Abstract
The incidence of keratinocyte-derived nonmelanoma skin cancers is increasing worldwide because of cumulative recreational exposure to sunlight. At present, no specific molecular markers are available for assessing the progression of premalignant actinic keratoses to invasive cutaneous squamous cell carcinoma (SCC). We examined the role of the Serpin family in skin SCCs. Expression profiling of cutaneous SCC cell lines (n = 8) revealed up-regulation of SerpinA1 compared with normal epidermal keratinocytes (n = 5). Analysis with quantitative RT-PCR showed that the mean level of SerpinA1 mRNA was markedly up-regulated in cutaneous SCC cell lines (n = 8) compared with in normal keratinocytes. SerpinA1 production by SCC cells was dependent on p38 mitogen-activated protein kinase activity and was up-regulated by epidermal growth factor, tumor necrosis factor-α, interferon-γ, and IL-1β. Immunostaining of tissue arrays with 148 human tissue samples revealed tumor cell–associated expression of SerpinA1 in 19 of 36 actinic keratoses, 22 of 29 Bowen's disease samples, 67 of 71 sporadic SCCs, and all 12 recessive dystrophic epidermolysis bullosa–associated SCCs examined. Moreover, tumor cell–associated SerpinA1 staining was detected in all chemically induced mouse skin SCCs studied (n = 17). Overexpression of SerpinA1 mRNA was also detected by quantitative RT-PCR in chemically induced mouse skin SCCs (n = 14) compared with control tissues (n = 14). These data identify SerpinA1 as a novel tumor cell–associated biomarker for progression of cutaneous SCCs.
The incidence of melanoma and nonmelanoma skin cancer is increasing globally.1–3 Nonmelanoma skin cancers, including basal cell carcinoma (approximately 80%) and squamous cell carcinoma (SCC) (approximately 20%), are among the most common cancers worldwide, and SCC has been reported as the second most common cutaneous malignancy in the white population.1–3 Although early excision of cutaneous SCC is associated with a favorable outcome, for patients with metastatic disease (6%), the long-term prognosis is poor.4 Important risk factors for cutaneous SCC include exposure to UV radiation, immunosuppression, and chronic skin ulceration.1–3 An example of the latter is individuals with recessive dystrophic epidermolysis bullosa (RDEB), who often develop rapidly progressing cutaneous SCCs at sites of chronic ulceration and scarring.5,6 At present, no specific molecular markers for progression of cutaneous SCC are available. Such biomarkers would be valuable in clinical practice for early detection of individual cutaneous SCCs with a high risk of progression and metastasis.
Serine protease inhibitors (serpins) constitute the largest and most broadly distributed superfamily of protease inhibitors described in humans, with the two largest clades of the 36 serpins consisting of extracellular molecules “clade A” and intracellular serpins “clade B.”7,8 Serpin peptidase inhibitor clade A member 1 (SerpinA1), also known as α1-proteinase inhibitor or α1-antitrypsin (AAT), is a highly effective inhibitor of neutrophil elastase, which also inhibits the activity of plasmin, thrombin, trypsin, chymotrypsin, and plasminogen activator.7,8 Patients with AAT deficiency carry an increased risk of emphysema and liver disease.9 Another member of the serpin superfamily with medical importance is serpin peptidase inhibitor clade A member 3 (SerpinA3), also known as α1-antichymotrypsin (ACT), which displays inhibitory function toward neutrophil cathepsin G and mast cell chymase and serves as an inflammatory response molecule and an acute-phase reactant protein.7,8 Structural variants of ACT protein have been implicated in Alzheimer's disease,10,11 and combined deficiency of AAT and ACT increases the risk of chronic liver disease.12 Elevated expression of AAT is associated with the invasive and metastatic potential and poor prognosis in lung, colorectal, and gastric carcinoma.13–16 In addition, ACT is expressed at high levels in gastric and salivary gland cancer and in malignant melanoma.16–18
Herein, we examined Serpin expression in cutaneous SCCs. The results show that SerpinA1 is expressed by cutaneous SCC cells in culture and by tumor cells in SCCs of the skin. The level of expression is low in premalignant lesions of skin (actinic keratoses) and is clearly elevated in invasive cutaneous SCCs. In addition, tumor cell–associated SerpinA1 staining was detected in mouse skin SCCs. These results identify SerpinA1 as a novel tumor cell–associated diagnostic biomarker for progression of cutaneous SCC.
Materials and Methods
Ethical Issues
The use of archival tissue specimens and the collection of normal skin and SCC tissues was approved by the Ethics Committee of the Hospital District of Southwest Finland, Turku, Finland. Before surgery, each patient gave their informed consent, and the study was conducted according to the Declaration of Helsinki.
The animal experiments were approved by the State Provincial Office of Southern Finland.
Cell Cultures
Human cutaneous SCC cell lines (n = 8) were established from surgically removed SCCs of skin.19–21 SCC cells were cultured in Dulbecco's modified Eagle's medium supplemented with 6 mmol/L glutamine, nonessential amino acids, and 10% fetal calf serum.20,21 HaCaT, a spontaneously immortalized, nontumorigenic human epidermal keratinocyte–derived cell line,22 and the Ha-ras–transformed tumorigenic HaCaT cell lines A5, II4, and RT323,24 (a gift from Dr. Norbert E. Fusenig, German Cancer Research Center, Heidelberg, Germany) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. G418 (200 μg/mL) was added to the medium of the Ha-ras–transformed HaCaT cell lines.23,24 Normal human epidermal keratinocytes (NHEKs) were cultured from nonmalignant skin of patients (n = 4) undergoing surgery for mammoplasty at Turku University Hospital, Turku, Finland. In addition, normal keratinocytes were purchased from PromoCell (Heidelberg, Germany). Keratinocytes were cultured in keratinocyte basal medium 2, supplemented with SingleQuots (Cambrex Bioscience, Walkersville, MD), as previously described.20,21
Human Tissue Samples
Tissue collection was performed at the Department of Pathology, Turku University Hospital.6,25
Mouse Skin Chemical Carcinogenesis
Normal (n = 5), acetone-treated (n = 2), and hyperplastic skin (n = 6) and SCC (n = 17) samples were collected from skin of FVB/N HanHsd mice (maintained at the Laboratory Animal Centre, University of Oulu, Oulu, Finland). Mouse skin carcinogenesis was induced as previously described.26 Briefly, to induce hyperplasia, the shaved dorsal skin was treated four times, at 2-day intervals, with 5 μg of 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma-Aldrich, St Louis, MO,) in 100 μL of acetone. The control mice were treated in a similar way four times with acetone. To induce skin tumors, a single dose of 100 μg of 7,12-dimethylbenz[α]anthracene (DMBA, Sigma-Aldrich) in 100 μL of acetone was administered topically on the shaved dorsal skin, followed by weekly TPA treatments (5 μg in 100 μL of acetone) for 20 weeks. Mice were monitored weekly for the appearance of tumors and were sacrificed at the end of the experiment at week 32 or earlier when the tumor load was excessive, invasive carcinomas appeared, or the diameter of an individual tumor exceeded 10 mm. Approximately 10% of the benign papillomas progress to malignant SCCs in this model. Tumors and skin samples were removed and fixed in fresh phosphate-buffered 4% paraformaldehyde for 24 hours at 4°C and were embedded in paraffin. The TPA-treated skin and SCC samples were evaluated on the basis of H&E-stained sections.
