Abstract
Gene-targeted deletion of the immediate early responsive gene X-1 (IEX-1) results in a significant increase in systemic arterial hypertension, but the underlying mechanism is not understood. Studies of arterial reactivity in isolated aortas revealed normal endothelium-dependent and independent vasorelaxation and vasoconstriction, but reduced cAMP-dependent vasorelaxation in the absence of IEX-1. This defect in cAMP signaling was also evident in endothelium-denuded aortic rings, consistent with the enhancement of mitochondrial O2− production only in IEX-1 deficient VSMCs, not in endothelial cells. Excessive production of reactive oxygen species at mitochondria (mROS) augmented the expression of Gαi2, suppressing cAMP production in VSMCs. The role of mROS in the up-regulation of Gαi2 leading to the development of hypertension was supported by the ability of antioxidant or pertussis toxin to restore the cAMP-dependent vasorelaxation to a normal level and reverse established hypertension in IEX-1 homozygous knockout (KO) mice. Our results suggest that hypertension in IEX-1 KO mice may arise primarily from impaired cAMP-signaling induced by over production of mROS in VSMCs, and demonstrate a causal relationship between mitochondrial dysfunction and cAMP-dependent vasorelaxation.
Keywords: hypertension, IEX-1, ROS, Gαi2, mitochondria
Introduction
Systemic hypertension markedly increases the risk of death from stroke, ischemic heart and vascular diseases. Approximately 50 million people in the United States are hypertensive1, and 30~50% of them inherit one or more susceptibility genes2. In accordance to this, various single gene variants have been consistently identified in hypertensive individuals by genome-wide linkage analysis. For instance, the C825T allele carrier status of the GNB3 gene has been shown to positively associate with an increasing risk of hypertension in Caucasians3. The GNB3 gene encodes the β3 subunit of heterotrimeric GTP-binding proteins and a C825T allele leads to enhanced Gαi protein activation in individuals at risk3,4. The finding is consistent with a well documented role of G-protein-mediated signals in the control of vascular tone, endothelial function, and renal handling of salt and water4–6.
The immediate early responsive gene X-1 (IEX-1) is a stress-induced gene7, and it is abundantly expressed in aortas and small blood vessels in humans as well as in mice8,9. Vascular smooth muscle cells (VSMCs) and cardiac myocytes rapidly express IEX-1 in response to mechanical stress and pressure overload10,11, probably to counteract hypertrophy in cardiovascular tissues. In support, over-expression of IEX-1 prevents the hypertrophic response of these tissues to biomechanical strain10,11. These observations, in conjunction with the development of hypertension in IEX-1-deficient mice12,13, argue strongly a role for IEX-1 in the regulation of vascular function, but the underlying mechanism is not known.
We recently showed that IEX-1 targeted the mitochondrial F1F0-ATPase inhibitor (IF1) for degradation, giving rise to a high level of F1F0-ATPase activity, concomitant with reduced production of mitochondrial reactive oxygen species (mROS)12,14. Conversely, absence of IEX-1 increased IF1 expression and reduced mitochondrial ATPase activity in aortic tissues12. Impaired mitochondrial function and mROS homeostasis occur frequently in cardiovascular disorders such as endothelial dysfunction and hypertension15–17. And, hypertensive patients of different ethnic origins have been reported to bear one or more mitochondrial mutations18–20. Despite these cues about a possible linkage of mitochondrial malfunction to hypertension in both humans and animal models, there is a lack of a mechanistic understanding of how mitochondrial dysfunction contributes to hypertension15,18,20,21.
Our present study demonstrates that IEX-1 deficiency up-regulates Gαi2 protein in VSMCs as a result of exaggerated ROS production at mitochondria. Increased Gαi2 expression inhibits cAMP-dependent vascular smooth muscle relaxation and contributes to hypertension in the animal. The study directly links mitochondrial dysfunction to cAMP-regulated vascular tone and helps us to understand how mitochondrial malfunction causes systemic hypertension.
Materials and Methods
Animals
IEX-1 knockout (KO) and wild type (WT) control mice on a mixed 129Sv/C57BL/6 background were generated by gene-targeted deletion in our lab as detailed in the supplemental materials (please see http://hyper.ahajournals.org). The animals were housed in conventional cages in the animal facilities of Massachusetts General Hospital (MGH) in compliance with institutional guidelines and only male mice were studied. All studies were reviewed and approved by the MGH Subcommittee of Research Animal Studies.
Statistical analysis
All data are expressed as mean ± standard errors of measurement (SEM). Two-Way or One-Way repeated measures ANOVA was used to compare vascular reactivity (figures 4&5) and blood pressure (BP) (figures 1B and 7E) or the level of cAMP (figure 6) and Gαi2 mRNA in VSMCs (figures 8D, E and F), respectively. The lucigenin data among different groups (figure 2M) and the influence of single treatment on BP in two groups (figure 1) was compared by one way ANOVA. Statistical significance between two groups was determined with a Student's two-tailed t test (figure 7C and 7D). A value of p<0.05 was considered significant.
