Abstract
The increasing incidence of breast cancer brain metastasis in patients with otherwise well-controlled systemic cancer is a key challenge in cancer research. It is necessary to understand the properties of brain-tropic tumor cells to identify patients at risk for brain metastasis. Here we attempt to identify functional phenotypes that might enhance brain metastasis. To obtain an accurate classification of brain metastasis proteins, we mapped organ-specific brain metastasis gene expression signatures onto an experimental protein-protein interaction network based on brain metastatic cells. Thirty-seven proteins were differentially expressed between brain metastases and non-brain metastases. Analysis of metastatic tissues, the use of bioinformatic approaches, and the characterization of protein expression in tumors with or without metastasis identified candidate markers. A multivariate analysis based on stepwise logistic regression revealed GRP94, FN14, and inhibin as the best combination to discriminate between brain and non-brain metastases (ROC AUC = 0.85, 95% CI = 0.73 to 0.96 for the combination of the three proteins). These markers substantially improve the discrimination of brain metastasis compared with ErbB-2 alone (AUC = 0.76, 95% CI = 0.60 to 0.93). Furthermore, GRP94 was a better negative marker (LR = 0.16) than ErbB-2 (LR = 0.42). We conclude that, in breast carcinomas, certain proteins associated with the endoplasmic reticulum stress phenotype are candidate markers of brain metastasis.
Brain metastases occur in 10% to 15% of breast cancer patients with advanced disease.1–3 It can be assumed that up to 30% of metastatic breast cancer patients will undergo brain metastasis during the course of their disease.4,5 This rate is increasing, which can be linked to greater survival in patients receiving chemotherapy and to the fact that it is difficult to cross the blood-brain barrier with current systemic treatments.6–8 The difficulties in managing brain metastasis therapy result in a median survival of 7 months, with brain metastasis being the cause of death or a major contributing factor in 68% of patients.9 Thus, there is a need for both prevention and improved treatment of brain metastasis.2,3
The association of ErbB-2 overexpression with brain metastasis has been attributed to both the inability of a humanized antibody such as trastuzumab to penetrate the blood-brain barrier10 and the longer life span of patients receiving therapy that improves visceral disease control.11 A longer life can lead to the onset of late tumor spread to the central nervous system. The predilection of ErbB-2+ tumor cells for the central nervous system has also been reported.12 Thus, ErbB-2 may affect the development of breast cancer and increase the potential for brain metastasis.
The development of metastasis in the central nervous system depends on the interaction of tumor cells with host defenses and the brain microenvironment, which, surrounded by the blood-brain barrier and lacking lymphatic drainage, differs from lung, liver, lymph node, or bone microenvironments.13 Moreover, microenvironmental factors at the metastatic foci may affect the response of tumors to chemotherapy and may condition drug resistance.14 Unraveling the biological pathways that drive brain metastasis promises insight into how to limit or prevent this deadly aspect of cancer progression.
Our aim was to identify proteins involved in the progression of brain metastasis. Recently, a strategy based on mapping expression profiles with protein interactions has been described.15 The authors show that it is possible to extract relevant biological information about deregulated functions and the relationship between them, and to identify molecules that could be helpful as metastatic markers or therapeutic targets. We compared data obtained from an experimental protein-protein interaction network (PPIN),16 which identifies biological pathways contributing to the organ-specific phenotype of brain metastatic cells, with gene expression profile data17 obtained from published transcriptomic analysis of 23 human breast cancer metastasis samples excised from various anatomical locations, including the brain. To compare the expression and network data sets, we mapped the expression values of each gene onto its corresponding protein in the network and searched for proteins whose activities are highly discriminative of brain metastasis. Protein expression analysis of tissues from metastatic human brain and primary breast tumors provided candidate markers of brain metastasis in both ErbB-2+ and ErbB-2− breast carcinomas.
Materials and Methods
Sample Collection
The Breast Cancer Committee of the Catalan Institute of Oncology and the University Hospital of Bellvitge supplied samples from patients diagnosed between 1988 and 2006. The series of 122 breast cancers included 71 consecutive primary ductal breast carcinomas at initial diagnosis from metastatic patients in treatment at the time of the study, with one or several organs affected (Table 1), and 51 patients with positive lymph nodes at surgery without metastatic progression after a minimum follow-up duration of 5 years. Three patients had brain as the unique metastasis location and 10 patients had dissemination also at bone (n = 7), lung (n = 6), and liver (n = 4). A total of 48 tumors with bone metastasis, 23 with liver metastasis, and 31 with lung metastasis were included.
Table 1.
Distribution and Combinations of the Various Metastases from Breast Cancer Tumors Included in the Tissue Array Analysis
| Metastatic involvement of organs | ||||
|---|---|---|---|---|
| Brain | Bone | Liver | Lung | Total (no.) |
| In each organ (no.) | ||||
| 13 | 48 | 23 | 31 | |
| As a unique organ [no. (%)] | ||||
| 3 (23) | 11 (23) | 4 (17) | 3 (10) | 21 |
| Multimetastatic combinations | ||||
| × | × | 4 | ||
| × | × | 0 | ||
| × | × | 1 | ||
| × | × | × | 2 | |
| × | × | × | 1 | |
| × | × | × | 0 | |
| × | × | 5 | ||
| × | × | 5 | ||
| × | × | × | 4 | |
| × | × | 2 | ||
| × | × | × | × | 2 |
| Other multimetastatic combinations⁎ | ||||
| 24 | ||||
Total number of patients with metastasis: 71.
One or more metastases in combination with other organs (lymph nodes, skin, pleura, esophagus, and vagina).
To optimize each immunohistochemical analysis, the corresponding control tissues for the expression of each protein were also used. To validate protein expression, we included in the analysis six brain metastasis samples matched with the corresponding ductal breast carcinoma to validate protein expression. As a validation set, we used a series of 295 breast tumors for which the transcriptomic data were publicly available.18,19
Identification of Brain Metastasis Candidate Markers
The strategy for identifying novel cancer candidates has been described elsewhere.20 The general procedure of the study, the steps of the analysis, and the levels of protein expression measured are shown as a flow chart in Figure 1A.
Figure 1.
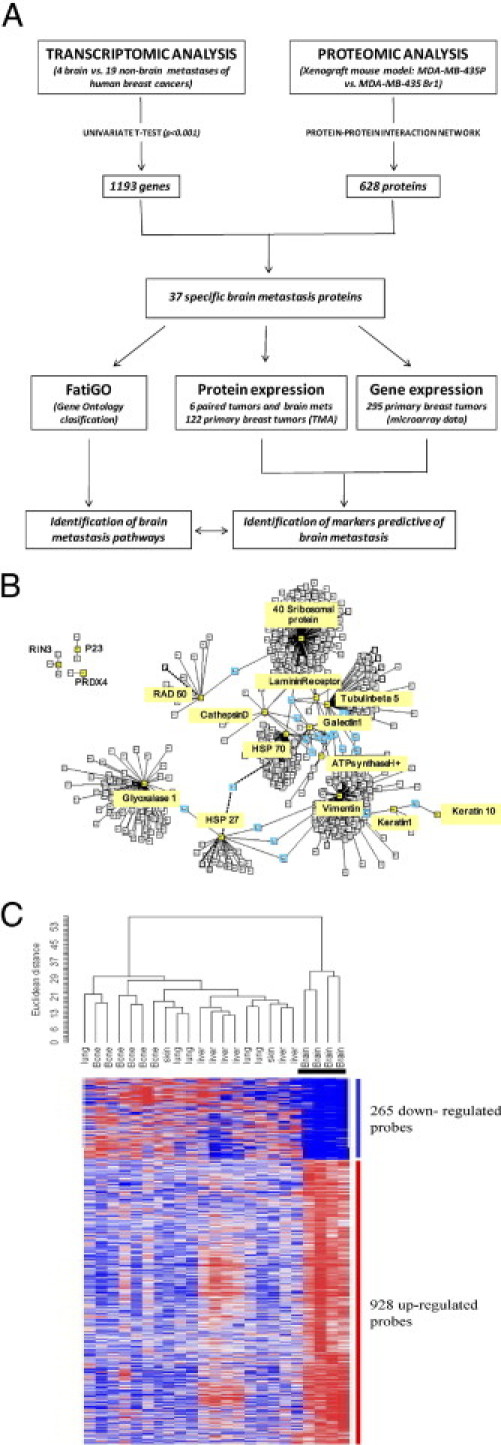
Identification of candidate genes and pathways. A: Study design flow chart. B: The protein-protein interaction network (PPIN) for interacting proteins identified by mass spectrometry.16 Root proteins are in yellow boxes and linker proteins in blue boxes. C: Specific signature of brain metastasis. Hierarchical clustering of a series of 23 breast cancer metastases using 1193 genes from the MetaBre brain-specific signature.17
Experimental Proteomic Analysis and Protein Interaction Network Analysis
To identify brain metastasis-associated proteins, we used a prior proteomic analysis that compared differential expression of proteins between 435-P and 435-Br1 cells.16 Briefly, the proteins differentially expressed by two-dimensional gel electrophoresis (Amersham Ettan DIGE system; GE Healthcare, Little Chalfont, UK) in 435-Br1 cells were identified by peptide mass fingerprinting spectra recorded by a Voyager STR MALDI-TOF system (Applied Biosystems, Foster City, CA) in positive reflector mode with delayed extraction. The spectra were analyzed using the m/z software package (ProteoMetrics, New York, NY). Proteins were identified against a nonredundant database (NCBInr) using online MASCOT search tool (http://www.matrixscience.com/search_form_select.html).