RNA Extraction and cDNA Preparation
Twenty-four hours before the RNA extraction, the culture media of cells was replaced by serum-free culture media. Total RNA was isolated from cultured cells and tissue samples using the RNeasy kit (Qiagen, Chatworth, CA) according to the manufacturer's protocol. cDNA was reverse transcribed from 1 μg of RNA using reverse transcriptase M-MLV RNase H without reverse transcriptase and random hexamers (Promega, Helsinki, Finland).20,21
Affymetrix Microarray Analysis
The gene expression profiling of cutaneous SCC cell lines (n = 8) and NHEKs from healthy individuals (n = 5) for the entire serpin family was performed using Affymetrix Human U133 Plus 2.0 GeneChips (Affymetrix Inc., Santa Clara, CA) at the Finnish Microarray and Sequencing Centre, Turku Center for Biotechnology. Normalization of the arrays was performed using robust multiarray average assay, Chipster software (CSC, Espoo, Finland). The mean signal level of NHEKs was used as the control for the signal level of each cell line. The sequence specificity of Affymetrix probes was verified by Basic Local Alignment Search Tool search.
Quantitative Real-Time PCR
The primers and probes were designed based on human sequences for the genes tested and purchased from Oligomer (Helsinki, Finland): SerpinA1 forward primer, 5′-CACCGTGAAGGTGCCTATGATG-3′; SerpinA1 reverse primer, 5′-GGCATTGCCCAGGTATTTCATC-3′; SerpinA1 probe, 5′-Fam-TAGGCATGTTTAACATCCAGCACTGTA-Tamra-3′; SerpinA3 forward primer, 5′-GCATCACCTGACTATACCT-3′; SerpinA3 reverse primer, 5′-GCATTGCCTGTGTACTTC-3′; and SerpinA3 probe, 5′-Fam-TACTTCCGGGACGAGGAGCTGTC-BHQ1-3′. One set of primers and probe were designed for murine SerpinA1 mRNAs based on homologous sequence in the coding regions of five murine SerpinA1 genes (a-e): murine SerpinA1 forward primer, 5′-CCTCAACAGACCAGACAGTGA-3′; murine SerpinA1 reverse primer, 5′-CTCCACCAGCTTCAGGTCAT-3′; and murine SerpinA1 probe, 5′-Fam-CTGCAGCTGAGCACAGGCAATGG-BHQ1-3′.
RT-PCR was performed using an ABI 7700 sequence detector (Applied Biosystems, Warrington, UK) as outlined previously.20,21 The levels of β-actin and glyceraldehyde-3-phosphate dehydrogenase mRNA (as housekeeping genes) were determined as described previously.21
Western Blot Analysis
SerpinA1 production by cutaneous SCC cell lines and NHEKs was analyzed by Western blotting of conditioned culture media after fractionation by 10% SDS-PAGE27 using polyclonal rabbit anti-human AAT (SerpinA1; DakoCytomation, Glostrup, Denmark). Equal protein loading was ensured by Western blot analysis of corresponding cell lysates with an antibody against β-actin (A1978; Sigma-Aldrich).
Regulation of SerpinA1 Expression by SCC Cells
To investigate the regulation of SerpinA1 expression with inflammatory cytokines and growth factors, the cutaneous SCC cell line (UT-SCC-118) was cultured and serum starved for 24 hours before treatment with epidermal growth factor (EGF; 25 ng/mL) (Roche Diagnostics GmbH, Mannheim, Germany), tumor necrosis factor-α (TNF-α; 20 ng/mL) (Sigma-Aldrich), interferon-γ (IFN-γ; 100 U/mL) (Promega), and IL-1β (10 ng/mL) (Calbiochem, San Diego, CA). Total RNA was isolated and cDNAs were prepared for quantitative real-time PCR as described previously herein. Western blot analysis of the conditioned media for SerpinA1 was performed as described previously herein. As loading control, cell lysates were analyzed for β-actin by Western blotting, as mentioned previously herein.
To study the role of mitogen-activated protein kinase (MAPK) signaling on the expression of SerpinA1, SCC cell cultures were serum starved for 24 hours and then treated with MEK1/2 inhibitor PD98059 (30 μmol/L) or with p38 MAPK inhibitor SB203580 (10 μmol/L) (both from Calbiochem) for 24 hours. SerpinA1 levels in conditioned medium were determined as described previously herein. The functions of the inhibitors PD98059 and SB203580 were verified by Western blot analysis of the cell lysates with antibodies specific for phospho-ERK1/2 and phospho-CREB (both from Cell Signaling Technology Inc., Beverly, MA), respectively.25,27
IHC Analysis
The tissue used for immunohistochemical (IHC) staining consisted of archival formalin-fixed, paraffin-embedded (FFPE) tissue samples as follows: actinic keratosis (n = 36), Bowen's disease (n = 29), sporadic UV-induced cutaneous SCC (n = 71), and RDEB-associated cutaneous SCC (n = 12).6,25 To enhance the comparability and reliability of the IHC analysis results, we used an automated immunostaining device and the tissue microarray (TMA) technique. TMA blocks were generated from the original paraffin blocks using a tissue arrayer (Beecher Instruments Inc., Sun Prairie, WI), as previously described.28 Immunolabeling was performed on the 5-μm-thick TMA sections using an automated immunostaining device (Ventana Medical Systems SA, Illkirch, France) for analysis of the human tissue samples. Mouse skin SCC tumors and mouse control tissue samples were analyzed as whole sections. We used, as a primary antibody, polyclonal rabbit anti-human SerpinA1 antibody, which cross-reacts with rodent SerpinA1, 1:800 (ATT, code No. A0012; DakoCytomation). The primary antibody was detected using the Ventana ultraView Universal DAB detection kit and the Ventana amplification kit (Ventana Medical Systems SA). All the samples were examined independently by light microscopy by two observers (M.F. and A.K.). For semiquantitative evaluation of SerpinA1 immunostainings, membranous and cytoplasmic SerpinA1 stainings in tumor cells were considered positive. SerpinA1 immunostaining was scored semiquantitatively as follows: negative (−), weak (+), moderate (++), and strong (+++). For negative control stainings, the primary antibody was omitted and replaced by PBS.