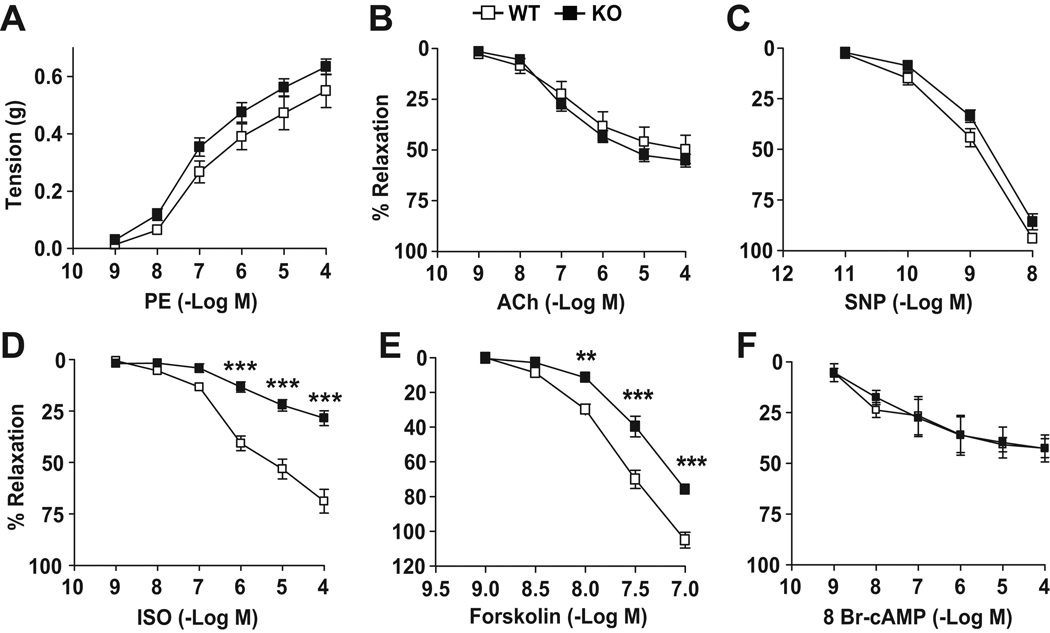
Figure 4. Impaired cAMP-dependent vascular relaxation in IEX-1 KO mice.
Shown are the cumulative concentration responses of aortic rings to phenylephrine (PE) (A), acetylcholine (Ach) (B), sodium nitroprusside (SNP) (C), isoproterenol (ISO) (D), forskolin (E), and 8 bromo-cyclic AMP (8 br-cAMP) (F). Aortic rings were isolated from 12-wk-old WT and IEX-1 KO mice. Data are expressed as mean percents ± SEM of absolute tension (A) or relaxation (B–F) relative to the contraction induced by 106 M PE; n = 12 in A–D and 8 in E and F. * p<0.05, ** p<0.01 and ***p<0.001 respectively, in the presence or absence of IEX-1.
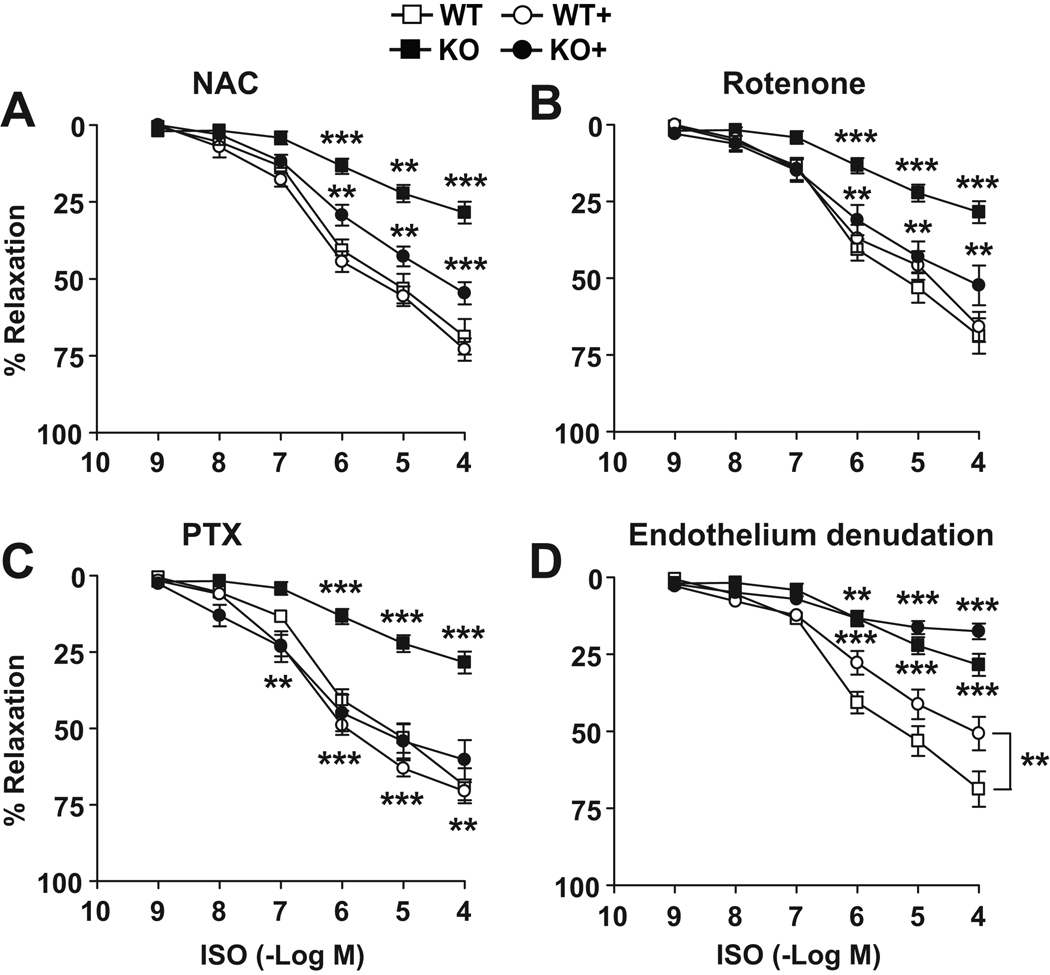
Figure 5. Impaired cAMP-dependent vascular relaxation in an endothelium-independent manner.
Shown are the cumulative concentration responses to ISO of aortic rings isolated from 12-wk-old IEX-1 KO and WT mice in the absence and presence of NAC (A), rotenone (B), PTX (C) or endothelium (D). Aortic rings were incubated for 2 hrs with NAC or rotenone and for 1 hr with PTX or endothelium denudation before measuring the response to ISO. Data are expressed as mean % ± SEM of relaxation relative to the contraction induced by 106 M PE; n = 8. * p<0.05, ** p<0.01 and *** p<0.001, respectively, in the presence or absence of the anti-oxidants, PTX or IEX-1.