The protein network was based on 17 proteins known to be differentially expressed between 435-P breast cancer cells and the brain metastatic variant 435-Br1. We used PIANA21 to combine data from DIP 2006.01.16, MIPS 2006.01, HPRD 2005.09.13, BIND 2006.01, and the human interactions from two high-throughput experiments. The final PPIN included 628 proteins from 13 known seeds (interacting proteins) identified by MALDI-TOF (Figure 1B).
Human Brain Metastasis Transcriptomic Data
The protein-network approach for identifying markers of brain metastasis was based on results from a previously analyzed microarray hybridization using the GeneChip human genome U133 Plus 2.0 array (Affymetrix, High Wycombe, UK; Santa Clara, CA), which includes more than 47,000 transcripts and variants, according to standard protocols for RNA extraction and probe preparation.17 Briefly, to process and normalize Affymetrix chips, robust multichip averaging RMA algorithms were used.22 All these computations were performed with the Bioconductor package version 2.0.23 Expression profiles were analyzed with BRB Array tools, version 3.3beta3 (Molecular Statistics and Bioinformatics Section, Biometric Research Branch, Division of Cancer Treatment and Diagnosis, NIH-National Cancer Institute, Bethesda, MD).
The univariate t-test was used to identify genes differentially expressed in four brain metastases and metastases in organs other than the brain (5 lung, 6 liver, 2 skin, and 6 osteolytic bone metastases) (Figure 1C). Differences were considered significant when P < 0.001. This stringent threshold was used to limit the number of false positives. These data sets, under the identification number GSE11078, are freely available from the Gene Expression Omnibus (GEO) repository at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov).
Identification of Candidate Genes and Pathways
Gene expression levels obtained from the microarray experiments were mapped onto the network proteins, assuming that a protein might be differentially expressed if the gene encoding for it was found to be differentially expressed at the RNA level. Differential gene expression was found for 556 of the 658 proteins in the initial PPIN.
To classify proteins by function, we used FatiGO software, an online tool for detecting significant associations between gene ontology terms (GO) and groups of genes.24
TMAs and IHC
Tissue microarrays (TMAs) were prepared from three representative areas of the tumor that were carefully selected from H&E-stained sections of 122 donor blocks (S.B. and S.H.). Core cylinders, 2 mm in diameter, were removed from each tumor with a skin-biopsy punch and were deposited into recipient paraffin blocks using a specific arraying device (Beecher Instruments, Sun Prairie, WI), as described elsewhere.25 Sections (3-μm thick) of the resulting microarray block were cut and used for immunohistochemical (IHC) analysis after being transferred to glass slides.
Experimental conditions, positive control tissues, and the characteristics and source of the antibodies used are listed in Table 2. Staining optimization, evaluation parameters, and analyses were established by two pathologists (P.L.F. and S.B.) who were blinded to the clinical status.
Table 2.
Antibodies and Corresponding Conditions for IHC
| Antibody | Clone | Supplier⁎ | Protocol | Cellular expression | Control tissue |
|---|---|---|---|---|---|
| GRP 94 | sc-1794 (C-19) | SCB | 1/2000† | Endoplasmic reticulum | Breast carcinoma |
| TRAF2 | SM7106P (clon 33A1293; 205–222 aa) | Acris | 1/100 O/N† | Cytoplasm | Breast carcinoma |
| FN14 | sc-27143 (C-13) | SCB | 1/3000† | Membrane | Kidney, heart |
| INHA | MCA951ST (R1) | AbD S | 1/50† | Cytoplasm | Testis |
| TOP1 | ab3825 (401–600 aa) | Abcam | 1/100‡ | Nuclei, cytoplasm | Colorectal tumor |
| VAV2 | sc-20803 (H-200) | SCB | 1/1000† | Cytoplasm | Pancreas |
| GFAP | Z0334 | Dako | 1/8000† | Cytoplasm | Brain (astrocytes) |
| TEM 8 | ab21270 | Abcam | 1/2000† | Cytoplasm, membrane | Brain tumor endothelium |
| ARFGAP | SP1402P | Acris | 1/1000† | Cytoplasm | Testis |
| EIF3s8 | ab19359 (N-terminal 1–50 aa) | Abcam | 1/1000 O/N† | Cytoplasm | Kidney |
| BAT 8 | G-6919 | Sigma | 1/250† | Cytoplasm | Lymph node |
O/N, antibody is incubated overnight.
Suppliers: Abcam, Cambridge, UK; AbD S, AbD Serotec, MorphoSys UK, Oxford, UK; Acris, Acris Antibodies, Herford, Germany; SCB, Santa Cruz Biotechnology, Santa Cruz, CA; Sigma, Sigma-Aldrich, St. Louis, MO.
Retrieved in Na-citrate buffer.
Retrieved in Tris/EDTA.
Antigens were retrieved by heating in a pressure cooker for 7 minutes in the appropriate buffer. Primary antibodies were diluted in Dako real antibody diluent buffer (Dako, Glostrup, Denmark; Carpinteria, CA): Tris buffer, pH 7.2, 15 mmol/L NaN3. LSAB+ system-horseradish peroxidase (Dako) was used, including biotinylated anti-rabbit, anti-mouse, and anti-goat immunoglobulins in PBS; streptavidin conjugated to horseradish peroxidase in PBS; and liquid 3–3′ diaminobenzidine in chromogen solution. A polyclonal antibody anti-ErbB2 (A0485; Dako) was used with an ultraView detection kit in an automatic staining system (Ventana Benchmark XT; Roche, Tucson, AZ).
Statistical Analysis
To evaluate the correlation of protein expression with brain metastasis, immunostained samples were graded on a three-category scale (negative, weak positive, and strong positive). The marker was catalogued as overexpressed in strong-positive samples. The association of brain metastasis for each marker was tested using a two-sided Fisher's exact test and summarized by calculating the sensitivity among tumors that developed metastasis, and calculating the specificity among tumors without metastasis, for strong-positive values. Positive and negative likelihood ratios (LR) were also calculated as integrated predictive indexes, as was the area under the ROC curve (AUC). Markers were assessed using a multivariate logistic regression model in a forward stepwise procedure to identify the best combination to discriminate brain metastasis. Because ErbB-2 is a known metastasis risk factor, an analysis including ErbB-2 as the baseline was also performed, as well as a stratified analysis of each candidate marker within ErbB-2+ and ErbB-2− tumors. In all of the analyses, associations were considered significant when P < 0.05. No multiple testing correction was done in this analysis, because the search for the best combination of markers started from a very small set of candidates.
Results
Identification of Specific Brain Metastasis Proteins
We mapped human brain metastasis expression profiles with a PPIN to maximize accuracy in the classification of brain metastasis proteins.
The signature of brain genes was catalogued as the organ-specific metastasis signature (BOSMS) with a hierarchical clustering that clearly distinguishes among the different metastases.17 The BOSMS contained 1193 genes (MetaBre) after the one-versus-all (ONA) class comparisons identified genes differentially expressed in the 4 brain metastases versus the 19 metastases to other organs.
Integrating genomic and proteomic analyses, we matched the BOSMS with the PPIN,16 and obtained 37 organ-specific proteins (Table 3): seven underexpressed and 30 overexpressed. The FatiGO classifier based on GO terms grouped proteins as follows: 13 nucleic acid metabolism proteins (48%), 10 translation proteins (37%), seven cell death proteins (26%), and six modification and folding proteins (22%), as well as a miscellany of metabolic, transport and signaling proteins, some of them with multiple functions (Figure 2). The cellular components of the analysis were as follows: 74% intracellular organelles, 51% cytoplasm, 22% ribonucleoprotein complex proteins, and 15% proteins intrinsic to membrane.
Table 3.
