maturation, fertilization and then development of the very early embryo (reviewed in (Mastroianni, 1999). To fulfill this role, the oviduct must undergo cyclic changes involving the growth and regression of its epithelial cell layer (Abe and Oikawa 1993; Abe et al. 1999). Cyclic changes in the ratio of ciliated to secretory epithelial cells (Shirley and Reeder 1996), their overall height (Murray and DeSouza 1995) and secretory output (Nichol et al. 1992; Gardner et al. 1996) are all reported. As such, the oviduct is a dynamic organ, reliant on processes such as regulated cell death or apoptosis to maintain cellular homeostasis and therefore function and fertility.
The epithelial cells of the oviduct also function as a barrier to infection and disease, with exposure to an array of foreign pathogens at mating possible. Again, the process of apoptosis plays a key role in maintaining cellular homeostasis, however in this case in the defense against pathogenic infection (Antoni et al. 1995). In fact, the most common sexually-transmitted bacterial pathogens, Chlamydia trachomatis and Neisseria gonorrhoea are known to target a variety of apoptotic processes within this tissue (Fan et al. 1998; Binnicker et al. 2003). These obligate intracellular parasites manipulate signaling pathways in an intricate balance to both exploit and protect the host (Koomey 2001; Zhong 2009).
Given that regulated apoptotic processes are required for the maintenance of a normal, patent oviduct and therefore fertility, and that sexually transmitted infections are known to impair oviductal function by a targeted disruption of this same cascade, it appeared to us that specific apoptotic processes could be targeted in the future for the management of oviductal diseases, especially those induced by sexually transmitted pathogens. Unfortunately, very little information is currently available on the normal cyclic pattern of apoptotic gene expression in the oviduct, effectively hampering our ability to fully interpret the results of many studies. This, in turn, impairs our ability to translate results to the clinical setting as well as refine animal models for further research.
To address this, we designed a study that would utilize real-time PCR based super-arrays to simultaneously determine the level of expression of many genes within the apoptotic pathway. The objective of this study was to determine a profile of apoptotic genes within the oviduct that are regulated during the normal estrous cycle of the mouse, thus providing knowledge that will facilitate future research in the prevention and/or control of apoptotic-regulated oviductal dysfunction. Genes of both the intrinsic and extrinsic pathways were examined, the intrinsic pathway being activated from stressors within a cell that is destined for destruction, whereas the extrinsic pathway is invoked via external, receptor mediated signals (reviewed in Delhalle, 2003). To complement this gene expression analysis, we then evaluated the presence of both the intact and cleaved form of four of the caspases by western blotting. Activation of these caspases marks one of the final functional processes in the apoptotic pathway. Both initiator and effector caspases were detected, confirming regulated apoptosis throughout the estrous cycle in the oviduct of the mouse.
MATERIALS AND METHODS
Animals
CD1 mice were purchased from Harlan Inc. (Harlan, IN) and maintained under controlled lighting (14 h light/10 h dark) with continuous access to food and water. Animal procedures are described for each experiment below and were approved by the University of Kentucky Animal Care and Use Committee according to NIH guidelines for the ethical use of animals in research.
Staging of estrous cycles
Beginning at 6–8 weeks of age, estrous cycles were staged in CD1 females by analysis of vaginal cytology. The vagina was flushed daily, at the same time each morning, with 0.9% sodium chloride using a bent, blunted borosilicate glass pipette. To acclimatize the mice to the procedure, smears were collected for the first 7–10 days without classification. Thereafter, vaginal smears were collected in individual wells of 24-well culture plates (Costar, Corning, NY) and classified according to well established morphological guidelines (Goldman et al. 2007; Caligioni 2009). Smears were visualized under a Motic AE21 inverted microscope (Motic Instruments, Canada) and a digital image recorded for later reference. A total of 6 mice were killed on each day of the estrous cycle and all mice were killed at 1 pm on a designated day. Immediately before sacrifice, a final confirmatory smear was collected. Mice were allocated to a particular day only after a consistent and repeatable pattern of cycling activity was recorded. Mice were killed by asphyxiation with carbon dioxide followed by cervical luxation and the reproductive tracts exteriorized for tissue collection. Ovaries and oviducts were retrieved, separated and snap frozen on dry ice. Blood was collected by cardiac puncture and stored overnight at 4°C before serum was harvested by centrifugation.
Concentrations of oestradiol in the serum of cycling mice
The concentration of oestradiol was determined in the serum of mice killed for the analysis of gene expression by super-array (3 mice per day). Radioimmunoassays were performed bythe University of Virginia Center for Research in ReproductionLigand Assay and Analysis Core using a Beckman Coulter antibody, as described by others (O’Brien et al. 2006). Because the concentration of oestradiol that was measured in samples collected on each day other than proestrus was at, or below, the listed sensitivity of this radioimmunoassay, no statistical analysis was performed and the results are not reported herein.