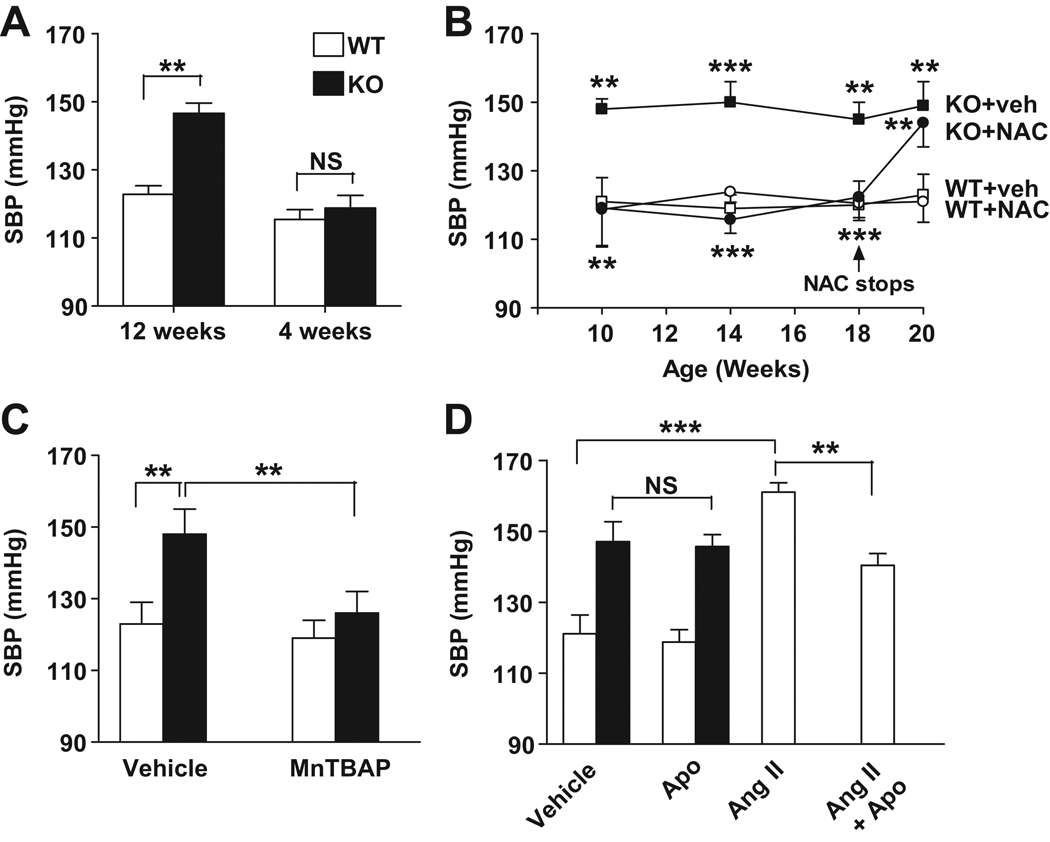
Figure 1. Normalization of BP in IEX-1 KO mice by anti-oxidant treatment.
A. Increased SBP in 12-wk-old but not in 4-wk-old mice. SBP was measured noninvasively in awake IEX-1 KO (filled) and age-matched WT (blank) mice at the indicated age. B. NAC completely prevents hypertension. IEX-1 KO and WT mice at 3 wks of age were given NAC (10 g/L) or vehicle (veh) with drinking water for 15 wks. NAC or vehicle was later omitted from the drinking water (arrow) and SBP was measured again after two wks of treatment. C and D. Reduction of SBP in IEX-1 KO mice by MnTBAP but not by NADPH oxidase inhibitor apocynin. IEX-1 KO and WT mice at 12 wks of age were daily given 5 mg MnTBAP/kg/d or vehicle control by intraperitoneal injection (C), or fed 1.5 mmol/L apocynin (Apo) or vehicle in drinking water for two wks (D). As a positive control for the efficacy of apocynin, WT mice were infused with angiotensin II (Ang II) at a dose of 0.7mg/kg/d via an implanted osmotic minipump, with or without apocynin in their drinking water for two wks. Data are expressed as mean ± SEM of SBP (mmHg); n = 6 in A and B and 5 in C and D. ** p<0.01 and ***p<0.001 with or without treatment or in the presence or absence of IEX-1.
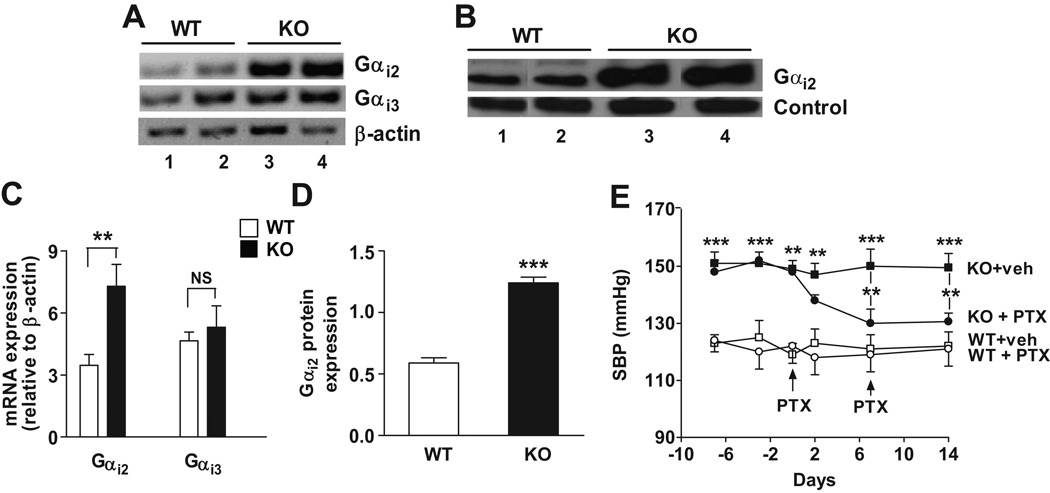
Figure 7. Increased expression of Gαi2 in IEX-1-deficient vasculature contributes to the elevated BP.