Statistical Analysis
To evaluate the gene expression differences between the groups, we used Student's t-test. Statistical analysis of the immunostaining results was performed by comparing the differences in immunoreactivity between different tissue types using the nonparametrical Pearson χ2 test. For statistical analysis, semiquantitative immunostaining results were regrouped in a way that negative (−) and weak (+) staining results were considered as one group and moderate (++) and strong (+++) semiquantitative results were considered as the other group.
Results
SerpinA1 Expression Is Up-Regulated in Cutaneous SCC Cells
Expression profiling of the entire Serpin gene family in primary (n = 5) and metastatic (n = 3) cutaneous SCC cell lines and primary NHEKs from healthy individuals (n = 5) was performed using oligonucleotide-based microarrays. The analysis revealed expression of several distinct Serpin gene family members in NHEKs (Figure 1A). In addition, several serpin family members (SerpinA1, SerpinA3, SerpinB5, SerpinB6, SerpinE1, SerpinE2, and SerpinH1) were also expressed at high levels in primary and metastatic skin SCC cells. Comparison of the Serpin gene expression levels between epidermal keratinocytes and cutaneous SCC cell lines revealed elevated expression of SerpinA1 and SerpinA3 in SCC cells compared with that in normal keratinocytes (Figure 1A).
Figure 1.
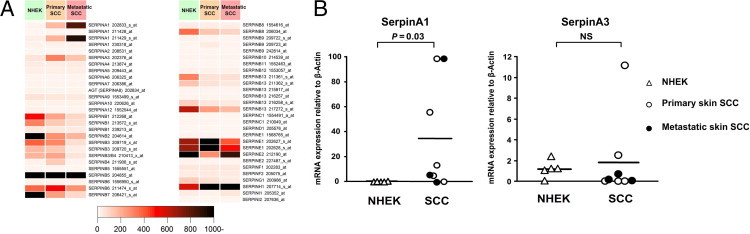
Overexpression of SerpinA1 in cutaneous SCC cell lines. A: Oligonucleotide array (Affymetrix)–based analysis of Serpin expression in primary (n = 5) and metastatic (n = 3) human cutaneous SCC cell lines and in primary NHEKs from different individuals (n = 5). B:SerpinA1 and SerpinA3 mRNA levels in SCC cell lines and normal keratinocytes, as in A, were quantified by real-time PCR, and the levels were corrected for β-actin mRNA levels in the same samples. Horizontal bars represent the mean SerpinA1 expression level for each group. Statistical significance was determined by Student's t-test. NS, not significant.
To verify the microarray-based results, the expression of SerpinA1 and SerpinA3 mRNAs in cutaneous SCC cells and NHEKs was analyzed by quantitative real-time PCR and was corrected for β-actin mRNA levels in the same samples. The results revealed marked and significant (50-fold; P = 0.03) up-regulation of SerpinA1 mRNA in cutaneous SCC cell lines compared with that in NHEKs (Figure 1B). In contrast, most cutaneous SCC cell lines showed low expression of SerpinA3 mRNA, whereas notable expression of SerpinA3 was observed in NHEKs (Figure 1B). No significant difference in the levels of SerpinA3 mRNA were detected between cutaneous SCC cells and NHEKs (P = 0.66).
SerpinA1 Production Is Up-Regulated in Cutaneous SCC Cells
Production of SerpinA1 protein by cutaneous SCC cells and epidermal keratinocytes was also examined by Western blot analysis of conditioned media of the cultures. Presence of the specific 51-kDa band corresponding to SerpinA1 was noted in aliquots of conditioned media of all cutaneous SCC cell lines (Figure 2A). In contrast, only one of five NHEK cell lines produced detectable levels of SerpinA1 (Figure 2A).
Figure 2.
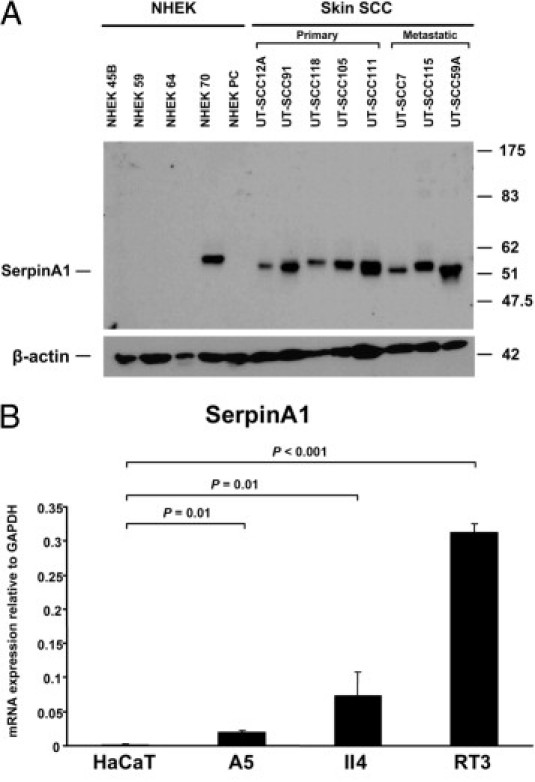
SerpinA1 overexpression in cutaneous SCC cells and tumorigenic ras-transformed HaCaT cell lines in culture. A: SerpinA1 levels in conditioned media of NHEKs and cutaneous SCC cell lines were determined by Western blot analysis. β-Actin levels were determined in the corresponding cell lysates as the loading control. Migration positions of molecular weight markers (in kilodaltons) are shown on the right. B:SerpinA1 mRNA levels were determined using quantitative real-time PCR in an immortalized nontumorigenic cell line (HaCaT) derived from NHEKs and in three Ha-ras–transformed HaCaT cell lines (A5, II4, and RT3). A5 is a benign tumorigenic HaCaT cell line, II4 forms invasive malignant tumors, and RT3 cells form metastatic SCCs in vivo. The mRNA levels were corrected for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels in the same samples (n = 4). Data are given as mean ± SD. Statistical difference was determined by Student's t-test.