Expression of mRNA for Cyp17a1 and Cyp19a1 in the ovaries of cycling mice
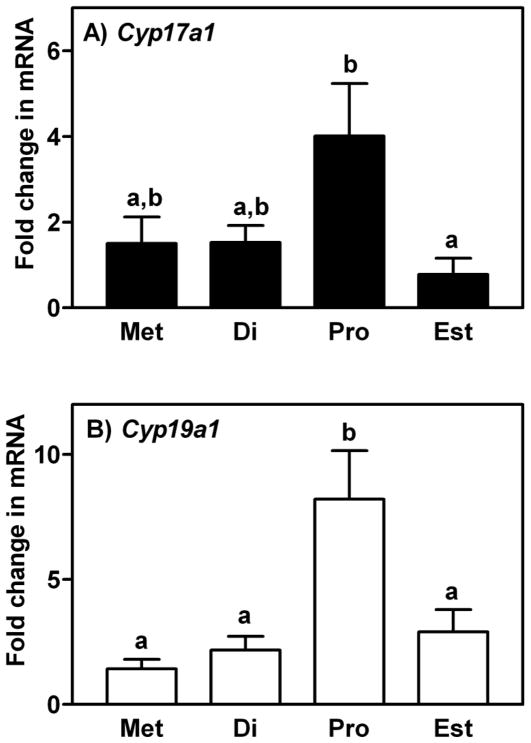
Real-time PCR was performed to determine the level of expression of mRNA for Cyp17a1 (cytochrome P450, family 17, subfamily a, polypeptide 1) and Cyp19a1 (cytochrome P450, family 19, subfamily a, polypeptide 1; aromatase) in the ovaries of each mouse that was sacrificed (Figure 1). Analysis of the level of mRNA for these two steroidogenic genes was used as another means of confirmation that the estrous cycle of each mouse had been staged correctly, as described by others (Soumano et al. 1996; Hinshelwood et al. 2005). Real-time PCR was performed using SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA) and gene specific primer pairs (Cyp17a1 forward 5′-TGG TCA TAT GCA TGC CAA CT-3′ and reverse 5′-GAG CGT AGA CAG ATC TCG GG-3′; Cyp19a1 forward 5′-GTC CTG GCT ACT GTC TGG GA-3′ and reverse 5′-CAA ATG CTG CTT GAT GGA CT-3′) on a Bio-Rad IQ5 system, as described previously (Al-Alem et al. 2007; Bridges et al. 2010; Jeoung et al. 2010). Protocol conditions consisted of denaturation at 95 °C for 30 seconds, followed by 40 cycles at 94 °C for 30 sec, 55 °C for 30 sec and 72 °C for 45 sec with a final dissociation (melting) curve analysis. The relative level of expression of each mRNA was standardized against L19 (forward 5′-TGG TTG GAT CCC AAT GAG AC-3′ and reverse 5′-GTC TGC CTT CAG CTT GTG GAT-3′) as a housekeeping gene and analyzed by the 2−ΔΔCT method (Livak and Schmittgen 2001).
Figure 1.
Expression of mRNA encoding A) Cyp17a1 and B) Cyp19a1 in the ovaries of mice sacrificed at each day of the estrous cycle. Data are the means ± SEM of three samples per day. Levels of mRNA were obtained by real-time PCR, expressed as fold changes and analyzed by one-way ANOVA. For each mRNA within a panel, values with different superscript letters differ (P < 0.05).
Analysis of gene expression by real-time PCR super-array
Temporal changes in the level of expression of genes that regulate apoptotic processes was determined in whole oviducts collected from naturally cycling mice by a super-array, real-time PCR analysis. Oviducts were collected from three mice at each day of the estrous cycle and the paired oviducts from each mouse were handled as individual samples for this analysis. Total RNA was isolated from each sample using Trizol (Invitrogen, Carlsbad, CA)and then purified using RNeasy (Qiagen, Valencia, CA) following the manufacturer’sdirections. Potential genomic DNA contamination was eliminated by treatment with DNase. The mouse apoptosis RT2 Profiler PCR Arrays and RT2 Real-Timer SyBR Green reagent were purchased from SuperArray Bioscience Corporation (Frederick, MD). Each super-array (96-well plate) contains the primers to identify 84 key genes involved in apoptosis as well as 5 housekeeping genes. A genomic DNA contamination control, reverse transcription controls and positive PCR controls are also included. The RT2 First strand kit (SuperArray Bioscience) contained all the reagents required to reverse transcribe the total RNA to cDNA and eliminate potential genomic DNA. Real-time PCR was then performed on a Bio-Rad IQ5 system, as described previously (Al-Alem et al. 2007; Bridges et al. 2010; Jeoung et al. 2010). All experimental arrays passed the designated quality controls for reproducibility, reverse transcription efficiency and (lack of) genomic DNA contamination. The relative level of expression of each mRNA was standardized against GAPDH as a housekeeping gene and analyzed by the 2−ΔΔCT method (Livak and Schmittgen 2001).