The levels of Gαi2 and Gαi3 in aortic tissues isolated from 12-wk-old IEX-1 KO and WT mice were analyzed by RT-PCR (A) and compared by densitometric analysis of individual bands relative to control β-actin (C). Gαi2 protein expression in cell membranes prepared from aortas was evaluated by immunoblotting using anti-Gαi2 antibody (B). Band density was analyzed with a non-specific 75 kDa membrane-protein serving as a control (D). Each lane represents the sample from one mouse illustrated in A and B. Data in C and D are expressed as mean arbitrary units ± SEM of band density; n = 3. E. Reduction of SBP in IEX-1 KO mice by PTX treatment. IEX-1 KO and WT control mice at 12 wks of age were treated once a week with PTX (20 µg/kg intraperitoneally) or vehicle (veh) for two wks (arrows). Arterial SBP was monitored for two wks by radiotelemetry. Data are expressed as mean ± SEM of SBP (mmHg); n = 5. ** p<0.01 and *** p<0.001 in the presence or absence of IEX-1 or PTX treatment.
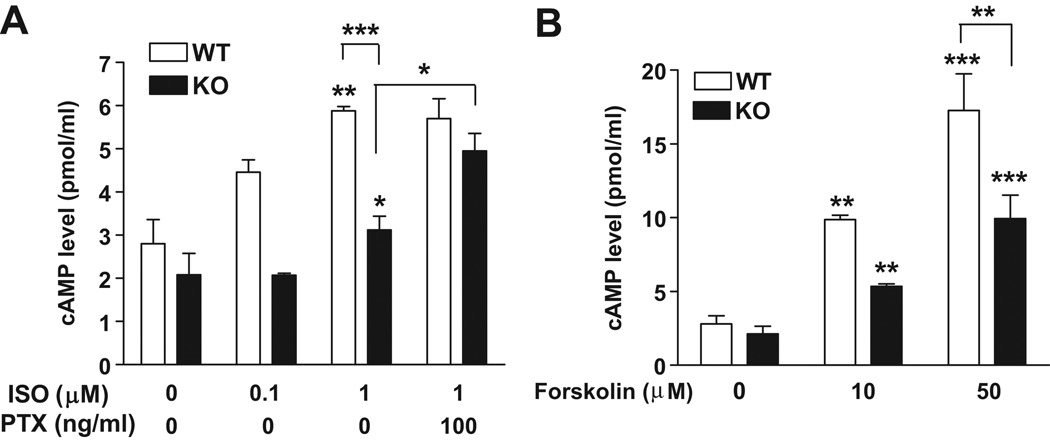
Figure 6. Impaired cAMP production in IEX-1 deficient VSMCs in response to ISO or forskolin.
An increase in intracellular cAMP level in response to ISO (A) or forskolin (B) in a dose dependent manner in both WT and IEX-1 deficient VSMCs. Preincubation with PTX for 1 hr restored cAMP production in KO cells to a WT level in response to ISO (1 µM). Data are expressed as mean ± SEM of cAMP concentration (pmol/ml); n = 3. * p<0.05 and ** p<0.01 in the presence or absence of IEX-1 or with or without an indicated stimulus.
Figure 2. Increased superoxide production in IEX-1-deficient vasculature.
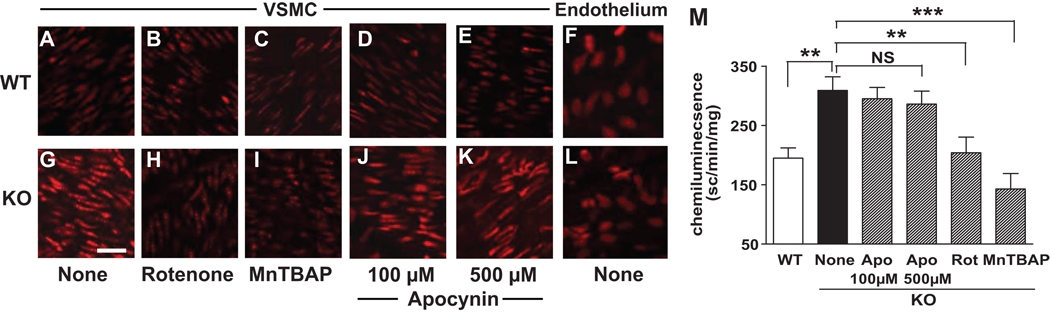
Dihydroethidium fluorescence intensity was greater in VSMCs in aorta of IEX-1 KO mice (G) relative to WT counterparts (A), but the intensity was comparable in the endothelium of the two strains of mice (F and L). The fluorescence intensity in IEX-1 KO VSMCs was diminished to a WT level by pretreatment with 1 µM rotenone (B and H) or 10 µM MnTBAP (C and I), but not with apocynin (D, E, J, and K) for 45 min. On representative result of three experiments performed with similar results. Lucigenin chemiluminescence was also greater in aortic rings obtained from IEX-1 KO mice, but it was diminished significantly after treatment with 1 µM rotenone (Rot) or 10 µM MnTBAP, but not with 100 or 500 apocynin (Apo) for 30 min (M). Data are expressed as mean ± SEM of scintillation counts per min/mg of dried tissue; n = 6. ** p<0.01 and ***p<0.001 in the presence or absence of the indicated anti-oxidant or IEX-1. Magnification: 40×. Bar = 25 µM
Extended methods can be found in online supplement (please see http://hyper.ahajournals.org)
RESULTS
Normalization of blood pressure in IEX-1 KO mice by anti-oxidants
IEX-1 KO mice were generated by gene-targeted deletion of IEX-1 as detailed in the supplemental materials (Figure S1, please see http://hyper.ahajournals.org). The mice appear healthy and lack any gross developmental abnormalities. However, all of them developed systemic hypertension by 8–10 wks of age, but none of the mice younger than 4 wks of age did so (Figure 1A). The average systolic and diastolic BP at 12 wks of age were 148±3 and 114±4 mmHg, respectively, in IEX-1 KO mice as compared to 122±3 and 90±5 mmHg in WT mice (p<0.01 for both systolic and diastolic blood pressure) (Figure 1A, data not shown). Despite persistent hypertension, there was no abnormality in the histology of the kidney or in the vascular morphology in the aorta or 2nd order mesenteric arteries in the mice when examined at 12 wks of age (Figure S2, please see http://hyper.ahajournals.org).