Expression of SerpinA1 Correlates with Tumorigenic Potential of Transformed Keratinocytes
The previous results suggest that the expression of SerpinA1 in cutaneous SCC cells is induced as a result of malignant transformation of epidermal keratinocytes. To further investigate this hypothesis, we examined the expression of SerpinA1 mRNA in an immortalized nontumorigenic cell line (HaCaT) derived from human epidermal keratinocytes and in three Ha-ras–transformed HaCaT cell lines (A5, II4, and RT3), which represent an in vitro model for different stages of SCC tumor progression. Specifically, A5 is a benign tumorigenic HaCaT-derived cell line, II4 forms invasive malignant tumors, and RT3 cells form metastatic tumors in vivo.23,24 Nontumorigenic HaCaT cells, with inactivation of both alleles of p53 tumor suppressor, express low levels of SerpinA1 mRNA (Figure 2B). In contrast, significantly higher expression of SerpinA1 mRNA was noted in Ha-ras–transformed HaCaT cell lines, and the expression correlated with tumorigenic potential because it was lowest in the A5 cell line and highest in the RT3 cell line (Figure 2B). These results suggest that SerpinA1 serves as a marker for the malignant transformation of NHEKs to the aggressive and metastatic forms of SCC.
Expression of SerpinA1 by Cutaneous SCC Cells Is Enhanced by EGF, TNF-α, IFN-γ, and IL-1β
The histologic features of cutaneous SCC are characterized by infiltration of tumor microenvironment by inflammatory cells in the peritumoral area. To elucidate the regulation of SerpinA1 expression, we treated primary cutaneous SCC cells in culture for 24 hours with growth factors and cytokines (EGF, TNF-α, IFN-γ, and IL-1β) present in the tumor microenvironment of cutaneous SCCs. The mean expression level of SerpinA1 mRNA was markedly up-regulated in EGF-, TNF-α–, IFN-γ–, and IL-1β–treated cultures compared with untreated control cells, with IFN-γ showing the most potent effect (Figure 3A). The conditioned media of the corresponding cultures were also analyzed by Western blotting for the production of SerpinA1. Treatment of SCC cells with EGF, TNF-α, IFN-γ, or IL-1β resulted in potent enhancement of SerpinA1 production compared with untreated control cultures (Figure 3B).
Figure 3.
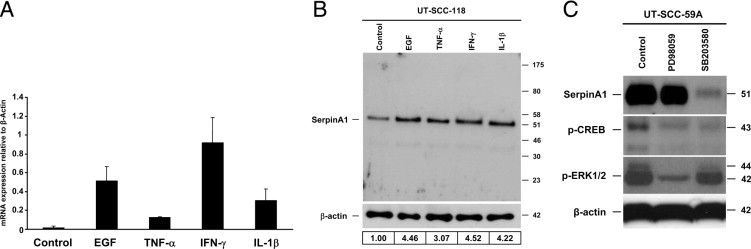
Up-regulation of SerpinA1 expression in cutaneous SCC cells by EGF, TNF-α, IFN-γ, IL-1β, and p38 signaling pathway. A: Cutaneous SCC cells (UT-SCC-59A) in culture were treated with EGF (25 ng/mL), TNF-α (20 ng/mL), IFN-γ (100 IU/mL), and IL-1β (10 ng/mL) for 24 hours. The expression levels of SerpinA1 mRNA were determined using quantitative RT-PCR, and the levels were corrected for β-actin mRNA in the same samples (n = 2). Data are given as mean ± SD. B: The conditioned media of the cultures in A were collected, and SerpinA1 levels were determined by Western blot analysis. The level of β-actin was determined in the corresponding cell lysates as an indicator of equal loading. The mean of SerpinA1 levels from three separate experiments are shown below the Western blots relative to levels in untreated control cells (1.00). Migration positions of molecular weight markers (in kilodaltons) are shown on the right. C: Cutaneous SCC cells (UT-SCC-59A) in culture were treated with MEK1/2 inhibitor PD98059 (30 μmol/L) and p38 inhibitor SB203580 (10 μmol/L) for 24 hours. The conditioned media were analyzed for SerpinA1 by Western blot analysis. Cell lysates were analyzed for the levels of phosphorylated CREB (p-CREB) and phosphorylated ERK1/2 (p-ERK1/2) to indicate the appropriate function of SB203580 and PD98059, respectively. The levels of β-actin were determined as a marker of the equal protein level.
SerpinA1 Expression by Cutaneous SCC Cells Is Regulated by p38 MAPK
To further investigate the regulation of SerpinA1 expression in cutaneous SCC cells, we treated primary cutaneous SCC cells with the inhibitor of MEK1/2 (PD98059) or the inhibitor of p38α and p38β MAPKs (SB203580) for 24 hours. Basal SerpinA1 expression by the cutaneous SCC cells was potently down-regulated by SB203580 compared with the untreated control cells (Figure 3C). In contrast, in parallel cultures treated with PD98059, production of SerpinA1 was not markedly different from that in untreated controls, although a potent reduction in ERK1/2 activation was noted as a marker for the effect of PD98059 (Figure 3C). These results implicate p38α MAPK in the regulation of SerpinA1 expression in cutaneous SCC cells because p38β is not expressed in cutaneous SCC cells.20
Expression of SerpinA1 by Tumor Cells in Human Cutaneous SCCs in Vivo Correlates with Tumor Progression
The results presented previously herein show that the expression of SerpinA1 is specifically up-regulated by tumor cells derived from cutaneous SCCs. To investigate the role of SerpinA1 in progression of skin SCCs in vivo, we first analyzed its expression in TMAs consisting of UV-induced premalignant lesions of skin (actinic keratoses) (n = 36), in situ SCCs of skin (Bowen's disease) (n = 29), and UV-induced sporadic cutaneous SCCs (n = 71) by IHC analysis. Positive immunostaining for SerpinA1 was noted in all human premalignant and malignant tissue types studied (actinic keratosis, Bowen's disease, and sporadic SCC), but the staining intensities varied between the tissue types, and the semiquantitative analysis revealed substantial up-regulation of SerpinA1, especially in invasive sporadic SCCs (Figure 4 and Table 1).