Identities of 37 Brain Metastasis-Specific Proteins Matched in the Proteomic and Transcriptomic Analyses of Human Brain Metastasis
| Gene symbol | UniProtKB ID | Protein name | Function | P value | Network position (linked to) |
|---|---|---|---|---|---|
| Up-Regulated | |||||
| RPL13 | Q3KQT8 | 60S ribosomal protein L13 (breast basic conserved protein 1) | Protein biosynthesis | 0.0008 | 40S ribosomal protein s12 |
| RPS10 | P46783 | 40S ribosomal protein S10 | Protein biosynthesis | 0.0005 | |
| RPL5 | P46777 | 60S ribosomal protein L5 | Protein biosynthesis | 0.0002 | |
| EIF5 | P55010 | Eukaryotic translation initiation factor 5 | Protein biosynthesis | 0.0007 | |
| EIF3C (prev. EIF3S8) | Q99613 | Eukaryotic translation initiation factor 3, subunit 8 | Protein biosynthesis | 0.00002 | |
| EEF1D | P29692 | Eukaryotic translation elongation factor 1-delta, isoform 2 | Signal transduction | 0.0006 | |
| EEF1D | Q96I38⁎ | Eukaryotic translation elongation factor 1-delta, isoform 1 | Signal transduction | 0.0006 | |
| PARF (syn. C9orf86) | Q8IWK1† | Putative GTP-binding protein Parf [alt.: C9orf86 protein (fragment)] | Signal transduction | 0.0001 | |
| INHA | P05111 | Inhibin alpha chain | Signal transduction | <0.000001 | |
| CLN3 | Q13286 | Protein CLN3 | Protein folding | 0.0008 | |
| FAM3A | P98173 | Protein FAM3A precursor (2–19 protein) | No function | 0.0009 | |
| PARF (syn. C9orf86) | Q9BU21† | Putative GTP-binding protein Parf (alt.: C9orf86 protein) | No function | 0.0001 | |
| TUBB2A | P05218 | Tubulin beta-2 chain | Structural | 0.0004 | Root protein |
| TBCD | Q96E74 | Tubulin-specific chaperone D | Structural | 0.00005 | Tubulin beta-2 chain |
| MCM4 | P33991 | DNA replication licensing factor MCM4 | DNA binding | 0.0004 | |
| ARFGAP1 | Q8N6T3 | ADP-ribosylation factor GTPase-activating protein 1 | Transport | 0.0003 | |
| EHMT2 (syn. BAT8) | Q96KQ7 | Histone-lysine N-methyltransferase EHMT2 (alt.: HLA-B-associated transcript 8) | Methylation | 0.0008 | |
| RNF25 | Q96BH1 | Ring finger protein 25 | Ubiquitinization | 0.0002 | |
| HMG20B | Q9P0W2 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1-related | DNA binding | 0.00001 | Vimentin |
| SIRT6 | Q8N6T7 | Sirtuin 6 | Amino acid metabolism | 0.000004 | |
| GFAP | P14136 | Glial fibrillary acidic protein | Structural | 0.0001 | |
| TOP1 | Q9UJN0‡ | DNA topoisomerase I | DNA binding | 0.00001 | |
| CRAMP1L (syn. C16orf34, KIAA1426) | Q96RY5 | Protein cramped-like (alt.: uncharacterized protein KIAA1426) | DNA binding | 0.0003 | Glyoxalase I |
| C9orf84 | Q5VXU9 | Uncharacterized protein C9orf84 | No function | 0.0009 | |
| C16orf34 | Q9H910 | Hematological and neurological expressed 1-like protein | No function | 0.0003 | |
| MSH6 | P52701 | DNA mismatch repair protein MSH6 | DNA repair | 0.00002 | RAD50 |
| TCERG1 | O14776 | Transcription elongation regulator 1 | DNA binding | 0.00004 | HSP 70 |
| HSP90B1 (prev. TRA1; syn. GRP94) | P14625 | 94kDa glucose regulated protein (alt.: GRP94) | Protein folding | 0.0009 | LINKER (laminin receptor 67 kDa and HSP 27) |
| TRAF2 | Q12933 | TNF-receptor associated factor 2 | Signal transduction | 0.00007 | PRDX4 |
| TNFRSF12A (syn. FN14) | Q9NP84 | TNF-receptor superfamily member 12A (alt.: fibroblast growth factor-inducible immediate-early response protein 14; alt.: FN14) | Receptor | 0.0001 | TRAF2 |
| Down-Regulated | |||||
| RPS12 | P25398 | 40S ribosomal protein S12 | Protein Biosynthesis | 0.0006 | Root protein |
| RPS23 | P62266 | 40S ribosomal protein S23 | Protein biosynthesis | 0.000001 | 40S ribosomal protein s12 |
| DNM3 | Q6P2G1 | Dynamin 3 | Protein biosynthesis | 0.0008 | |
| SERPINB9 | P50453 | Serpin B9 | Signal transduction | 0.0007 | Tubulin beta-2 chain |
| CREB1 | Q53X93 | cAMP responsive element binding protein 1, isoform A | Transcription | 0.000005 | Vimentin |
| CREB1 | P16220 | cAMP responsive element binding protein 1, isoform B | Transcription | 0.00005 | |
| AOC3 | Q16853 | Vascular adhesion protein-1 | Cell adhesion | 0.0004 | Glyoxalase I |
alt., alternative protein name; prev., previous approved gene symbol; syn., gene symbol synonym appearing in the literature.
Q96I38 is a secondary accession number. The primary (citable) accession number is number is P29692.
Figure 2.
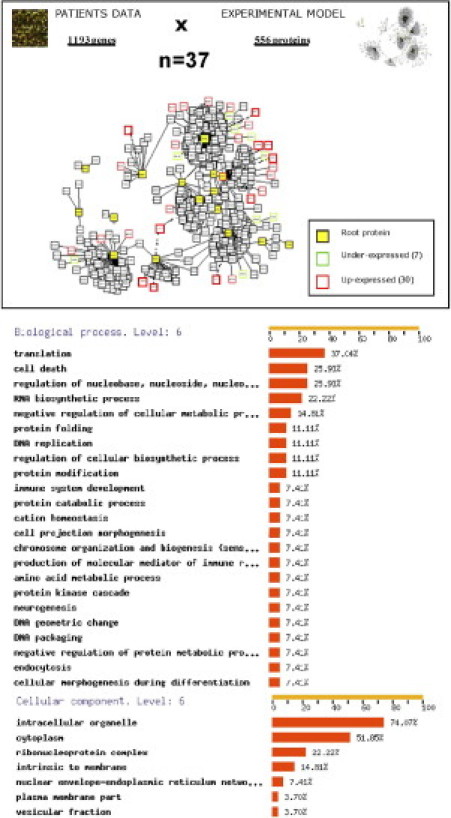
The PPIN analysis was performed for 556 proteins matched with 1193 differentially expressed brain metastasis genes (transcriptomic comparison of brain metastases versus other metastases), yielding 37 pairs corresponding to 7 underexpressed and 30 overexpressed organ-specific proteins. FatiGO, an online tool for finding significant associations of gene ontology-terms with groups of genes,24 shows the preponderant functions of significant proteins in clusters of coexpression. The classification by function was performed using GO level 6.
We graphically represented the brain organ-specific metastasis phenotype (Figure 3) in the PPIN-based functional approach from protein interaction databases, providing a novel hypothesis for pathways involved in brain metastasis progression. Indeed, five functions from the PPIN were predominant: i) DNA binding and repair; ii) protein folding and chaperones, which engage one more DNA binding protein (O14776); iii) structural cytoskeleton, which engages four new DNA binding proteins (Q9P0W2, P33991, Q53X93, and Q9UJN0), two new signal transcription factors (P50453 and P16220), one ubiquitinization protein (Q96BH1), one amino acid metabolism protein (Q8N6T7), and one protein involved in methylation (Q96KQ7); iv) protein biosynthesis, which engages four new signal transduction factors (P29692, Q96I38, Q8IWK1, and P05111); and v) vesicle transport, which engages one protein (Q8N6T3).
Figure 3.
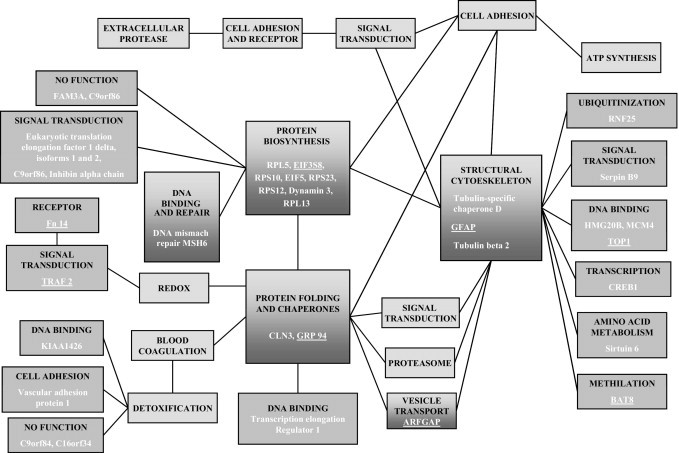
Functional classification of the PPIN. Proteins in functional clusters are grouped within a single box containing root and linker proteins. Functions are indicated in black type; the 37 brain metastasis proteins are indicated in white type. Boxes in light gray indicate the previous network16 of brain metastatic cells; boxes in dark gray indicate new functions added from the transcriptomic analysis; boxes shaded from light to dark represent redundant functions identified in proteomics and transcriptomic analysis. Proteins that were validated by IHC are underlined.