Detection of Caspases by Western Blotting
Levels of intact and cleaved Caspases-3, −6, −9 and 12 (CASP3, CASP6, CASP9 and CASP12) were assessed in whole oviducts collected from naturally cycling mice by western blotting. Oviducts were collected from cyclic mice as described above and lysed by homogenization in radio-immunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, MA). Again, three mice were sacrificed at each day of the estrous cycle and the oviducts of each mouse were handled as an individual sample. The concentration of protein in each sample was determined using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) and protein (50 μg per lane) separated on 10% precast SDS polyacrylamide gels before being transferred to polyvinylidene fluoride (PVDF) membranes (both from Bio-Rad, Hercules, CA) using a semi-dry transfer. Non-specific binding sites were blocked with 5% non-fat milk at room temperature for 2 h and membranes were incubated with antibodies purchased from Cell Signaling Technology (Danvers, MA) that were generated against Capase-3 (full length, #9662 and cleaved, #9664), Caspase-6 (full length, #9762 and cleaved, #9761), Caspase-9 (full length, #9504 and cleaved, #9509) and Caspase-12 (detects full length and cleaved, #2202), each at a 1:2000 dilution, overnight at 4 °C. Each blot was later incubated with an anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) polyclonal goat antibody (diluted 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) to verify equal protein loading. The immuno-reaction was detected by incubating each blot with the appropriate peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:4000; Cell Signaling Technology) or donkey anti-goat secondary antibody (1:5000; Santa Cruz) for 2 h at room temperature and images acquired using an enhanced chemiluminescence kit (Amersham Pharmacia, Freiburg, Germany).
Statistical Analysis
All data sets were tested for homogeneity of variance and normality. If these criteria were met, the effect of day of the estrous cycle on gene expression was analyzed by one-way ANOVA followed by a post-hoc Student Newman-Keuls test when significant (P < 0.05). When these criteria were not met, a nonparametric Kruskal-Wallis test was utilized. These analyses were performed using SigmaStat 3.5 (SystatSoftware, Inc. Point Richmond, CA). The results presented in Supplemental Table I were obtained by analysis of sequential daily changes in gene expression by Students t-test. Those results are provided to grant the reader a more complete understanding of apoptotic gene expression profiles in the murine oviduct.
RESULTS
Components of the Intrinsic Pathway
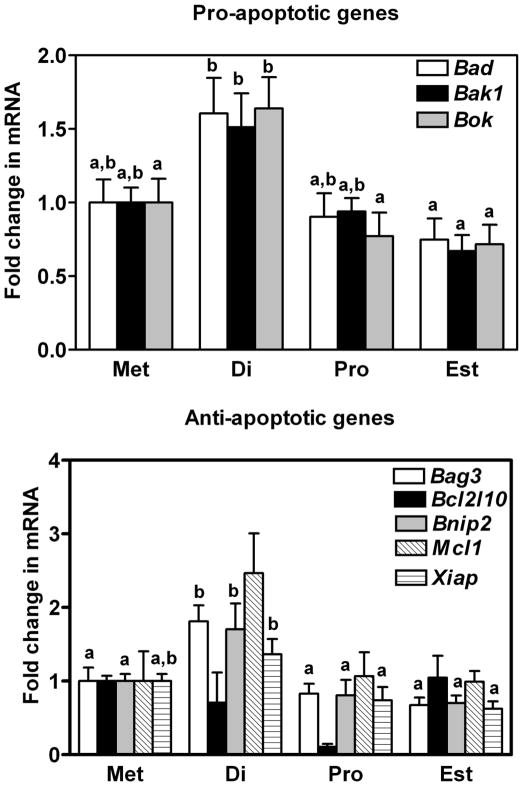
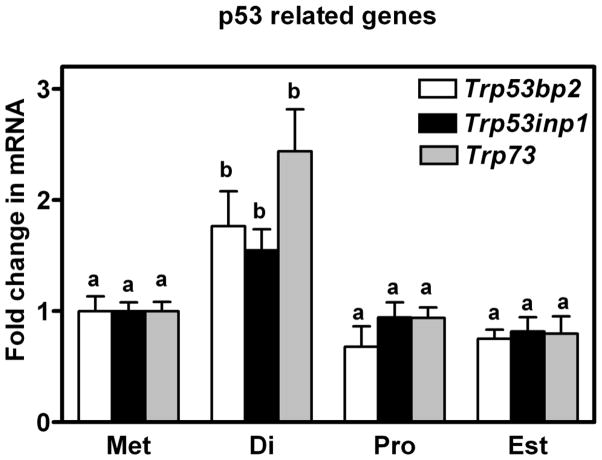
The level of expression of mRNA encoding three pro-apoptotic (Bad: BCL2-associated agonist of cell death; Bak1: BCL2-antagonist/killer 1; and Bok: BCL2-related ovarian killer protein) and four anti-apoptotic (Bag3: BCL2-associated athanogene 3; Bcl2l10: Bcl2-like 10; Bnip2: BCL2/adenovirus E1B interacting protein; Mcl1: myeloid cell leukemia sequence 1) genes of the intrinsic apoptotic pathway is shown in Figure 2 (the anti-apoptotic Xiap is described later in the results). Five of these seven genes were observed to undergo a cyclic regulation in their level of gene expression with mRNA consistently higher in oviducts collected on the day of diestrus (P < 0.05). Temporal differences in the expression of mRNA for 3 other intrinsic genes, in this case inclusive to the p53 family, were also observed and are shown in Figure 3. Expression of mRNA for Trp73 (transformation related protein 73) as well as Trp53inp1 (transformation related protein 53 inducible nuclear protein 1) and Tr53bp2 (transformation related protein 53 binding protein 2), all pro-apoptotic genes, were transiently increased in oviducts at diestrus when compared to other days of the estrous cycle (P < 0.05).