Figure 4.
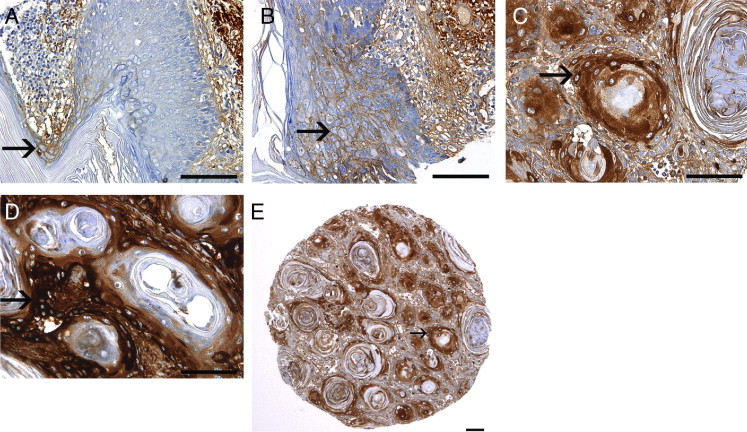
Expression of SerpinA1 by tumor cells in human cutaneous SCCs in vivo correlates with tumor progression. SerpinA1 expression was determined by IHC analysis in sections of TMAs consisting on UV-induced premalignant lesions (actinic keratoses), in situ SCCs (Bowen's disease), sporadic cutaneous SCCs, and RDEB-associated cutaneous SCCs, which represent clinically early metastasizing and aggressive forms of skin SCC. A: In actinic keratoses, SerpinA1 staining is typically either absent or weak (arrow). B: In Bowen's disease, weak membranous SerpinA1 immunostaining is noted (arrow). C: In sporadic UV-induced human cutaneous SCCs, clear cytoplasmic SerpinA1 staining is noted (arrow). D: Strongest SerpinA1 staining is detected in RDEB-associated cutaneous SCCs (arrow). E: Overview of the TMA sample of sporadic UV-induced human cutaneous SCCs shows clear cytoplasmic SerpinA1 staining in the tumor cells (arrow). Scale bars = 100 μm.
Table 1.
Increased Staining Intensity for SerpinA1 in Human Cutaneous SCCs
| SerpinA1 staining intensity |
||||
|---|---|---|---|---|
| Tissue sample | − | + | ++ | +++ |
| Actinic keratosis (n = 36) | 17 | 16 | 3 | 0 |
| Bowen's disease (n = 29) | 7 | 19 | 3 | 0 |
| Sporadic SCCs (n = 71) | 4 | 34 | 26 | 7 |
| RDEB-associated SCCs (n = 12) | 0 | 0 | 3 | 9 |
Number of tissue specimens staining positive for SerpinA1 by IHC analysis in UV-induced premalignant lesions (actinic keratosis), in situ SCC (Bowen's disease), UV-induced sporadic SCCs, and RDEB-associated SCCs. Intensity of immunostaining is scored as follows: negative (−), weak (+), moderate (++), and strong (+++). SerpinA1 staining intensity is significantly more abundant in sporadic SCCs than in Bowen's disease and actinic keratosis (P = 0.001, Pearson χ2 test) and in RDEB-associated SCCs than in sporadic SCCs (P = 0.002, Pearson χ2 test).
The weakest SerpinA1 staining was observed in premalignant epithelial cells in actinic keratosis; in 33 of 36 cases (92%), SerpinA1 staining was negative (−) or weak (+) (Figure 4A and Table 1). Here, the immunostaining for SerpinA1 was analyzed in the actual premalignant cells, whereas stromal staining for SerpinA1 in the dermal layer was ignored because this staining mainly represents serum-derived AAT produced in the liver (Figure 4, A and B). In the tumor cells of Bowen's disease samples, SerpinA1 staining was either negative or weak in 26 of 29 cases (90%) (Figure 4B). A trend toward stronger SerpinA1 staining in Bowen's disease compared with actinic keratosis was noted because only 24% of Bowen's disease samples were negative for SerpinA1 whereas 47% of actinic keratoses were negative. However, there was no statistically significant difference between actinic keratoses and Bowen's disease when all staining intensities were taken into account (P > 0.05) (Table 1). In sporadic cutaneous SCCs, moderate (++) or strong (+++) cytoplasmic staining for SerpinA1 was noted in the tumor cells in 33 of 71 cases (46%) (Figure 4, C and E). SerpinA1 staining was significantly more abundant in invasive sporadic SCCs than in in situ Bowen's disease and actinic keratosis (P = 0.001) (Table 1).
SerpinA1 expression was also examined in RDEB-associated SCCs, a subset of cutaneous SCCs characterized by aggressive behavior. The most abundant SerpinA1 expression of all SCCs examined was noted in RDEB-associated SCCs because there was clear or strong cytoplasmic staining in all 12 cases (100%) studied (Figure 4D). Moreover, up-regulation of SerpinA1 expression was significantly more abundant in RDEB-associated SCCs than in sporadic SCCs (P = 0.002) (Table 1).