Additional IHC experiments were performed on six matched breast cancer tumor-brain metastasis samples from patients, to corroborate in human brain metastasis the expression of 11 proteins representative of the functions involved. These proteins were chosen on the basis of commercial availability of antibodies (Table 2 and Figure 4). The IHC analysis validated the expression of GRP94, TRAF2, FN14, TOP1, VAV2, GFAP, TEM8, BAT8, and ARFGAP proteins in brain metastasis. In addition, some of these proteins were also expressed in the corresponding primary breast carcinomas, suggesting their functional involvement from the primary tumor to the brain metastasis.
Figure 4.
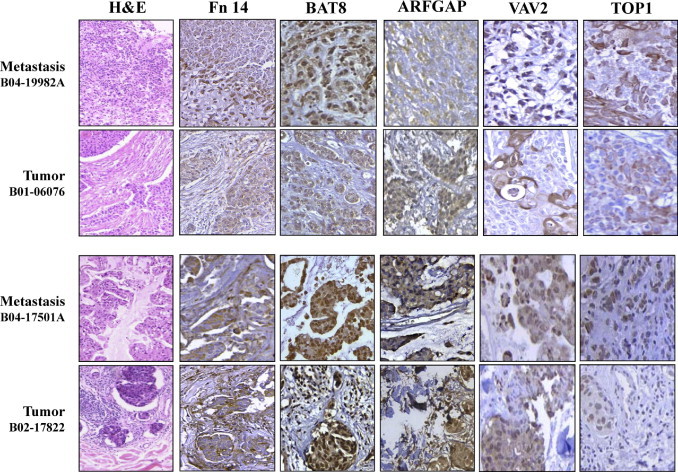
Validation at the protein expression level (brown) in matched tumor-brain metastasis samples by means of IHC analysis to identify representative functional-type proteins in representative paraffin-embedded tumor-brain metastasis pairs. H&E staining of each tissue is shown as viewed by light microscopy. Original magnification: ×10 (H&E stain); ×20 (all others).
We searched for references to brain metastasis signatures in published genomic data from experimental and clinical breast cancer and metastasis analysis. From our list of genes, only seven appeared in previous lists of gene expression profiling predicting clinical outcomes of breast cancer26–35 (Table 4): EEF1D, MCM4, RPL5, RPS12, and CLN326 and also FAM3A and TBCD.27 GFAP, encoding a ubiquitous protein in the central nervous system, also appeared in a list of genes differentially expressed between brain and bone breast cancer metastasis.35
Table 4.
In silico Validation of the Endoplasmic Reticulum Stress Phenotype, Taking Into Account Previous Experimental And Clinical Reports
| Reference | Array platform | Description of sample | Gene signature | Match to present study |
|---|---|---|---|---|
| 26 | Agilent 24479 60-mer oligos | 97 Samples from LN− patients | 231 Prognosis reporters (risk of distant metastasis) | 0 |
| 430 Brca1 reporters | 3 (EEF1D, MCM4, RPL5) | |||
| 2460 ER− reporters | 3 (CLN3, MCM4, RPS12) | |||
| 30 | Rosetta inkjet (24479 genes; breast adenocarcinoma) oligonucleotide microarray | 279 Primary tumors of diverse types (lung, breast, prostate) | 128 Genes able to distinguish patients with good versus poor prognosis | 0 |
| 27 | Multiple gene expression signatures “metagenes” | 86 LN+ breast cancer patients | 143 Predictors of lymph node metastasis | 0 |
| 165 Predictors of breast cancer recurrence | 2 (FAM3A, TBCD) | |||
| 28 | Affymetrix U133A 25-mer oligos | LN− and LN+ patients with invasive breast cancer | 76-Gene signature to distinguish LN− primary breast cancer to develop distant metastasis within 5 years | 0 |
| 33 | Affymetrix U133A | 82 Breast cancer patients (primary tumors) | 95 Genes predictors of lung metastasis | 0 |
| 31 | Agilent 24479 60-mer oligos | 161 Patients in stage I and II breast cancer with age <53 years | 70-Gene predictor of local recurrence | 0 |
| 29 | Agilent 22575 60-mer oligos | 135 Tumor samples (no criteria for selection) | 70-Gene prognostic signature (risk of metastasis) | 0 |
| 32 | Operon 70-mer two-color 21239 probes | 35 Patients: primary tumor and lymph node metastasis paired samples | 79 Differentially expressed genes between primary samples and metastasis samples | 0 |
| 35 | Affymetrix U133A | 8 Bone metastases, 18 brain metastases and 3 primary tumors | 51 Brain metastasis specific genes (versus bone metastasis) | 1 (GFAP) |
| 19 | Affymetrix U133A | CN34-BrM2 and MDA231-BrM2 brain metastatic cell lines. | 17 Genes whose expression was correlated with brain relapse | 0 |
| 368 Breast cancer primary tumors | 26 Genes whose expression was increased in brain metastatic cell lines but not in bone or lung metastatic cell lines | 0 |
LN, lymph node.
These findings indicate that cells metastasizing in brain were enriched in cell structure, chaperones, stress and redox regulation, and intracellular transport proteins. The organ-specific character of this functional signature was also found in the transcriptomic data from breast cancer brain metastasis (Figure 5 and Table 5). From these, the most differentially expressed in brain metastasis, compared with metastases in other organs, were GRP94 (P = 0.002), FN14 (P = 0.002), ARFGAP1 (P = 0.003), TRAF2 (P = 0.003), and PDGFRA (0.002) genes. In contrast, other functions had no relevant expression in brain; for example, amino acid metabolism genes were overexpressed only in liver.
Figure 5.
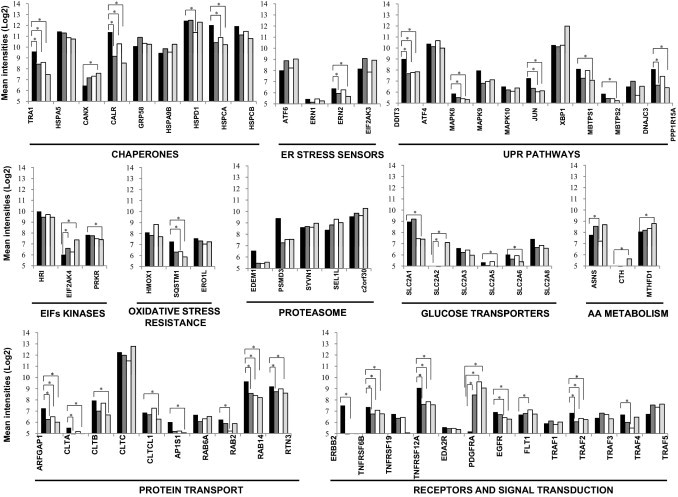
Differentially expressed genes in brain metastasis (black), compared with metastases in other organs: lung (dark gray), bone (white), and liver (light gray), based on a Mann-Whitney test calculated for each gene using the normalized log intensities (see further in Table 5). *P < 0.05, statistically significant different expression between bracketed organs.
Table 5.