Figure 2.
Expression of mRNA encoding A) Bad, Bak1 and Bok, and B) Bag3, Bcl2l10, Bnip2, Mcl1 and Xiap in the oviducts of mice sacrificed at each day of the estrous cycle. Data are the means ± SEM of three samples per day. Levels of mRNA were obtained using mouse apoptosis RT2 Profiler PCR Arrays by the 2−ΔΔCT method. Data was analyzed by one-way ANOVA. For each mRNA within a panel, values with different superscript letters differ (P < 0.05).
Figure 3.
Expression of mRNA encoding Trp53bp2, Trp53inp1 and Trp73 in the oviducts of mice sacrificed at each day of the estrous cycle. Data are the means ± SEM of three samples per day. Levels of mRNA were obtained using mouse apoptosis RT2 Profiler PCR Arrays by the 2−ΔΔCT method. Data was analyzed by one-way ANOVA. For each mRNA, values with different superscript letters differ (P < 0.05).
Components of the Extrinsic Pathway
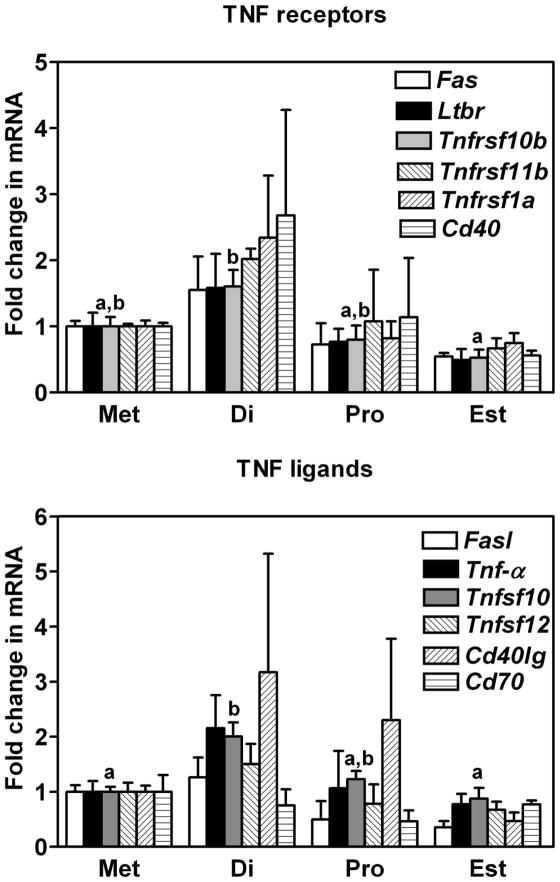
Consistent with our observation that several members of the intrinsic pathway of apoptosis exhibited a cyclic regulation to their gene expression, analysis of extrinsic factors uncovered temporal changes as well. The expression of mRNA for Tnfsf10 (tumor necrosis factor (ligand) superfamily, member 10) and Tnfrsf10b (tumor necrosis factor (receptor) superfamily, member 10b) a ligand and receptor within the TNF family of extrinsic factors, were found to differ. Levels of mRNA for both these genes were higher at diestrus when compared to the day of estrus, 48 h later (P < 0.05, Figure 4). Analysis of gene expression from day to day (t-test; Supplemental Table 1) uncovered additional TNF family members that may warrant further investigation. Inclusive to these were mRNA encoding two receptors (Fas: TNF receptor superfamily member 6; and Cd40: Cd40 antigen) and one ligand (Cd40lg: Cd40 ligand). No statistically significant difference was detected in the expression of mRNA encoding Ltbr (lymphotoxin B receptor), Fasl (Fas ligand, TNF superfamily, member 6), TNF-α (tumor necrosis factor) or Cd70 (Cd70 antigen).