SerpinA1 Expression Correlates with Progression of Mouse Skin SCCs
To gain further insight into the role of SerpinA1 in the progression of cutaneous SCCs, we used the well-characterized mouse model of chemically induced skin carcinogenesis.26 Immunostaining of the normal mouse skin, vehicle (acetone)-treated mouse skin, hyperplastic mouse skin, and chemically induced cutaneous mouse SCC was performed using specific anti-human SerpinA1 antibody, which cross-reacts with murine SerpinA1. No SerpinA1 expression was noted in epidermal keratinocytes in normal untreated control skin or in vehicle-treated skin (n = 5; Figure 5, A and B, and Table 2). Focal immunostaining detected in the acellular superficial epidermal layer (ie, stratum corneum) was considered nonspecific (Figure 5A). As a positive internal control, SerpinA1 immunostaining was noted in the dermal layer of mouse skin, representing serum-derived AAT (Figure 5, A–C). The dermal layer in the negative-control stained region remained blank (Figure 5E). In TPA-treated mouse skin characterized by epidermal hyperplasia (n = 6), weak staining for SerpinA1 was noted in the keratinocytes (Figure 5C and Table 2). Prominent staining for SerpinA1 was detected in tumor cells in all mouse cutaneous SCCs studied, although the staining intensity varied from weak to strong (n = 17; Figure 5D and Table 2). The SerpinA1 immunostaining was distributed mainly in the center of the tumor in 8 of 17 cases (47%) and throughout the tumor in 9 of 17 cases (53%). Staining for SerpinA1 in SCC tumor cells was significantly stronger compared with that in all nonmalignant mouse skin samples as a group (P = 0.002). These results provide evidence of a similarly important role for SerpinA1 as a biomarker in progression of human and murine cutaneous SCCs.
Figure 5.
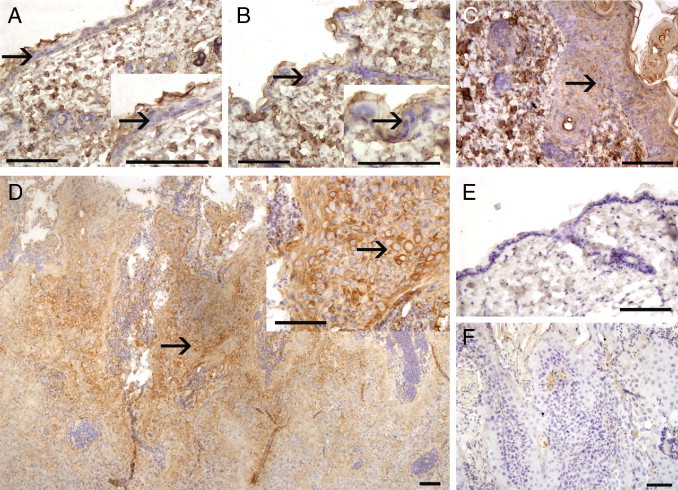
SerpinA1 expression correlates with progression of chemically induced mouse skin SCCs. A: In untreated control mouse skin, epidermal keratinocytes do not express SerpinA1 (arrow), whereas nonspecific staining is noted in the acellular superficial epidermal layer (stratum corneum). Higher magnification of the same area is shown in the inset. B: Treatment with vehicle (acetone) did not cause any histologic changes in mouse skin and did not induce SerpinA1 expression in epidermal keratinocytes (arrow). Nonspecific staining in the stratum corneum is detected. Higher magnification of the same area is shown in the inset. C: Treatment of mouse skin with TPA four times induced hyperplasia of the epidermis, and, typically, weak staining of keratinocytes for SerpinA1 was detected (arrow). D: Significant up-regulation of SerpinA1 expression was noted in DMBA-TPA–induced mouse skin SCC compared with the preceding nonmalignant samples. Clear cytoplasmic staining for SerpinA1 was noted in the mouse SCC tumor cells (arrow). Overview of the whole section shows that immunopositive cells are distributed mainly throughout the tumor. Higher magnification of the same area is shown in the inset. E: Negative control staining of untreated mouse skin. F: Negative control staining of DMBA-TPA–induced mouse skin SCC. Scale bars = 100 μm.
Table 2.
Increased Staining Intensity for SerpinA1 in Chemically Induced SCCs in Mouse Skin
| SerpinA1 staining intensity |
||||
|---|---|---|---|---|
| Tissue sample | − | + | ++ | +++ |
| Untreated mouse skin (n = 5) | 5 | 0 | 0 | 0 |
| Acetone-treated mouse skin (n = 2) | 2 | 0 | 0 | 0 |
| TPA-treated mouse skin (n = 6) | 1 | 3 | 2 | 0 |
| Mouse SCC (n = 17) | 0 | 6 | 9 | 2 |
Number of tissue specimens staining positive for SerpinA1 by IHC analysis in untreated mouse skin, vehicle (acetone)-treated skin, TPA-treated hyperplastic skin, and SCCs. Intensity of immunostaining is scored as follows: negative (−), weak (+), moderate (++), and strong (+++). SerpinA1 staining intensity is significantly more abundant in mouse SCC than in nonmalignant skin samples (P = 0.002, Pearson χ2 test).
To verify the IHC analysis findings, RNA samples derived from DMBA-TPA–treated mouse skin SCC (n = 14), hyperplastic TPA-treated skin (n = 6), and untreated control skin (n = 8) as control were analyzed using quantitative RT-PCR for SerpinA1 mRNA expression. Accordingly, the mean expression level of the SerpinA1 mRNA was significantly higher in chemically induced mouse skin SCCs than in normal or phorbol ester–treated hyperplastic skin (Figure 6). No significant difference was detected between control and TPA-treated skin.
Figure 6.
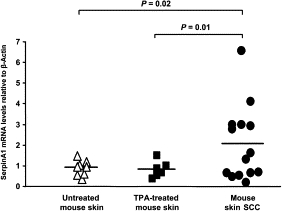
Overexpression of SerpinA1 in chemically induced mouse skin SCCs. SerpinA1 mRNA levels in RNAs from untreated control mouse skin (n = 8), TPA-treated hyperplastic skin (n = 6), and DMBA-TPA–induced mouse skin SCC (n = 14) were quantified by real-time PCR, and the levels were corrected for murine β-actin mRNA levels in the same samples. Statistical significance was determined by Student's t-test. Horizontal bars represent the mean SerpinA1 expression level for each group. Mean ± SD values: control, 0.93 ± 0. 36; TPA, 0.84 ± 0.39; and SCC, 2.08 ± 1.81.