Differentially Expressed Genes in Brain Metastasis versus Non-Brain Metastases
| UniProtKB ID | Protein name | Gene symbol | Metastasis⁎ |
P value† | |
|---|---|---|---|---|---|
| Brain | Non-brain | ||||
| Chaperones | |||||
| P14625 | 94kDa glucose-regulated protein (alt.: GRP94) | HSP90B1 (prev. TRA1) | 9.59 | 8.10 | 0.002 |
| P11021 | 78kDa glucose-regulated protein | HSPA5 | 11.44 | 10.95 | 0.168 |
| P27824 | Calnexin | CANX | 6.45 | 7.35 | 0.035 |
| P27797 | Calreticulin | CALR | 11.38 | 9.22 | 0.006 |
| P30101 | 58kDa glucose-regulated protein (alt.: p58; ERp57; ERp60) | PDIA3 (prev. GRP58) | 7.82 | 4.05 | 0.003 |
| P38646 | 75kDa glucose-regulated protein | HSPA9 (prev. HSPA9B; syn. GRP75) | 9.46 | 9.91 | 0.465 |
| P10809 | 60kDa heat shock protein, mitochondrial | HSPD1 | 10.47 | 9.71 | 0.144 |
| P07900 | Heat shock protein 90kDa alpha (cytosolic), class A member 1 | HSP90AA1 (prev. HSPCA) | 12.03 | 10.52 | 0.006 |
| P08238 | Heat shock protein 90kDa alpha (cytosolic), class B member 1 | HSP90AB1 (prev. HSPCB) | 11.93 | 11.06 | 0.168 |
| Endoplasmic reticulum stress sensors | |||||
| P18850 | Activating transcription factor 6 | ATF6 | 8.01 | 8.70 | 0.256 |
| O75460 | Serine/threonine-protein kinase/endoribonuclease IRE1 (alt.: inositol-requiring protein 1; IRE1a) | ERN1 | 5.44 | 5.27 | 0.441 |
| Q76MJ5 | Serine/threonine-protein kinase/endoribonuclease IRE2 (alt.: inositol-requiring protein 2; IRE1b) | ERN2 | 6.39 | 5.93 | 0.038 |
| Q9NZJ5 | Eukaryotic translation initiation factor 2-alpha kinase 3 (alt.: PRKR-like endoplasmic reticulum kinase) | EIF2AK3 (syn. PERK) | 5.02 | 4.85 | 0.155 |
| UPR pathways | |||||
| P35638 | DNA damage-inducible transcript 3 protein | DDIT3 (syn. CHOP, GADD153) | 9.01 | 7.69 | 0.006 |
| P18848 | cAMP-dependent transcription factor ATF-4 | ATF4 | 10.38 | 10.27 | 0.626 |
| P45983 | Mitogen-activated protein kinase 8 (alt.: c-Jun N-terminal kinase 1) | MAPK8 | 5.86 | 5.38 | 0.009 |
| P45984 | Mitogen-activated protein kinase 9 (alt.: c-Jun N-terminal kinase 2) | MAPK9 | 7.96 | 6.95 | 0.144 |
| P53779 | Mitogen-activated protein kinase 10 (alt.: c-Jun N-terminal kinase 3) | MAPK10 | 6.50 | 6.26 | 0.441 |
| P05412 | Transcription factor AP-1 (alt.: proto-oncogene c-Jun) | JUN | 7.27 | 6.08 | 0.006 |
| P17861 | X-box-binding protein 1 | XBP1 | 10.27 | 10.85 | 0.417 |
| Q14703 | Membrane-bound transcription factor site-1 protease (alt.: endopeptidase S1P) | MBTPS1 | 8.10 | 7.35 | 0.062 |
| O43462 | Membrane-bound transcription factor site-2 protease (alt.: endopeptidase S2P) | MBTPS2 | 5.85 | 5.31 | 0.155 |
| Q13217 | DnaJ homolog subfamily C member 3 (alt.: Protein kinase inhibitor p58) | DNAJC3 | 4.87 | 4.43 | 0.006 |
| O75807 | Protein phosphatase 1 regulatory subunit 15A (alt.: growth arrest and DNA damage-inducible protein GADD34) | PPP1R15A (syn. GADD34) | 8.08 | 6.74 | 0.009 |
| EIF kinases | |||||
| Q9BQI3 | Heme-regulated inhibitor | HRI | 9.98 | 9.51 | 0.33 |
| Q9P2K8 | Eukaryotic translation initiation factor 2-alpha kinase 4 (alt.: GCN2-like protein) | EIF2AK4 | 6.02 | 6.73 | 0.052 |
| P19525 | Interferon-induced, double-stranded RNA-activated protein kinase (alt.: protein kinase RNA-activated) | EIF2A2 (syn. PKR, PRKR) | 7.84 | 7.52 | 0.061 |
| Oxidative stress resistance | |||||
| P09601 | Heme oxygenase 1 | HMOX1 | 8.10 | 8.02 | 0.516 |
| Q13501 | Sequestosome-1 | SQSTM1 | 7.27 | 6.15 | 0.004 |
| Q96HE7 | ERO1-like protein alpha | ERO1L | 7.56 | 7.19 | 0.18 |
| Proteasome | |||||
| Q92611 | ER degradation-enhancing alpha-mannosidase-like 1 | EDEM1 | 6.53 | 5.41 | 0.088 |
| O43242 | 26S proteasome regulatory subunit S3 | PSMD3 | 9.38 | 7.45 | 0.043 |
| Q86TM6 | E3 ubiquitin-protein ligase synoviolin | SYVN1 | 8.59 | 8.77 | 0.441 |
| Q9UBV2 | Protein sel-1 homolog 1 (alt.: suppressor of lin-12-like protein 1) | SEL1L | 8.36 | 9.05 | 0.871 |
| Q96DZ1 | Endoplasmic reticulum lectin 1 (alt.: XTP3-transactivated gene B protein; XTP-3) | ERLEC1 (prev. C2orf30) | 9.54 | 9.93 | 0.57 |
| Glucose transporters | |||||
| P11166 | Solute carrier family 2, facilitated glucose transporter member 1 (alt.: GLUT-1) | SLC2A1 | 8.96 | 7.97 | 0.081 |
| P11168 | Solute carrier family 2, facilitated glucose transporter member 2 (alt.: GLUT-2) (liver) | SLC2A2 | 3.43 | 4.64 | 0.123 |
| P11169 | Solute carrier family 2, facilitated glucose transporter member 3 (alt.: GLUT-3) (brain) | SLC2A3 | 6.60 | 6.13 | 0.871 |
| P22732 | Solute carrier family 2, facilitated glucose transporter member 5 (alt.: GLUT-5) | SLC2A5 | 5.33 | 5.07 | 0.074 |
| Q9UGQ3 | Solute carrier family 2, facilitated glucose transporter member 6 (alt.: GLUT-6) | SLC2A6 | 6.03 | 5.60 | 0.074 |
| Q9NY64 | Solute carrier family 2, facilitated glucose transporter member 8 (alt.: GLUT-9) | SLC2A8 | 7.43 | 6.72 | 0.035 |
| Amino acid metabolism | |||||
| P48067 | Sodium- and chloride-dependent glycine transporter 1 (alt.: GlyT-1) | SLC6A9 | 5.06 | 4.78 | 0.035 |
| P08243 | Asparagine synthetase | ASNS | 7.78 | 8.15 | 0.49 |
| P32929 | Cystathionine gamma-lyase | CTH | 4.06 | 4.75 | 0.144 |
| P11586 | Methylenetetrahydrofolate dehydrogenase | MTHFD1 | 8.07 | 8.48 | 0.035 |
| Protein transport | |||||
| Q8N6T3 | ADP-ribosylation factor GTPase-activating protein 1 | ARFGAP1 | 7.25 | 6.21 | 0.003 |
| P09496 | Clathrin light chain A | CLTA | 5.51 | 4.80 | 0.009 |
| P09497 | Clathrin light chain B | CLTB | 4.66 | 4.11 | 0.015 |
| Q00610 | Clathrin heavy chain 1 | CLTC | 12.25 | 12.07 | 0.685 |
| P53675 | Clathrin heavy chain 2 | CLTCL1 | 6.86 | 6.72 | 0.33 |
| P61966 | AP-1 complex subunit sigma-1A (alt.: sigma-adaptin 1A) | AP1S1 (prev. CLAPS1) | 6.02 | 5.12 | 0.009 |
| P20340 | Ras-related protein Rab-6A | RAB6A | 6.66 | 6.30 | 0.074 |
| P61019 | Ras-related protein Rab-2A | RAB2A (prev. RAB2) | 9.67 | 8.73 | 0.043 |
| P61106 | Ras-related protein Rab-14 | RAB14 | 9.65 | 8.38 | 0.002 |
| O95197 | Reticulon-3 | RTN3 | 9.19 | 8.73 | 0.019 |
| Receptors and signal transductors | |||||
| P04626 | Receptor tyrosine-protein kinase erbB-2 | ERBB2 | 7.50 | 4.17 | 0.009 |
| O95407 | Tumor necrosis factor receptor superfamily member 6B | TNFRSF6B | 7.37 | 6.85 | 0.003 |
| Q9NS68 | Tumor necrosis factor receptor superfamily member 19 | TNFRSF19 | 5.14 | 4.53 | 0.006 |
| Q9NP84 | Tumor necrosis factor receptor superfamily member 12A (alt.: FN14) | TNFRSF12A (syn. FN14) | 9.07 | 7.68 | 0.002 |
| Q9HAV5 | Ectodysplasin-A2 receptor (alt.: tumor necrosis factor receptor superfamily member 27) | EDA2R (syn. TNFRSF27) | 5.57 | 5.41 | 0.516 |
| P16234 | Alpha-type platelet-derived growth factor receptor | PDGFRA | 5.19 | 9.09 | 0.002 |
| P00533 | Epidermal growth factor receptor | EGFR | 6.92 | 6.42 | 0.012 |
| P17948 | Vascular endothelial growth factor receptor 1 | FLT1 | 7.09 | 7.47 | 0.088 |
| Q13077 | TNF receptor-associated factor 1 | TRAF1 | 5.91 | 5.99 | 0.655 |
| Q12933 | TNF receptor-associated factor 2 | TRAF2 | 6.85 | 6.20 | 0.003 |
| Q13114 | TNF receptor-associated factor 3 | TRAF3 | 6.40 | 6.63 | 0.417 |
| Q9BUZ4 | TNF receptor-associated factor 4 | TRAF4 | 6.60 | 5.39 | 0.035 |
| O00463 | TNF receptor-associated factor 5 | TRAF5 | 6.75 | 7.55 | 0.330 |
alt., alternative name (proteins); prev., previously approved symbol (genes).
Based on a Mann-Whitney U-test calculated for each gene using normalized log-intensities.
Statistically significant differences are highlighted in bold face type.
After mapping transcriptomic into proteomic analyses, we concluded that molecules involved in protein folding and chaperones might connect different functions and presumably act by rescuing cells from endoplasmic reticulum stress responses.
Expression of Endoplasmic Reticulum Stress Phenotype in Breast Cancer Primary Tumors Is Associated with Brain Metastasis Progression
Because proteins were also expressed in primary tumors, to estimate the probability of specific brain metastasis outcomes we further analyzed the proteins in a series of primary breast carcinomas using TMA technology. We considered a marker to be positive when strong expression was detected, to avoid false positives, and taking into account the known expression in a control tissue (Figure 6).