Figure 4.
Expression of mRNA encoding A) Fas, Ltbr, Tnfrsf10b, Tnfrsf11b, Tnfrsf1a and Cd40 and B) Fasl, Tnf-α, Tnfsf10, Tnfsf12, Cd40lg and Cd70 in the oviducts of mice sacrificed at each day of the estrous cycle. Data are the means ± SEM of three samples per day. Levels of mRNA were obtained using mouse apoptosis RT2 Profiler PCR Arrays by the 2−ΔΔCT method. Data was analyzed by one-way ANOVA. For each mRNA within a panel, values with different superscript letters differ (P < 0.05).
The Caspase Cascade
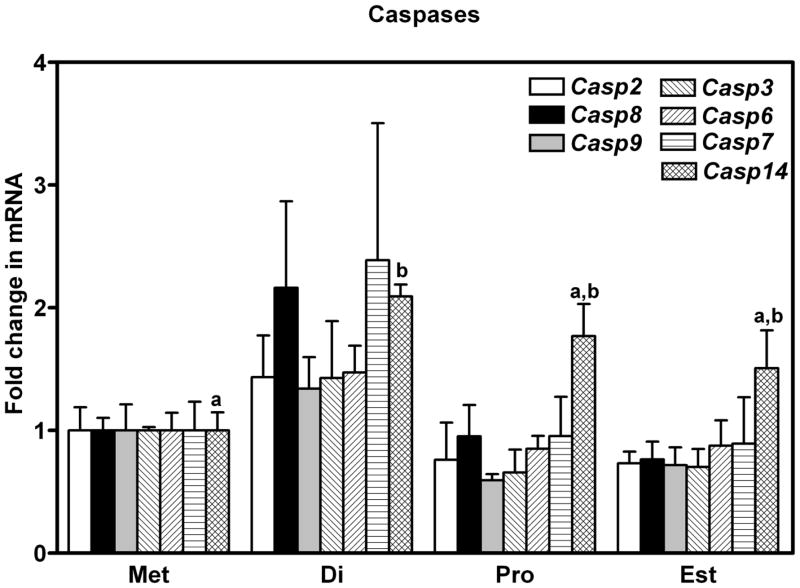
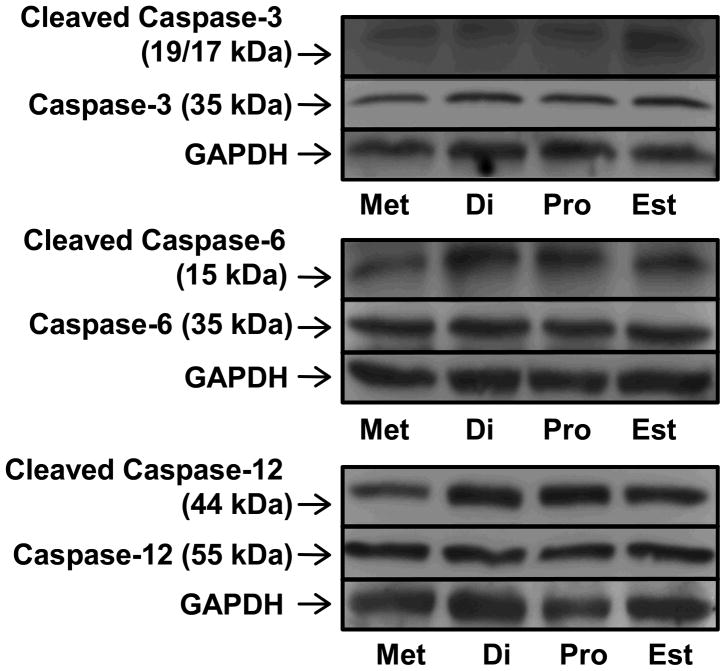
No dramatic differences in the expression of mRNA for members of the caspase familly were observed during the estrous cycle. Expression of mRNA for Casp14 (Caspase-14) was higher in oviducts collected from mice at diestrus than metestrus (P < 0.05, Figure 5), and the level of mRNA encoding Xiap (X-linked inhibitor of apoptosis), an inhibitor of Caspase-3, −7 and −9, was higher at diestrus than proestrus or estrus (P < 0.05, Figure 2). Cleaved CASP3, CASP6 and CASP12 were all detected by western blotting, along with their uncut precursors (Figure 6), with no dramatic temporal pattern of expression apparent. Interestingly, of the two bands (17 and 19 kDa) that were expected for cleaved CASP3, the 17 kDa band was only observed when oviducts were collected from mice sacrificed at estrus. Cleaved CASP9 was not detected by western blotting (not shown).