To investigate whether hypertension in IEX-1 KO mice was attributable to deregulated mROS production, we treated the mice, along with age-matched WT mice, with a ROS scavenger, N-acetylcysteine (NAC)12,14 by including it in daily drinking water at 10g/L for 15 wks starting at 3 wks of age. IEX-1 KO mice treated with vehicle alone, similar to the untreated mice, had a significantly elevated systolic blood pressure (SBP) at 10 wks of age (148±3 mmHg) that remained elevated throughout the study. Inclusion of NAC in the drinking water clearly prevented IEX-1 KO mice from developing hypertension (Figure 1B) and their SBP remained normal until 18 wks of age as long as NAC was given (Figure 1B). Yet, these mice developed hypertension within two wks after removal of NAC from their drinking water, SBP increasing from 121±6 to 144±7 mmHg (p<0.01).
We next treated IEX-1 KO mice with Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) to confirm a role for excess O2− production in the hypertension. MnTBAP is a membrane-permeable superoxide dismutase mimetic that has been shown to scavenge superoxide within mitochondria22. Daily intraperitoneal injection of 5 mg MnTBAP/kg for two wks reduced SBP from 148±7 to 126±6mmHg in 12-week-old IEX-1 KO mice (p<0.01, Figure 1C). However, treatment with apocynin for two wks failed to lower SBP in the mice (Figure 1D), ruling out that cell membrane-associated NAD(P)H oxidases are involved in the hypertension, as apocynin blocks the association of p47phox and p67phox with the gp91phox subunit within the membrane NAD(P)H oxidase complex and thus inhibits this oxidase specifically. In control WT mice, the same apocynin treatment significantly attenuated the development of angiotensin (Ang) II-induced hypertension (p<0.01, Figure 1D)23, verifying the efficacy of the drug we used.
Enhanced mROS formation in IEX-1 KO mice
The importance of IEX-1 in regulating mitochondrial O2− homeostasis12 and in maintaining a normal SBP promoted us to assess vascular O2− levels in the absence of IEX-1 using two independent methods, dihydroethidium (DHE) fluorescence and lucigenin chemiluminescence. Confocal microscopic analyses of ex-vivo aortas demonstrated significantly greater ethidium bromide fluorescence and hence increased O2− production in VSMCs in the absence than in the presence of IEX-1 (Figures 2G versus 2A). But, such an increase was not seen in the endothelium (Figures 2F versus 2L). A similar increase in O2− formation was also observed in aortas isolated from IEX-1 KO mice when measured by lucigenin-enhanced chemiluminescence (P<0.01, figure 2M). Pretreatment of aortic rings with either rotenone, a specific inhibitor for the complex I of the mitochondrial respiratory chain (Figures 2B vs. 2H), or MnTBAP (Figures 2C vs. 2I), but not with apocynin (Figures 2D & 2E vs. 2J & 2K) significantly reduced both ethidium bromide fluorescence intensity and lucigenin chemiluminescence counts (Figure 2M) in aortas prepared from IEX-1 KO mice. Interestingly, this elevated level of O2− production in VSMCs was also evident in the aorta of 4-wk-old IEX-1 KO mice as compared to age-matched WT mice (Figure S3, please see http://hyper.ahajournals.org).
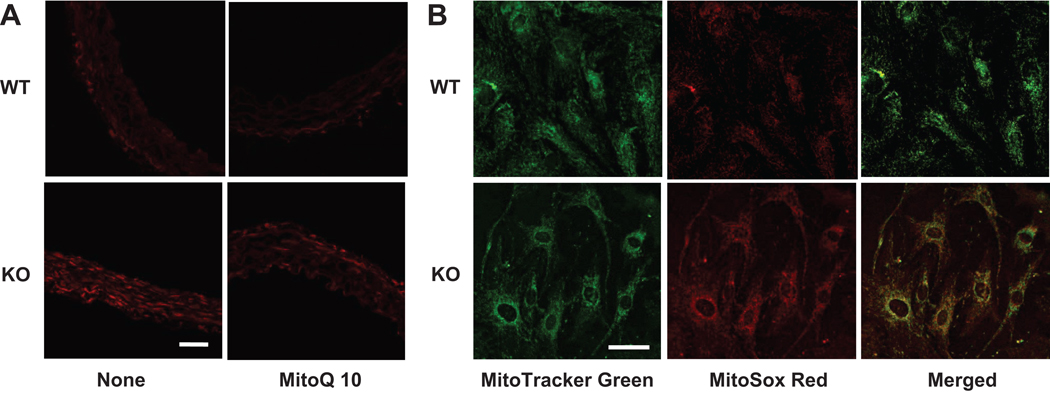
To corroborate that mitochondria were the source of excessive O2− production in the absence of IEX-1, we employed a mitochondrion-specific hydroethidine-derivative fluorescent dye MitoSox Red to detects O2− levels in ex-vivo aortas24 and significantly greater MitoSox fluorescence in the absence than in the presence of IEX-1 (Figures 3A, lower versus upper panel). A similar increase in mitochondrial O2− formation was also observed in cultured VSMCs isolated from the aortas of IEX-1 KO mice when compared to WT counterparts (Figure 3B, lower vs. upper panel). Increased MitoSox fluorescence in IEX-1 deficient cells displayed a typical patter of mitochondrial staining, intensifying around cell nuclei, and it was colocalized with MitoTracker Green, a fluorescent mitochondrial marker, as implicated by yellow-orange fluorescence when the two images were merged (Figure 3B). Moreover, pretreatment of ex-vivo aortic rings with a mitochondrion-specific antioxidant MitoQ 10 (100 nM)25 for 2 hrs significantly reduced MitoSox fluorescence intensity in the absence of IEX-1 (Figure 3A). These data, along with our previous study demonstrating enhanced degradation of IF1 by IEX-113, confirm that mitochondria in VSMCs are the source for the elevated O2− production in IEX-1 KO arterial vessels.