Figure 6.
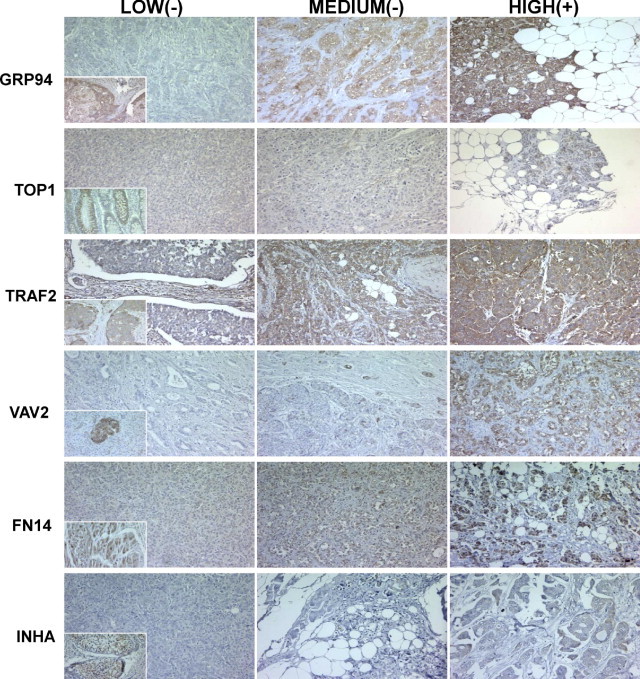
ERS response phenotype in breast cancer at first diagnosis. Representative tabulation of protein expression in breast cancer tissues. Tissues are shown as viewed by light microscopy. Low and medium intensities of staining were considered negative for semiquantitative purposes, and only tumors with high intensity staining were considered positive. Insets: Standard positive control tissue sample used in each determination. Original magnification, ×10.
Statistical analysis of the data showed significant associations between brain metastasis progression and high expression of GRP94 (P < 0.0001), TRAF2 (P < 0.001), and FN14 (P < 0.0001). As expected, ErbB-2 expression was associated with brain metastasis with a high significance (P < 0.0001): 8/13 (62%) breast cancers that progressed to brain metastasis were positive, versus 12% and 5% of breast carcinomas that relapsed in other locations or without metastasis (7/57 and 2/42, respectively). ErbB-2 expression was also associated with the absence of hormone receptors: ER, 55% versus 30% (6/11 and 29/98, respectively, P = 0.016); PR, 73% versus 39% (8/11 versus 37/95, respectively, P = 0.009). A slight association with histological grade (HG) was also observed: HG III 58% versus 47% (7/12 versus 45/96, P = 0.105). In addition, we did not find correlation between lung, bone, or liver metastasis and high expression of these proteins.
We calculated the positive and negative likelihood ratios (LR) to assess the predictive accuracy of each molecule as a brain metastasis marker, considering the sensitivity and the specificity of each (Table 6). The highest predictive value for the presence of metastasis was ErbB-2 expression (positive LR = 6.7, P < 0.0001), followed by FN14 (positive LR = 3.01, P = 0.001), GRP94 (positive LR = 1.89 P = 0.003), and TRAF2 (positive LR = 1.67, P = 0.055). Furthermore, GRP94 was the best negative predictive marker (negative LR = 0.16), followed by TRAF2 (negative LR = 0.35), FN14 (negative LR = 0.40), and ErbB-2 (negative LR = 0.42). Thus, the absence of the endoplasmic reticulum stress (ERS) response phenotype in tumors predicted the absence of brain metastasis.
Table 6.
Risk of Brain Metastasis Associated with Each Marker in Breast Cancer
| Brain metastasis marker | UniProtKB ID | Sensitivity⁎ | Specificity⁎ | LR |
P value† | |
|---|---|---|---|---|---|---|
| Pos | Neg | |||||
| ErbB-2 | P04626 | 8/13 (61.5) | 90/99 (90.9) | 6.70 | 0.42 | <0.0001 |
| GRP94 | P14625 | 12/13 (92.0) | 55/107 (51.4) | 1.89 | 0.16 | 0.003 |
| FN14 | Q9NP84 | 9/13 (69.2) | 80/104 (77.0) | 3.01 | 0.40 | 0.001 |
| TRAF2 | Q12933 | 9/11 (81.8) | 45/88 (51.1) | 1.67 | 0.35 | 0.055 |
| VAV2 | P52735 | 2/13 (15,4) | 95/107 (88.8) | 1.38 | 0.95 | 0.65 |
| TOP1 | Q9UJN0‡ | 4/13 (30.8) | 91/105 (86.6) | 2.30 | 0.80 | 0.11 |
| Inhibin | P05111) | 0/13 (0) | 97/107 (90.7) | 0 | 1.10 | 0.60 |
LR, likelihood ratio; Neg, negative LR; Pos, positive LR.
Variation in denominators derives from missing values in the IHC (eg, tissue lost, unviable staining, or background).
Fisher's exact test, 2-sided.
Secondary accession number. The primary (citable) accession number is number is P11387.
For a validation set, we used a series of 295 breast tumors for which the transcriptomic data were publicly available.18,19 As expected, the highest predictive value was ErbB-2 expression (positive LR = 8.27, P < 0.00001). Moreover, TRAF2 (positive LR = 2.36, P = 0.026), GRP94 (positive LR = 1.72 P = 0.028), and FN14 (positive LR = 1.78, P = 0.044) were associated with brain metastasis.
A multivariate analysis based on stepwise logistic regression retained GRP94, FN14, and inhibin as the best combination to discriminate brain metastasis. The AUC value for this combination was 0.85 (95% CI = 0.73 to 0.96), substantially better than that for ErbB-2, which was the variable more strongly associated with brain metastasis (AUC = 0.76, 95% CI = 0.58 to 0.93). The model that combined ErbB-2, GRP94, FN14, and inhibin expression further increased the discrimination of metastatic disease in brain (AUC = 0.91, 95% CI = 0.77 to 1.00). The ROC curves for the three models are shown in Figure 7.
Figure 7.
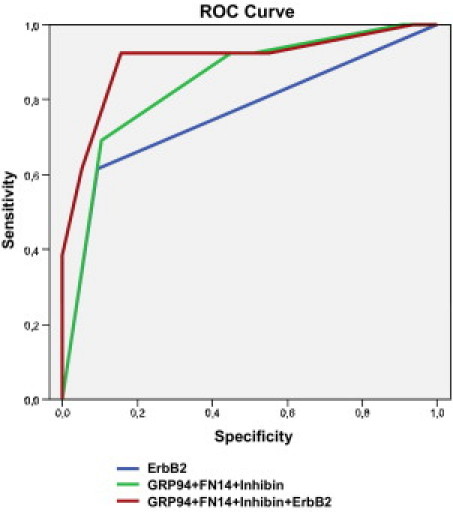
The area under the ROC curve (AUC) obtained with the integrated predictive indexes. Markers were assessed in a multivariate logistic regression model using a forward stepwise procedure to identify the best combination to predict brain metastasis. For ErbB-2 alone, AUC = 0.76; for GRP94, FN14, and inhibin in combination, AUC = 0.85; and for ErbB-2, GRP94, FN14, and inhibin in combination, AUC = 0.91.
We also performed a stratified analysis to check the relationship between ErbB-2 positivity and ERS response phenotype in binary combinations (Table 7). In ErbB-2− tumors, FN14 had a high negative likelihood ratio to predict the absence of brain metastasis progression (LR = 0.26, sensitivity = 0.8, P = 0.015).
Table 7.
Risk of Brain Metastasis Associated with Each Marker in Breast Cancer with Regard to ErbB-2 Expression
| Brain metastasis marker⁎ | ErbB-2+ |
ErbB-2− |
||||
|---|---|---|---|---|---|---|
| Sensitivity† | Specificity† | P value‡ | Sensitivity† | Specificity† | P value‡ | |
| Novel markers | ||||||
| GRP94 | 8/8 (100) | 2/9 (22.2) | 0.47 | 4/5 (80) | 48/89 (53.9) | 0.19 |
| FN14 | 5/8 (62.5) | 6/9 (66.7) | 0.35 | 4/5 (80) | 68/88 (77.3) | 0.015 |
| TRAF2 | 6/7 (85.7) | 5/9 (55.6) | 0.15 | 3/4 (75) | 36/71 (50.7) | 0.62 |
| Traditional markers | ||||||
| ER | 3/7 (42.9) | 6/8 (75) | 0.61 | 2/4 (50) | 21/83 (25.3) | 0.28 |
| PR | 2/7 (28.6) | 6/7 (85.7) | 1.0 | 1/4 (25) | 29/81 (35.8) | 0.15 |
| Histologic grade III | 6/8 (75.0) | 3/9 (33.3) | 1.0 | 1/4 (25) | 42/45 (93.3) | 0.62 |
For novel markers, UniProtKB identifiers are as follows: GRP94, P14625; FN14, Q9NP84; TRAF2, Q12933. For traditional markers: estrogen receptor ER, P03372; progesterone receptor PR, P06401.