Figure 5.
Expression of mRNA encoding Caspases 2, 3, 6, 7, 8, 9 and 14 in the oviducts of mice sacrificed at each day of the estrous cycle. Data are the means ± SEM of three samples per day. Levels of mRNA were obtained using mouse apoptosis RT2 Profiler PCR Arrays by the 2−ΔΔCT method. Data was analyzed by one way ANOVA. For each mRNA, values with different superscript letters differ (P < 0.05).
Figure 6.
Detection of full length and cleaved CASP3, CASP6 and CASP12 in the oviducts of mice sacrificed at each day of the estrous cycle. The images are representative of three western blots (replicates) performed for each caspase with GAPDH used as a loading control.
DISCUSSION
Sexually-transmitted pathogens such as C. trachomatis and N. gonorrhoea target apoptotic processes within the oviduct (Fan et al. 1998; Binnicker et al. 2003). In this report, we analyzed the pattern of expression of many of the genes involved in the apoptotic cascade over the natural estrous cycle of the laboratory mouse. It is our belief that by gaining an increased understanding of the normal genetic pathways involved in maintaining homeostasis and function of the oviduct, research investigating specific signaling pathways affected by infection with these bacterial agents can be designed more completely and the resultant data better extrapolated to the clinical setting. This may be especially pertinent given the increasing resistance to antibiotic therapy of infectious agents such as N. gonorrhoea (Tapsall et al. 2010; Whiley et al. 2010).
It is clear that sexually infectious intracellular pathogens have evolved the means to survive, utilizing us as hosts and adapting our bodies’ natural apoptotic processes to their advantage. Mechanisms whereby C. trachomatis can block host apoptosis include inducing the degradation of pro-apoptotic proteins from the mitochondria (Fan et al. 1998; Dong et al. 2005; Ying et al. 2005), inhibiting antigen presentation (Zhong et al. 2001; Kawana et al. 2007) and stabilizing proteins with an inhibitory apoptotic role (Rajalingam et al. 2006). Infection with N. gonorrhoea can induce the infiltration of immune cells and stimulate apoptosis at the epithelial cell interface (Witt et al. 1976). Specific membrane proteins are involved, including the bacterial PorB porin which allows a rapid calcium influx into the affected cell (Martin and Green 1995; Muller et al. 1999). Importantly, the regulated destruction of epithelial cells can allow pathogens access to deeper, uninfected tissues (McGee et al. 1981).
As expected, some commonality was identified among apoptotic genes exhibiting a cyclic pattern of expression and those targeted by apoptotic processes. For example, members of the well recognized Bcl-2 family include both pro- and anti-apoptotic members. Bim (BCL2-like 11, apoptosis facilitator) and Bmf (BCL2 modifying factor) are reported to mediate N. gonorrhoea-induced infection through the control of BAK1 and BAX (BCL2-associated X protein) (Kepp et al. 2009), and C. trachomatis was reported to resist apoptosis through the suppression or destruction of BIM, BAD and PUMA (BBC3, BCL2 binding component 3) proteins (Fischer et al. 2004). Of these, mRNA for Bak1 and Bad appeared to be the most regulated within an estrous cycle. It should be noted though that matching changes in gene expression to known pathogen-affected apoptotic processes is not the objective of the study herein. Rather, our aim was to provide a more complete overview of apoptotic genes that are regulated in a temporal manner over the course of an estrous cycle in the mouse.
Among the apoptotic genes differentially expressed over time, consistency was observed in their overall manner of regulation. With increasing concentrations of circulating estradiol, mice progressed from diestrus into proestrus and the expression of all the genes we identified as cyclic-regulated within the oviduct was decreased. In contrast, as mice progressed to metestrus and diestrus, mRNA for changing apoptotic genes was uniformly increased. A general trend in the regulation of apoptotic gene expression by ovarian steroids could be hypothesized by this temporal pattern. Indeed, the regulation of oviductal function by ovarian steroids was established many years ago (Mason 1952; Lehrman and Brody 1957), epithelial localization of the respective steroid receptors is well documented (Teilmann et al. 2006; Shao et al. 2007) and both estrogen and progesterone receptor mediated signaling of apoptotic mediators have been defined (Mintz et al. 2008; Yoshida et al. 2010).
It is also likely that the heterogeneity of cells within the oviduct and our limited sample size has decreased our ability to statistically identify some differences in gene expression across the estrous cycle. A striking example of this and what appears to be a gene negatively regulated by estradiol is the mouse homolog of Bcl2l10. Expression of mRNA for Bcl2l10 was ~10-fold higher in oviducts collected at estrus versus proestrus (Supplemental Table 1) and a search of the UCSC Genome Browser (Kent et al. 2002) indicated that there is an estrogen receptor binding site upstream of the Bcl2l10 promoter. Overall, the trend for gene expression to be increased at diestrus, regardless of whether the apoptotic gene was pro- or anti-apoptotic in function was an unexpected finding. However, with circulating steroids at basal levels and the reproductive cycle at a relatively quiescent stage, it could be construed that this is an opportune time to repair and ready the oviduct for the next ovulation.