Figure 3. An increase in mitochondrial superoxide production in IEX-1-deficient vasculature and cultured VSMCs.
Increased MitoSox fluorescence intensity was detected in vasculature in the absence compared to presence of IEX-1, but it was diminished to a WT level by pretreatment of the aortic rings ex-vivo with MitoQ 10 (100 nM) for 2 hrs (A). Shown in B are representative confocal microscopy images demonstrating an increase in MitoSOX Red fluorescence in cultured VSMCs isolated from the aorta of IEX-1 KO. The MitoSOX Red fluorescence was colocalized with MitoTracker Green as manifested by yellow fluorescence around the nuclei in merged images on the right panel. Magnification: 40×. Bar = 50 µM
Altered cAMP-mediated signaling and reduced vasorelaxation in IEX-1 KO mice
Excessive vascular O2− production by IEX-1 deficient VSMCs can impair arterial constriction or relaxation by reducing nitric oxide (NO) bioavailability and/or by interfering with cAMP-mediated signaling pathways26–28. We found that α1 adrenergic receptor-mediated vasoconstriction (Figure 4A) and endothelium-independent vasorelaxation (Figure 4C) both were not altered significantly in aortic segments dissected from 12-wk-old IEX-1 KO mice compared to age-matched WT control mice. There was also no difference in endothelium-dependent vasorelaxation between IEX-1 KO and WT mice (Figure 4B). Strikingly, the vasorelaxation response to the β-2 adrenergic receptor agonist isoproterenol (ISO) was blunted in IEX-1 KO mice as compared to WT mice (28±4 versus 69±6 % of maximum relaxation, p<0.001, figure 4D), suggesting impaired cAMP-dependent signaling in the absence of IEX-1. The reduced cAMP-dependent vasorelaxation occurred not only in 12-wk-old hypertensive mice, but also in 3~4-wk-old normotensive IEX-1 KO mice (21±2 vs. 50±3 % maximum relaxation, p<0.001, Figure S4 A–D, please see http://hyper.ahajournals.org). Consistent with a reduced cAMP-dependent response as a primary cause for an elevated BP was a significantly reduced relaxation response to the adenylyl cyclase activator forskolin in IEX-1 KO mice as compared to WT mice (75±2 versus 105±5 % of maximum relaxation, p<0.01, Figure 4E). However, no difference was observed in the vasodilator response to the cAMP analogue, 8-bromo cAMP between KO and WT mice (Figure 4F), suggesting that IEX-1 deficiency alters ISO-stimulated cAMP signaling at upstream of cAMP production.
We then investigated an involvement of mROS in the impaired cAMP-dependent vasorelaxation in IEX-1 KO mice. As shown in Figure 5 A & B, incubation of aortic rings with either NAC or rotenone for 2 hrs increased substantially the maximum relaxation to ISO in IEX-1 KO rings, whereas similar treatments had little impact on the response in WT rings. The β2 adrenergic receptor is known to couple with Gαs protein to activate adenylyl cyclase and the activity of Gαs can be counteracted by Gαi protein29. This, in line with the ability of ROS to increase the activity of Gαi, but not Gαs30, motivated us to examine whether aberrant cAMP-dependent signaling was attributed to altered Gαi activity in the absence of IEX-1. Pre-incubation of aortic rings with pertussis toxin (PTX), a specific inhibitor for Gαi/o, for 1 hr restored ISO-induced vasorelaxation in absence of IEX-1 to a WT level (60±6 vs. 28±4 %, p<0.01, figure 5C), confirming that altered Gαi activity accounts for the reduced response to ISO in IEX-1 KO mice. To pinpoint the defect in VSMCs, ISO-induced vasorelaxation responses were also evaluated in the endothelium-denuded aortic rings. Although endothelium removal resulted in the partial attenuation of the vasorelaxation responses to ISO in both WT and KO mice, the response to ISO was again blunted in IEX-1 KO mice to a degree that was comparable to the decrease seen with intact aortic rings (18±3 versus 51±5 % of maximum relaxation, p<0.001, figure 5D). The data suggest that impaired cAMP-dependent signaling in the vasculature of IEX-1 KO mice largely occurs in VSMCs.
Reduced cAMP production in IEX-1 deficient VSMCs
An aberrant vasorelaxation response to ISO and forskolin in IEX-1 KO mice indicates an abnormal receptor-dependent and –independent cAMP production in the aorta of these mice. To confirm this, we measured intracellular cAMP levels in response to ISO and forskolin in cultured VSMCs. No significant difference in the basal level of cAMP was observed between WT and IEX-1 KO VSMCs. These two types of VSMCs responded to ISO or forskolin in a dose-dependent fashion, but the degree of increase was compromised in IEX-1 deficient cells as compared to WT cells with each stimulus. The maximal percentage increase in the cAMP level in response to ISO (1 µM) or forskolin (50 µM) was 156±10 and 486±21 in IEX-1 KO cells as compared to 226±22 or 629±18 in WT cells, respectively (p<0.01, figure 6 A & B). The ISO-induced cAMP production in IEX-1 deficient VSMCs was restored to a WT level when the cells were pre-incubated with PTX, whereas the inhibitor influenced little over the level of cAMP production in WT cells (Figure 6A), consistent with up regulation of PTX-sensitive Gαi/o in VSMCs lacking IEX-1.
A critical role of Gαi expression in the absence of IEX-1
To corroborate up regulation of Gαi in vascular cells in the absence of IEX-1, we employed RT-PCR to evaluate the level of Gαi2 and Gαi3 expression in homogenized aortic tissues and found significantly greater levels of Gαi2 expression in IEX-1 KO mice than in WT mice, but a comparable level of Gαi3 expression in the two strains of mice (Figures 7A and C). Enhanced Gαi2 expression from IEX-1 KO mice was also confirmed by Western blot analysis of aortic membrane proteins (Figures 7B and D). The level of Gαi2 expression in IEX-1 KO VSMCs was reduced to a WT level when cells were cultured in the presence of NAC, rotenone or mitochondrial specific antioxidant Mito Q1025,31 (Figure S5, please see http://hyper.ahajournals.org), underscoring a role for ROS in the elevated level of Gαi2 expression in vascular tissues in IEX-1 KO mice. Foremost, a single dose of PTX treatment was able to lower the arterial BP in 12-wk-old IEX-1 KO mice after one week (Figure 7E) (Figure S6, please see http://hyper.ahajournals.org), while the same treatment affected little the BP in WT mice. These data collectively suggest a key role for mitochondrial O2− in regulation of Gαi2 expression and in cAMP-dependent vascular relaxation contributing to systemic hypertension in IEX-1 KO mice.