Discussion
Serpins constitute the largest and most broadly distributed superfamily of protease inhibitors described in humans. Members of this family, such as AAT, ACT, C1 inhibitor, and antithrombin, play a pivotal role in coagulation, inflammation, and turnover of extracellular matrix.7,8 Among the members of the serpin family, SerpinA1 and SerpinA3 have been reported to be overexpressed in various malignant tumors. High expression of the SerpinA1 gene has been found in papillary thyroid cancer,29,30 lung and prostate cancer,14,31 and colorectal adenocarcinoma.15 In a recent study, SerpinA1 was among 129 genes whose elevated expression was associated with progression of esophageal squamous dysplasia.32 Elevated levels of AAT have been detected in the serum of patients with SCC of the oral cavity.33 Furthermore, expression of SerpinA3 has been documented in lung adenocarcinoma34 and in tandem with SerpinA1 in human leukocyte antigen–positive cervical carcinoma.35
In this study, in an effort to discover novel biomarkers for the progression of cutaneous SCC, we analyzed expression of the entire serpin family in skin SCC cells using an oligonucleotide-based microarray technique. These results showed that SerpinA1 is markedly overexpressed in primary and metastatic cutaneous SCC cell lines compared with NHEKs. The overexpression of SerpinA1 mRNA was verified by quantitative real-time PCR analysis of RNAs derived from cutaneous SCC cell lines. The enhanced production of SerpinA1 protein by cutaneous SCC cells in culture was detected by Western immunoblotting. In addition, IHC analysis of TMAs consisting of a large panel (altogether 148) FFPE tissue samples from premalignant and malignant cutaneous skin tumors confirmed the findings on tumor cell–associated expression of SerpinA1 in cutaneous SCCs. Moreover, staining for SerpinA1 was weak or absent in premalignant lesions. Also, the IHC analysis using TMAs consisting of RDEB-associated cutaneous SCC tumors revealed strong tumor cell–associated SerpinA1 staining. The RDEB-associated SCC represents one of the most aggressive types of SCC, which makes the strong SerpinA1 staining in this subset of skin SCC particularly interesting. Based on these observations, we propose SerpinA1 as a novel biologic marker for the progression of cutaneous SCC.
We undertook two different experimental approaches to assess SerpinA1 as a putative marker for cutaneous carcinogenesis. First, we used an in vitro model of epidermal carcinogenesis and noted that the level of SerpinA1 expression increases, progressing from the epidermal keratinocyte–derived cell lines from immortalized nontumorigenic keratinocytes (HaCaT) (lower) toward Ha-ras–transformed HaCaT cell lines (higher). In addition, the level of SerpinA1 expression in Ha-ras–transformed HaCaT cell lines correlated with their tumorigenic potential, being lowest in the benign tumorigenic cell line (A5) and highest in RT3 cells, which form metastatic SCCs in vivo.23,24 These results also show that inactivation of p53 tumor suppressor, an early event in UV-induced epidermal carcinogenesis, is not sufficient to induce SerpinA1 expression in HaCaT cells, whereas additional activation of ras signaling pathway enhances basal SerpinA1 expression. Second, examination of DMBA-TPA–induced mouse skin SCCs, which harbor an activating H-ras1 mutation in addition to a p53 mutation,36 revealed prominent SerpinA1 expression in invasive SCCs and low expression in normal skin and hyperplastic skin lesions. The results of these two experiments imply that expression of SerpinA1 correlates with the malignant transformation of epidermal keratinocytes and provide further evidence of SerpinA1 as a biomarker for cutaneous SCC progression.
Infiltration of the tumor microenvironment with inflammatory cells is a typical histologic feature of cutaneous SCCs.1–3 Cytokines released by the inflammatory cells may promote SCC invasion and progression by stimulating the production of invasion-associated proteinases by tumor cells. Moreover, production of certain cytokines by tumor cells has been shown to regulate the local immune response against tumor cells.37,38 In the present study, we noted that the expression of SerpinA1 is enhanced by EGF and three distinct cytokines: TNF-α, IFN-γ, and IL-1β. In addition, we noted that the basal expression of SerpinA1 in SCC cells is regulated by the p38 MAPK signaling pathway. These findings are interesting with respect to previous studies showing the role of p38 MAPKs in growth and invasion of cutaneous SCCs.20,39
Although the mechanistic role of SerpinA1 in tumorigenesis is not fully understood, previous studies have identified certain tumorigenic properties for this protein. An antiapoptotic effect of AAT on lung endothelial cells via the inhibition of caspase-3 activity has been documented.40 In addition, AAT has been shown to inhibit natural killer cell activity.41,42 Another possible tumorigenic mechanism is the mitogenic action of C-terminal 26-residue peptide of SerpinA1, which has been shown to stimulate malignant cell proliferation with no significant effect on normal epithelial cells.42,43
Although primary cutaneous SCCs can usually be effectively treated by surgical excision, they harbor the potential for recurrence and metastasis, with the high possibility of morbidity and mortality.1–3 Because early diagnosis and excision of SCC is highly curable, prompt detection and screening are potentially lifesaving for patients. Particularly, among patients with a history and at high risk for skin cancer, regular screening is necessary to monitor the recurrence or persistence of the tumors.1–4 At present, only a few specific biomarkers for cutaneous SCC have been identified, including STAT3,44 E-cadherin,45 and matrix metalloproteinases 13,46 12,47 and 7.6,48 SerpinA1 has been previously suggested as a biomarker for the progression of insulinoma49 and thyroid cancer.50 In this study, we introduced SerpinA1 as a potential biomarker for the progression of cutaneous SCCs combined with other biomarkers mentioned previously herein.
In conclusion, the present findings show that the expression of SerpinA1 (AAT) is specifically up-regulated by cutaneous SCC cells in culture and in vivo. We also present evidence that the expression of SerpinA1 correlates with the malignant transformation of epidermal keratinocytes and their progression to invasive SCC. These results suggest that SerpinA1 could serve as a novel biomarker for the diagnosis of rapidly progressing cutaneous SCCs.
Acknowledgments
We thank Sari Pitkänen, Johanna Markola-Wärn, Sinikka Kollanus, and Sini Reponen for their skillful technical assistance; and Drs. John A. McGrath, Johann W. Bauer, Radana Königová, Márta Medvecz, Wolfgang Beckert, and Kathrin Sinemus for providing RDEB-associated SCC tissue samples.
Footnotes
Supported by grants from the Academy of Finland (projects 114409, 130224, and 137687), Turku University Hospital (projects 13336 and 13010), the Dystrophic Epidermolysis Bullosa Research Association, the Sigrid Jusélius Foundation, the Cancer Research Foundation of Finland, The Southwestern Finland Cancer Societies, and personal grants from the Centre for International Mobility, the Turku University Foundation, and the Finnish Cultural Foundation (M.F.) and from the Finnish Dermatological Society, the Finnish Society of Dermatopathology, and the K. Albin Johansson Foundation (A.K.).