An evaluation of functional activation of apoptosis within the cycling oviduct was included as a final objective in this study. Caspase activation is considered one of the most specific indicators of apoptosis (Gown and Willingham 2002) and hence, caspases-3, −6, −9 and −12 (CASP3, CASP6, CASP9 and CASP12), in their full length and cleaved states, were examined by western blotting, rather than the more ubiquitous TUNEL assay that detects DNA fragmentation. Caspase-9 and −12 are classed as initiator or pro-apoptotic caspases, whereas Caspases-3 and −6 are classed as downstream effector caspases that are cleaved and activated by these aforementioned initiators. The full length and cleaved forms of caspases-3, −6 and −12 were detected on each day of the estrous cycle, indicating that some level of functional apoptosis is ongoing within the oviduct throughout the entire reproductive cycle.
In summary, genes of the apoptotic pathway that are expressed within the cycling mouse oviduct show a temporal pattern in their level of expression. Genes of both the intrinsic and extrinsic pathways were readily detected and an overall trend for increased gene expression was observed at diestrus, however cleaved caspases signifying functional apoptosis could be detected on each day of the estrous cycle. With sexually-induced pathogens known to target apoptotic processes within the oviduct, it is hoped that a better understanding of the normal pattern of apoptotic gene expression will aid in the further study, diagnosis and or therapeutic intervention of apoptosis-related oviductal disease.
Supplementary Material
Acknowledgments
Grant Support: Supported by Start-up funds from the University of Kentucky, P20 RR15592 and K12 DA014040 from the National Institutes of Health.
References
- Abe H, Oikawa T. Observations by scanning electron microscopy of oviductal epithelial cells from cows at follicular and luteal phases. Anat Rec. 1993;235:399–410. doi: 10.1002/ar.1092350309. [DOI] [PubMed] [Google Scholar]
- Abe H, Onodera M, Sugawara S, Satoh T, Hoshi H. Ultrastructural features of goat oviductal secretory cells at follicular and luteal phases of the oestrous cycle. J Anat. 1999;195(Pt 4):515–521. doi: 10.1046/j.1469-7580.1999.19540515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrin. 2007;193:383–391. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- Antoni BA, Sabbatini P, Rabson AB, White E. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol. 1995;69:2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnicker MJ, Williams RD, Apicella MA. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell Microbiol. 2003;5:549–560. doi: 10.1046/j.1462-5822.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al Alem L, Na G, Su W, Gong MC, Jeoung M, Ko C. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010;22:780–787. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, Zhong G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005;73:1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, Ying S, Hacker G. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil Steril. 1996;65:349–353. doi: 10.1016/s0015-0282(16)58097-2. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- Hinshelwood MM, Shelton JM, Richardson JA, Mendelson CR. Temporal and spatial expression of liver receptor homologue-1 (LRH-1) during embryogenesis suggests a potential role in gonadal development. Dev Dyn. 2005;234:159–168. doi: 10.1002/dvdy.20490. [DOI] [PubMed] [Google Scholar]
- Jeoung M, Lee S, Hawng HK, Cheon YP, Jeong YK, Gye MC, Iglarz M, Ko C, Bridges PJ. Identification of a novel role for endothelins within the oviduct. Endocrinology. 2010;151:2858–2867. doi: 10.1210/en.2009-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana K, Quayle AJ, Ficarra M, Ibana JA, Shen L, Kawana Y, Yang H, Marrero L, Yavagal S, Greene SJ, Zhang YX, Pyles RB, Blumberg RS, Schust DJ. CD1d degradation in Chlamydia trachomatis-infected epithelial cells is the result of both cellular and chlamydial proteasomal activity. J Biol Chem. 2007;282:7368–7375. doi: 10.1074/jbc.M610754200. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Gottschalk K, Churin Y, Rajalingam K, Brinkmann V, Machuy N, Kroemer G, Rudel T. Bim and Bmf synergize to induce apoptosis in Neisseria gonorrhoeae infection. PLoS Pathog. 2009;5:e1000348. doi: 10.1371/journal.ppat.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey M. Implications of molecular contacts and signaling initiated by Neisseria gonorrhoeae. Curr Opin Microbiol. 2001;4:53–57. doi: 10.1016/s1369-5274(00)00164-8. [DOI] [PubMed] [Google Scholar]