Variation in denominators derives from missing values in the IHC (eg, tissue lost, unviable staining, or background).
Fisher's test.
Discussion
Fewer than 10% of breast cancer patients have detectable distant metastasis at diagnosis.36 Thus, it is necessary to understand the properties of brain-tropic tumor cells to identify patients with risk of brain metastasis and to effectively prevent it. Because we assume that metastasis colonization could already be underway at the time of diagnosis, the ERS response phenotype might be a predictive tool to help decide on treatment under the risk of brain metastasis progression.
The phenotype includes the overexpression of GRP94, FN14, and TRAF2, which are well correlated with brain metastasis in breast cancer patients. Our search for a multivariate panel of markers to predict brain metastasis revealed that the combination of GRP94, FN14, and inhibin together has a better discriminate accuracy than ErbB-2 alone, and that the best accuracy is obtained combining all four markers. Although all variables in these models were significantly associated with brain metastasis in the multivariate models, the increase in predictive accuracy measured by the difference in AUC was not (because of the small sample size in the present study). Indeed, TRAF2, which was associated with brain metastasis, had many missing values and could not be included in the multivariate analysis. The ERS response phenotype is indicative of a new tool to discriminate the risk of brain metastasis in both ErbB-2+ and ErbB-2− breast cancers. Moreover, the absence of the ERS response phenotype in tumors might predict the absence of metastasis.
These biomarkers can help in selection of treatment strategies, furthering the current ambitious aim of identifying treatment strategies that will cure patients with ErbB-2+ disease while ensuring minimal toxicity for each individual patient.37 Hicks et al38 reported that EGFR expression, like ErbB-2, predicted the development of brain metastasis. The incidence of brain metastasis in patients with breast cancer overexpressing ErbB-2 who are being treated with trastuzumab is double that in other breast cancer patients; one-third will develop central nervous system metastasis, and this often occurs when they are responding to therapy at other sites or have a stable disease.39 One of the clinical questions is whether this receptor remains a viable target after disease progression,40 and whether trastuzumab treatment can prevent brain metastasis or whether it encourages the development of metastatic cells that have crossed the blood-brain barrier.
GRP94 overexpression in brain metastasis might modulate ERS responses through activation of PERK, ATF6, and IRE1.41 Downstream from GRP94 activation, transcription of chaperones and protein degradation might increase in brain metastatic cells. (We are currently exploring these pathways in our laboratory.) Because therapy decisions should depend on the tumor phenotype, the known close correlation between brain metastasis potential and ERS response phenotype in primary tumors suggests that HSP90 and proteasome inhibitors might be alternatives for treatment of breast cancer patients with a high risk of brain metastasis.42 It has been reported that inhibition of HSP90, which helps expression and folding of its client proteins, such as ErbB-2, could simultaneously inhibit the expression of viable receptors.40 Furthermore, the expression of GRP94 has been associated with poor prognosis in gastric carcinomas,43 and with chemotherapy resistance of lung cancer cells and ovarian carcinoma cells.44
The switch from dormancy to growth of cancer cells in the brain may be dependent on stress response mechanisms, subsequent coordination of detoxification and redox pathways,16 and cytokines produced by glial cells, which may contribute, in a paracrine manner, to the final development of brain metastasis. We identified overexpression of the FN14 gene, a member of the tumor necrosis factor (TNF) superfamily of receptors.45 FN14 is an immediate early response gene whose expression is directly activated after exposure to growth factors in fibroblasts; it is up-regulated in migration-stimulated glioma cells in vitro, and it has been associated with high-grade tumors.46 Through activation of MAPK8/JNK and NF-κB, the TRAF proteins mediate signal transduction of the TNF receptor superfamily members47; they could connect ERS responses and FN14 signaling pathway activation. Because FN14 and TRAF2 are overexpressed in breast cancer tumors that develop brain metastasis, and in brain metastatic cells, the disruption of the TWEAK/FN14 feedback loop also emerges as a molecular targeting strategy.
FN14 overexpression can stimulate survival through interaction with the inhibitor-of-apoptosis proteins (IAPs).48 TNF-like weak inducer of apoptosis (TWEAK)/FN14 signaling recruits cIAP1-TRAF2 complex, which is then targeted for lysosomal degradation. Cell sensitivity to TWEAK correlates with sensitivity to synthetic IAP antagonist. Studies with FN14-overexpressing tumor cells that could be selectively destroyed using a TWEAK-cytotoxin protein fusion suggest that FN14 could be a new molecular target for treating metastasis.49 Indeed, the ERS response phenotype might indicate new opportunities in anticancer strategies for sanctuary sites and micrometastatic disease. Evidence of such phenotypes can be used to develop more specifically addressed therapies.
Microarray-based gene studies are difficult to interpret, because of the huge amount of data involved, and it is therefore a challenge to derive biological insights. We applied a PPIN-based approach that identifies markers not as individual genes but as subnetworks extracted from PPINs, thus providing a systemic view of the interactome. This method serves to filter information by picking out key protein functions as metastasis markers. Indeed, we have delineated a pathogenic mechanism of metastasis to the brain based on the information from a proteomic study of brain metastasis cellular proteins. Further work is needed to confirm the prognostic value of the ERS response phenotype in a second validation step that includes a large independent group to increase the statistic power of the study and to assess the usefulness of the ERS response phenotype as a predictive tool at first diagnosis.
To validate these markers, the main objective is to obtain a large collection of retrospective samples, far in excess of the typical numbers required to obtain statistical significance in the data. This could also lead to preventive therapy for brain metastases at initial diagnosis, not only in breast cancer patients, but also for other carcinomas with brain tropism.
Acknowledgments
We thank Dr. Marta Brell from the Neurosurgery Service and Dr. Sergio Herrero from the Pathology Service, both at the Bellvitge Hospital (C.S.U.B.), for their expert assistance and providing human metastasis samples. We also thank Mr. R. Rycroft for expert language advice. We acknowledge all of the partners of the MetaBre consortium for their collaboration and stimulating criticism: Marc Bracke (Ghent University Hospital, Belgium), Roberto Buccione (Consorzio Mario Negri Sud, Italy), Vincent Castronovo (University of Liège, Belgium), Philippe Clément-Lacroix (Prostrakan, France), Philippe Clézardin (INSERM, France), Suzanne Eccles (Institute of Cancer Research, UK), Anna Teti (University of l'Aquila, Italy), Maciej Ugorski (Wroclaw Agriculture University, Poland), and Gabri van der Pluijm (Leiden University Medical Center, The Netherlands).
Footnotes
Supported by grants from the Spanish Ministry of Health and Consumer AffairsFIS/PI041937 and FIS/PI071245, by the European Commission MetaBre contract No. LSHC-CT-2004-506049, and by the Spanish Ministry of Education and ScienceSAF2004-0188-E. B.O. and R.A. acknowledge grants from the Spanish Ministry of Education and Science (MEC BIO2005-00533 and MCyT BIO2002-0369), and P.L.F. from the Marató-TV3, RETICC from Instituto Carlos III, and Xarxa de Bancs de Tumors de Catalunya.