DISCUSSION
Our study demonstrates that in mice lacking IEX-1, an increase in Gαi2 expression induced by overproduction of mROS in VSMCs impairs cAMP-mediated vasorelaxation and contributes to systemic hypertension. The finding that a single molecular abnormality in VSMCs predisposes to developing systemic hypertension highlights the pivotal role of G protein-mediated signaling and mitochondrial activity in maintaining arterial BP. Hypertension in many animal models is often caused by impaired NO signaling, concurrent with vascular endothelial dysfunction, inflammation, and hypertrophy6,26, but the primary cause for this common disorder remains unclear in most patients. Strikingly, despite a persistent elevation of blood pressure, IEX-1 KO mice did not develop vascular hypertrophy and renal injury, and displayed normal NO-dependent signaling in both endothelium-dependent and independent manners, even at 18 months of age. This model may represent an early stage of systemic hypertension or a unique form of hypertension caused by a single gene variant, offering a unique opportunity to investigate contribution of other factors like inflammation, endothelial dysfunction to the full genesis of hypertension.
The most important finding of this study is that IEX-1 deficiency reduces systemic arterial vasodilation owing to increasing mROS production in VSMCs. Although the role of ROS generated by cell membrane-associated NAD(P)H oxidases in developing hypertension is well documented23,26,32, how deregulated ROS homeostasis at mitochondria causes hypertension remains elusive, despite considerable evidence demonstrating that they are correlated15,18,20,33. The present study shows that an increase in mROS production is associated with reduction in cAMP production in VSMCs and a diminished vascular relaxation response to ISO or forskolin in the absence of IEX-1. The reduction of cAMP-dependent vasodilation appears to be a primary cause, rather than an adaptive response to an elevated BP, since this phenotype is evident in young mice before they become hypertensive (Figure S4, please see http://hyper.ahajournals.org). The study thus unravels one of the mechanisms whereby deregulation of mitochondrial function enhances vascular tone and triggers the development of hypertension.
The second important finding of the present study is that elevated mROS production augmented Gαi2 expression that inhibited cAMP-dependent signaling in IEX-1 deficient VSMCs. ROS have been shown to up-regulate Gαi2 transcription in VSMCs of SHR28 and in K562 cells34 probably by activation of the promoter of the Gαi2 gene that contains transcriptional regulatory motifs for the redox-sensitive NF-κB and Nrf2 (nuclear factor erythroid 2-related factor) transcription factors34. Nishida et al. demonstrated that hydrogen peroxide (H2O2) specifically increased Gαi protein activity but not the activity of Gαs associated with the plasma membrane30. Interestingly, PTX has been previously reported to reduce BP effectively in established hypertension in SHR animals in a similar fashion as in the present study29,35. These previous reports and our current investigation provide a compelling evidence for an indispensable role for Gαi2 in the development of hypertension. The finding is of highly clinic relevance since a relatively high level of Gαi expression has been shown in association with an elevated arterial BP in humans36.
The present study identifies an important and a previously unappreciated role of IEX-1 in the maintenance of systemic vascular tone by the control of Gαi2 expression levels. IEX-1 deficiency increases mROS production and up regulates Gαi2 gene transcription in VSMCs, which in turn inhibits cAMP-dependent vascular relaxation leading to systemic hypertention in IEX-1 deficient mice. This study reveals one important mechanism whereby mitochondrial dysfunction and mROS overproduction can produce hypertension by altering cAMP signaling and augmenting vascular tone.
Perspective
The present study identifies a novel mechanism of hypertension development whereby IEX-1 deficiency impairs cAMP-signaling in VSMCs due to deregulated ROS homeostasis at mitochondria. IEX-1-deficiency-induced hypertension is devoid of abnormal NO signaling, vascular inflammation and hypertrophy, or renal abnormality commonly seen in other hypertension models. This mouse model thus provides us with a unique opportunity to investigate a causal relationship between VSMCs and endothelium or inflammation in the full genesis of hypertension.
Supplementary Material
Acknowledgments
We thank Dr. Kenneth D. Bloch for his critical reading, the members of the Bloch, Zapol and Wu groups for stimulating discussions and valuable comments, Dr. Marielle Scherrer-Crosbie for assistance with mouse echocardiography, Dr. Emmanuel Buys and Mr. Michael J Raher for help with radiotelemetry, and Dr. Hui Zheng for his statistical assistance. The authors are grateful to Dr. Michael Murphy from MRC Dunn Human Nutrition Unit, Cambridge, UK for the generous gift of MitoQ 10.