- Lehrman DS, Brody P. Oviduct response to estrogen and progesterone in the ring dove (Streptopelia risoria) Proc Soc Exp Biol Med. 1957;95:373–375. doi: 10.3181/00379727-95-23226. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Mason RC. Synergistic and antagonistic effects of progesterone in combination with estrogens on oviduct weight. Endocrinology. 1952;51:570–572. doi: 10.1210/endo-51-6-570. [DOI] [PubMed] [Google Scholar]
- McGee ZA, Johnson AP, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Mintz PJ, Habib NA, Jones LJ, Giamas G, Lewis JS, Bowen RL, Coombes RC, Stebbing J. The phosphorylated membrane estrogen receptor and cytoplasmic signaling and apoptosis proteins in human breast cancer. Cancer. 2008;113:1489–1495. doi: 10.1002/cncr.23699. [DOI] [PubMed] [Google Scholar]
- Muller A, Gunther D, Dux F, Naumann M, Meyer TF, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MK, DeSouza MM. Messenger RNA encoding an estrogen-dependent oviduct secretory protein in the sheep is localized in the apical tips and basal compartments of fimbria and ampulla epithelial cells implying translation at unique cytoplasmic foci. Mol Reprod Dev. 1995;42:268–283. doi: 10.1002/mrd.1080420303. [DOI] [PubMed] [Google Scholar]
- Nichol R, Hunter RH, Gardner DK, Leese HJ, Cooke GM. Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period. J Reprod Fertil. 1992;96:699–707. doi: 10.1530/jrf.0.0960699. [DOI] [PubMed] [Google Scholar]
- O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- Rajalingam K, Sharma M, Paland N, Hurwitz R, Thieck O, Oswald M, Machuy N, Rudel T. IAP-IAP complexes required for apoptosis resistance of C. trachomatis-infected cells. PLoS Pathog. 2006;2:e114. doi: 10.1371/journal.ppat.0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R, Egecioglu E, Weijdegard B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007;293:E1430–1442. doi: 10.1152/ajpendo.00384.2007. [DOI] [PubMed] [Google Scholar]
- Shirley B, Reeder RL. Cyclic changes in the ampulla of the rat oviduct. J Exp Zool. 1996;276:164–173. doi: 10.1002/(SICI)1097-010X(19961001)276:2<164::AID-JEZ10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Soumano K, Silversides DW, Doize F, Price CA. Follicular 3 beta-hydroxysteroid dehydrogenase and cytochromes P450 17 alpha-hydroxylase and aromatase messenger ribonucleic acids in cattle undergoing superovulation. Biol Reprod. 1996;55:1419–1426. doi: 10.1095/biolreprod55.6.1419. [DOI] [PubMed] [Google Scholar]
- Tapsall JW, Limnios EA, Abu Bakar HM, Darussalam B, Ping YY, Buadromo EM, Kumar P, Singh S, Lo J, Bala M, Risbud A, Deguchi T, Tanaka M, Watanabe Y, Lee K, Chong Y, Noikaseumsy S, Phouthavane T, Sam IC, Tundev O, Lwin KM, Eh PH, Goorant C, Goursaud R, Bathgate T, Brokenshire M, Latorre L, Velemu E, Carlos C, Leano S, Telan EO, Goh SS, Koh ST, Ngan C, Tan AL, Mananwatte S, Piyanoot N, Lokpichat S, Sirivongranson P, Fakahau M, Sitanilei H, Hung le V. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian regions, 2007–2008. Commun Dis Intell. 2010;34:1–7. [PubMed] [Google Scholar]
- Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191:525–535. doi: 10.1677/joe.1.06565. [DOI] [PubMed] [Google Scholar]
- Whiley DM, Goire N, Lambert SB, Ray S, Limnios EA, Nissen MD, Sloots TP, Tapsall JW. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J Antimicrob Chemother. 2010 doi: 10.1093/jac/dkq187. [DOI] [PubMed] [Google Scholar]
- Witt K, Veale DR, Finch H, Penn CW, Sen D, Smith H. Resistance of Neisseria gonorrhoeae grown in vivo to ingestion and digestion by phagocytes of human blood. J Gen Microbiol. 1976;96:341–350. doi: 10.1099/00221287-96-2-341. [DOI] [PubMed] [Google Scholar]
- Ying S, Seiffert BM, Hacker G, Fischer SF. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun. 2005;73:1399–1403. doi: 10.1128/IAI.73.3.1399-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, Sasaki H, Morikawa A, Maruo T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28:260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- Zhong G. Killing me softly: chlamydial use of proteolysis for evading host defenses. Trends Microbiol. 2009;17:467–474. doi: 10.1016/j.tim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Douglas AL, Hatch TP. Characterization of integration host factor (IHF) binding upstream of the cysteine-rich protein operon (omcAB) promoter of Chlamydia trachomatis LGV serovar L2. Mol Microbiol. 2001;41:451–462. doi: 10.1046/j.1365-2958.2001.02531.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.