References
- 1.Weil R.J., Palmieri D.C., Bronder J.L., Stark A.M., Steeg P.S. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luck A.A., Evans A.J., Green A.R., Rakha E.A., Paish C., Ellis I.O. The influence of basal phenotype on the metastatic pattern of breast cancer. Clin Oncol (R Coll Radiol) 2008;20:40–45. doi: 10.1016/j.clon.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Tosoni A., Ermani M., Brandes A.A. The pathogenesis and treatment of brain metastases: a comprehensive review. Crit Rev Oncol Hematol. 2004;52:199–215. doi: 10.1016/j.critrevonc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Tham Y.L., Sexton K., Kramer R., Hilsenbeck S., Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 5.Stemmler H.J., Heinemann V. Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist. 2008;13:739–750. doi: 10.1634/theoncologist.2008-0052. [DOI] [PubMed] [Google Scholar]
- 6.Carey L.A., Ewend M.G., Metzger R., Sawyer L., Dees E.C., Sartor C.I., Moore D.T., Graham M.L. Central nervous system metastases in women after multimodality therapy for high risk breast cancer. Breast Cancer Res Treat. 2004;88:273–280. doi: 10.1007/s10549-004-0999-3. [DOI] [PubMed] [Google Scholar]
- 7.Slimane K., Andre F., Delaloge S., Dunant A., Perez A., Grenier J., Massard C., Spielmann M. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004;15:1640–1644. doi: 10.1093/annonc/mdh432. [DOI] [PubMed] [Google Scholar]
- 8.Nathoo N., Chahlavi A., Barnett G.H., Toms S.A. Pathobiology of brain metastases. J Clin Pathol. 2005;58:237–242. doi: 10.1136/jcp.2003.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaal E.C., Niël C.G., Vecht C.J. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289–298. doi: 10.1016/S1474-4422(05)70072-7. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri D., Smith Q.R., Lockman P.R., Bronder J., Gril B., Chambers A.F., Weil R.J., Steeg P.S. Brain metastases of breast cancer. Breast Dis. 2006;26:139–147. doi: 10.3233/bd-2007-26112. [DOI] [PubMed] [Google Scholar]
- 11.Bendell J.C., Domchek S.M., Burstein H.J., Harris L., Younger J., Kuter I., Bunnell C., Rue M., Gelman R., Winer E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri D., Bronder J.L., Herring J.M., Yoneda T., Weil R.J., Stark A.M., Kurek R., Vega-Valle E., Feigenbaum L., Halverson D., Vortmeyer A.O., Steinberg S.M., Aldape K., Steeg P.S. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 13.Palmieri D., Chambers A.F., Felding-Habermann B., Huang S., Steeg P.S. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13:1656–1662. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 14.Gu B., España L., Méndez O., Torregrosa A., Sierra A. Organ-selective chemoresistance in metastasis from human breast cancer cells: inhibition of apoptosis, genetic variability and microenvironment at the metastatic focus. Carcinogenesis. 2004;25:2293–2301. doi: 10.1093/carcin/bgh272. [DOI] [PubMed] [Google Scholar]
- 15.Chuang H.Y., Lee E., Liu Y.T., Lee D., Ideker T. Network-based classification of breast cancer metastasis. Mol Syst Biol. 2007;3:140. doi: 10.1038/msb4100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin B., Aragues R., Sanz R., Oliva B., Boluda S., Martinez A., Sierra A. Biological pathways contributing to organ-specific phenotype of brain metastatic cells. J Proteome Res. 2008;7:908–920. doi: 10.1021/pr070426d. [DOI] [PubMed] [Google Scholar]
- 17.Landemaine T., Jackson A., Bellahcène A., Rucci N., Sin S., Abad B.M., Sierra A., Boudinet A., Guinebretière J.M., Ricevuto E., Noguès C., Briffod M., Bièche I., Cherel P., Garcia T., Castronovo V., Teti A., Lidereau R., Driouch K. A six-gene signature predicting breast cancer lung metastasis. Cancer Res. 2008;68:6092–6099. doi: 10.1158/0008-5472.CAN-08-0436. [DOI] [PubMed] [Google Scholar]
- 18.van de Vijver M.J., He Y.D., van'T Veer L.J., Dai H., Hart A.A., Voskuil D.W., Schreiber G.J., Peterse J.L., Roberts C., Marton M.J., Parrish M., Atsma D., Witteveen A., Glas A., Delahaye L., van der Velde T., Bartelink H., Rodenhuis S., Rutgers E.T., Friend S.H., Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 19.Bos P.D., Zhang X.H., Nadal C., Shu W., Gomis R.R., Nguyen D.X., Minn A.J., van de Vijver M.J., Gerald W.L., Foekens J.A., Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aragues R., Sander C., Oliva B. Predicting cancer involvement of genes from heterogeneous data. BMC Bioinformatics. 2008;9:172. doi: 10.1186/1471-2105-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragues R., Jaeggi D., Oliva B. PIANA: protein interactions and network analysis. Bioinformatics. 2006;22:1015–1017. doi: 10.1093/bioinformatics/btl072. [DOI] [PubMed] [Google Scholar]
- 22.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 23.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A.J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J.Y., Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Shahrour F., Minguez P., Tarraga J., Medina I., Alloza E., Montaner D., Dopazo J. FatiGO +: a functional profiling tool for genomic data: Integration of functional annotation, regulatory motifs and interaction data with microarray experiments. Nucleic Acids Res. 2007;35(Web Server issue):W91–W96. doi: 10.1093/nar/gkm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández P.L., Nayach I., Fernández E., Fresno L., Palacín A., Farré X., Campo E., Cardesa A. Tissue macroarrays (“microchops”) for gene expression analysis. Virchows Arch. 2001;438:591–594. doi: 10.1007/s004280100393. [DOI] [PubMed] [Google Scholar]
- 26.van 't Veer L.J., Dai H., van de Vijver M.J., He Y.D., Hart A.A., Mao M., Peterse H.L., van der Kooy K., Marton M.J., Witteveen A.T., Schreiber G.J., Kerkhoven R.M., Roberts C., Linsley P.S., Bernards R., Friend S.H. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 27.Nevins J.R., Huang E.S., Dressman H., Pittman J., Huang A.T., West M. Towards integrated clinico-genomic models for personalized medicine: combining gene expression signatures and clinical factors in breast cancer outcomes prediction. Hum Mol Genet. 2003;12(Spec No 2):R153–R157. doi: 10.1093/hmg/ddg287. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Klijn J.G., Zhang Y., Sieuwerts A.M., Look M.P., Yang F., Talantov D., Timmermans M., Meijer-van Gelder M.E., Yu J., Jatkoe T., Berns E.M., Atkins D., Foekens J.A. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 29.Naderi A., Teschendorff A.E., Barbosa-Morais N.L., Pinder S.E., Green A.R., Powe D.G., Robertson J.F., Aparicio S., Ellis I.O., Brenton J.D., Caldas C. A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene. 2007;26:1507–1516. doi: 10.1038/sj.onc.1209920. [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy S., Ross K.N., Lander E.S., Golub T.R. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 31.Nuyten D.S., Kreike B., Hart A.A., Chi J.T., Sneddon J.B., Wessels L.F., Peterse H.J., Bartelink H., Brown P.O., Chang H.Y., van de Vijver M.J. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y., Sun B., Li X., Zhang L., Niu Y., Xiao C., Ning L., Fang Z., Wang Y., Zhang L., Cheng J., Zhang W., Hao X. Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Res Treat. 2007;103:319–329. doi: 10.1007/s10549-006-9385-7. [Erratum appeared in Breast Cancer Res Treat 2007, 103:125–127] [DOI] [PubMed] [Google Scholar]
- 33.Minn A.J., Gupta G.P., Siegel P.M., Bos P.D., Shu W., Giri D.D., Viale A., Olshen A.B., Gerald W.L., Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bos P.M., Boon P.E., van der Voet H., Janer G., Piersma A.H., Brüschweiler B.J., Nielsen E., Slob W. A semi-quantitative model for risk appreciation and risk weighing. Food Chem Toxicol. 2009;47:2941–2950. doi: 10.1016/j.fct.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Klein A., Olendrowitz C., Schmutzler R., Hampl J., Schlag P.M., Maass N., Arnold N., Wessel R., Ramser J., Meindl A., Scherneck S., Seitz S. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 2009;276:212–220. doi: 10.1016/j.canlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Steeg P.S. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 37.Ignatiadis M., Desmedt C., Sotiriou C., de Azambuja E., Piccart M. HER-2 as a target for breast cancer therapy. Clin Cancer Res. 2009;15:1848–1852. doi: 10.1158/1078-0432.CCR-08-1844. [DOI] [PubMed] [Google Scholar]
- 38.Hicks D.G., Short S.M., Prescott N.L., Tarr S.M., Coleman K.A., Yoder B.J., Crowe J.P., Choueiri T.K., Dawson A.E., Budd G.T., Tubbs R.R., Casey G., Weil R.J. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 39.Lin N.U., Winer E.P. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 40.Piccart M. Circumventing de novo and acquired resistance to trastuzumab: new hope for the care of ErbB2-positive breast cancer. Clin Breast Cancer. 2008;8(Suppl 3):S100–S113. doi: 10.3816/cbc.2008.s.006. [DOI] [PubMed] [Google Scholar]
- 41.Dollins D.E., Warren J.J., Immormino R.M., Gewirth D.T. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28:41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlowski R.Z., Kuhn D.J. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 43.Zheng H.C., Takahashi H., Li X.H., Hara T., Masuda S., Guan Y.F., Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Wang S., Wangtao, Wang Y., Wang J., Jiang L., Li S., Hu X., Wang Q. Upregulation of GRP78 and GRP94 and its function in chemotherapy resistance to VP-16 in human lung cancer cell line SK-MES-1. Cancer Invest. 2009;27:453–458. doi: 10.1080/07357900802527239. [DOI] [PubMed] [Google Scholar]
- 45.Wiley S.R., Winkles J.A. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev. 2003;14:241–249. doi: 10.1016/s1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 46.Tran N.L., McDonough W.S., Savitch B.A., Sawyer T.F., Winkles J.A., Berens M.E. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Biol Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 47.Hu P., Han Z., Couvillon A.D., Kaufman R.J., Exton J.H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vince J.E., Chau D., Callus B., Wong W.W., Hawkins C.J., Schneider P., McKinlay M., Benetatos C.A., Condon S.M., Chunduru S.K., Yeoh G., Brink R., Vaux D.L., Silke J. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkles J.A., Tran N.L., Berens M.E. TWEAK and Fn14: new molecular targets for cancer therapy? Cancer Lett. 2006;235:11–17. doi: 10.1016/j.canlet.2005.03.048. [DOI] [PubMed] [Google Scholar]