Sources of Funding: This work is supported by National Institutes of Health (NIH) grants AI050822 and AI070785, and a Grant-in-Aid (0855762D) from the American Heart Association (to M.X.W.) and NIH CA75796 (to D.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Marteau JB, Zaiou M, Siest G, Visvikis-Siest S. Genetic determinants of blood pressure regulation. J Hypertens. 2005;23:2127–2143. doi: 10.1097/01.hjh.0000186024.12364.2e. [DOI] [PubMed] [Google Scholar]
- 3.Siffert W. G protein polymorphisms in hypertension, atherosclerosis, and diabetes. Annu Rev Med. 2005;56:17–28. doi: 10.1146/annurev.med.56.082103.104625. [DOI] [PubMed] [Google Scholar]
- 4.Siffert W. G-protein beta3 subunit 825T allele and hypertension. Curr Hypertens Rep. 2003;5:47–53. doi: 10.1007/s11906-003-0010-4. [DOI] [PubMed] [Google Scholar]
- 5.Feldman RD, Gros R. Defective vasodilatory mechanisms in hypertension: a G-protein-coupled receptor perspective. Curr Opin Nephrol Hypertens. 2006;15:135–140. doi: 10.1097/01.mnh.0000214772.96361.ef. [DOI] [PubMed] [Google Scholar]
- 6.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu MX. Roles of the stress-induced gene IEX-1 in regulation of cell death and oncogenesis. Apoptosis. 2003;8:11–18. doi: 10.1023/a:1021688600370. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Kobayashi T, Warner GM, Wu Y, Salisbury JL, Lingle W, Pittelkow MR. A novel immediate early response gene, IEX-1, is induced by ultraviolet radiation in human keratinocytes. Biochem Biophys Res Commun. 1998;253:336–341. doi: 10.1006/bbrc.1998.9692. [DOI] [PubMed] [Google Scholar]
- 9.Feldmann KA, Pittelkow MR, Roche PC, Kumar R, Grande JP. Expression of an immediate early gene, IEX-1, in human tissues. Histochem Cell Biol. 2001;115:489–497. doi: 10.1007/s004180100284. [DOI] [PubMed] [Google Scholar]
- 10.de Keulenaer GW, Wang Y, Feng Y, Muangman S, Yamamoto K, Thompson JF, Turi TG, Landschutz K, Lee RT. Identification of IEX-1 as a biomechanically controlled nuclear factor-kappaB target gene that inhibits cardiomyocyte hypertrophy. Circ Res. 2002;90:690–696. doi: 10.1161/01.res.0000012922.40318.05. [DOI] [PubMed] [Google Scholar]
- 11.Schulze PC, de Keulenaer GW, Kassik KA, Takahashi T, Chen Z, Simon DI, Lee RT. Biomechanically induced gene iex-1 inhibits vascular smooth muscle cell proliferation and neointima formation. Circ Res. 2003;93:1210–1217. doi: 10.1161/01.RES.0000103635.38096.2F. [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Zhi L, Hu W, Wu MX. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death Differ. 2009;16:603–612. doi: 10.1038/cdd.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer SL, Berndt TJ, Frank E, Patel JB, Redfield MM, Dong X, Griffin MD, Grande JP, van Deursen JM, Sieck GC, Romero JC, Kumar R. Elevated blood pressure and cardiac hypertrophy after ablation of the gly96/IEX-1 gene. J Appl Physiol. 2006;100:707–716. doi: 10.1152/japplphysiol.00306.2005. [DOI] [PubMed] [Google Scholar]
- 14.Akilov OE, Ustyugova IV, Zhi L, Hasan T, Wu MX. Enhanced susceptibility to Leishmania infection in resistant mice in the absence of immediate early response gene X-1. J Immunol. 2009;183:7994–8003. doi: 10.4049/jimmunol.0900866. [DOI] [PubMed] [Google Scholar]
- 15.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postnov YV, Orlov SN, Budnikov YY, Doroschuk AD, Postnov AY. Mitochondrial energy conversion disturbance with decrease in ATP production as a source of systemic arterial hypertension. Pathophysiology. 2007;14:195–204. doi: 10.1016/j.pathophys.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Ji Q, Ikegami H, Fujisawa T, Kawabata Y, Ono M, Nishino M, Ohishi M, Katsuya T, Rakugi H, Ogihara T. A common polymorphism of uncoupling protein 2 gene is associated with hypertension. J Hypertens. 2004;22:97–102. doi: 10.1097/00004872-200401000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz F, Duka A, Sun F, Cui J, Manolis A, Gavras H. Mitochondrial genome mutations in hypertensive individuals. Am J Hypertens. 2004;17:629–635. doi: 10.1016/j.amjhyper.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Zhu HY, Wang SW, Liu L, Li YH, Chen R, Wang L, Holliman CJ. A mitochondrial mutation A4401G is involved in the pathogenesis of left ventricular hypertrophy in Chinese hypertensives. Eur J Hum Genet. 2009;17:172–178. doi: 10.1038/ejhg.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronquist G, Soussi B, Frithz G, Schersten T, Waldenstrom A. Disturbed energy balance in skeletal muscle of patients with untreated primary hypertension. J Intern Med. 1995;238:167–174. doi: 10.1111/j.1365-2796.1995.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 22.Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 23.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 24.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 25.Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 26.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 28.Lappas G, Daou GB, Anand-Srivastava MB. Oxidative stress contributes to the enhanced expression of Gialpha proteins and adenylyl cyclase signaling in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens. 2005;23:2251–2261. doi: 10.1097/01.hjh.0000191905.26853.f1. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Anand-Srivastava MB. Inactivation of enhanced expression of G(i) proteins by pertussis toxin attenuates the development of high blood pressure in spontaneously hypertensive rats. Circ Res. 2002;91:247–254. doi: 10.1161/01.res.0000029969.39875.4b. [DOI] [PubMed] [Google Scholar]
- 30.Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H. G alpha(i) and G alpha(o) are target proteins of reactive oxygen species. Nature. 2000;408:492–495. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- 31.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 32.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 33.Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–253. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- 34.Arinze IJ, Kawai Y. Transcriptional activation of the human Galphai2 gene promoter through nuclear factor-kappaB and antioxidant response elements. J Biol Chem. 2005;280:9786–9795. doi: 10.1074/jbc.M414006200. [DOI] [PubMed] [Google Scholar]
- 35.Kost CK, Jr, Herzer WA, Li PJ, Jackson EK. Pertussis toxin-sensitive G-proteins and regulation of blood pressure in the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol. 1999;26:449–455. doi: 10.1046/j.1440-1681.1999.03058.x. [DOI] [PubMed] [Google Scholar]
- 36.Baritono E, Ceolotto G, Papparella I, Sartori M, Ciccariello L, Iori E, Calo L, Pessina AC, Semplicini A. Abnormal regulation of G protein alpha(i2) subunit in skin fibroblasts from insulin-resistant hypertensive individuals. J Hypertens. 2004;22:783–792. doi: 10.1097/00004872-200404000-00022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.