A.K. and P.R. are doctoral students in the National Graduate School of Clinical Investigation (CLIGS).
M.F. and A.K. contributed equally to this work.
References
- 1.Madan V., Lear J.T., Szeimies R.M. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 2.Rogers H.W., Weinstock M.A., Harris A.R., Hinckley M.R., Feldman S.R., Fleischer A.B., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Alam M., Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 4.Neville J.A., Welch E., Leffell D.J. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol. 2007;4:462–469. doi: 10.1038/ncponc0883. [DOI] [PubMed] [Google Scholar]
- 5.McGrath J.A., Schofield O.M., Mayou B.J., McKee P.H., Eady R.A. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116–123. doi: 10.1111/j.1600-0560.1992.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 6.Kivisaari A.K., Kallajoki M., Mirtti T., McGrath J.A., Bauer J.W., Weber F., Konigova R., Sawamura D., Sato-Matsumura K.C., Shimizu H., Csikos M., Sinemus K., Beckert W., Kähäri V.M. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778–785. doi: 10.1111/j.1365-2133.2008.08466.x. [DOI] [PubMed] [Google Scholar]
- 7.Silverman G.A., Bird P.I., Carrell R.W., Church F.C., Coughlin P.B., Gettins P.G., Irving J.A., Lomas D.A., Luke C.J., Moyer R.W., Pemberton P.A., Remold-O'Donnell E., Salvesen G.S., Travis J., Whisstock J.C. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 8.Law R.H., Zhang Q., McGowan S., Buckle A.M., Silverman G.A., Wong W., Rosado C.J., Langendorf C.G., Pike R.N., Bird P.I., Whisstock J.C. An overview of the serpin superfamily. Genome Biol. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrell R.W., Lomas D.A. Alpha1-antitrypsin deficiency: a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 10.Abraham C.R., Selkoe D.J., Potter H. Immunochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- 11.Kamboh M.I., Minster R.L., Kenney M., Ozturk A., Desai P.P., Kammerer C.M., DeKosky S.T. Alpha-1-antichymotrypsin (ACT or SERPINA3) polymorphism may affect age-at-onset and disease duration of Alzheimer's disease. Neurobiol Aging. 2006;27:1435–1439. doi: 10.1016/j.neurobiolaging.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon D., Kueppers F., Genta R.M., Klintmalm G.B., Khaoustov V.I., Yoffe B. Role of alpha-1-antichymotrypsin deficiency in promoting cirrhosis in two siblings with heterozygous alpha-1-antitrypsin deficiency phenotype SZ. Gut. 2002;50:730–732. doi: 10.1136/gut.50.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashiyama M., Doi O., Kodama K., Yokouchi H., Tateishi R. An evaluation of the prognostic significance of alpha-1-antitrypsin expression in adenocarcinomas of the lung: an immunohistochemical analysis. Br J Cancer. 1992;65:300–302. doi: 10.1038/bjc.1992.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karashima S., Kataoka H., Itoh H., Maruyama R., Koono M. Prognostic significance of alpha-1-antitrypsin in early stage of colorectal carcinomas. Int J Cancer. 1990;45:244–250. doi: 10.1002/ijc.2910450207. [DOI] [PubMed] [Google Scholar]
- 15.Tahara E., Ito H., Taniyama K., Yokozaki H., Hata J. Alpha 1-antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin in human gastric carcinomas: a retrospective immunohistochemical study. Hum Pathol. 1984;15:957–964. doi: 10.1016/s0046-8177(84)80125-2. [DOI] [PubMed] [Google Scholar]
- 16.Allgayer H., Babic R., Grutzner K.U., Beyer B.C., Tarabichi A., Schildberg F.W., Heiss M.M. Tumor-associated proteases and inhibitors in gastric cancer: analysis of prognostic impact and individual risk protease patterns. Clin Exp Metastasis. 1998;16:62–73. doi: 10.1023/a:1006564002679. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Jiang H., Dai D., Su M., Martinka M., Brasher P., Zhang Y., McLean D., Zhang J., Ip W., Li G., Zhang X., Zhou Y. Alpha 1 antichymotrypsin is aberrantly expressed during melanoma progression and predicts poor survival for patients with metastatic melanoma. Pigment Cell Melanoma Res. 2010;23:575–578. doi: 10.1111/j.1755-148X.2010.00715.x. [DOI] [PubMed] [Google Scholar]
- 18.Chomette G., Auriol M., Vaillant J.M., Kasai T., Niwa M., Mori M. An immunohistochemical study of the distribution of lysozyme, lactoferrin, alpha 1-antitrypsin and alpha 1-antichymotrypsin in salivary adenoid cystic carcinoma. Pathol Res Pract. 1991;187:1001–1008. doi: 10.1016/s0344-0338(11)81072-1. [DOI] [PubMed] [Google Scholar]
- 19.Lansdorf C.D., Grénman R., Bier H., Somers K.D., Kim S.Y., Whiteside T.L., Clayman G.L., Welkoborsky H.-J., Carey T. Cancer Cell Lines. In: Masters J., Palsson B., editors. Kluwer Academic Press; Dordrecht, Holland: 1999. pp. 185–255. [Google Scholar]
- 20.Junttila M.R., Ala-Aho R., Jokilehto T., Peltonen J., Kallajoki M., Grenman R., Jaakkola P., Westermarck J., Kähäri V.M. p38α and p38δ mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene. 2007;26:5267–5279. doi: 10.1038/sj.onc.1210332. [DOI] [PubMed] [Google Scholar]
- 21.Stokes A., Joutsa J., Ala-aho R., Pitchers M., Pennington C.J., Martin C., Premachandra D.J., Okada Y., Peltonen J., Grénman R., James H.A., Edwards D.R., Kähäri V.M. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16:2022–2035. doi: 10.1158/1078-0432.CCR-09-2525. [DOI] [PubMed] [Google Scholar]
- 22.Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boukamp P., Stanbridge E.J., Foo D.Y., Cerutti P.A., Fusenig N.E. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990;50:2840–2847. [PubMed] [Google Scholar]
- 24.Mueller M.M., Peter W., Mappes M., Huelsen A., Steinbauer H., Boukamp P., Vaccariello M., Garlick J., Fusenig N.E. Tumor progression of skin carcinoma cells in vivo promoted by clonal selection, mutagenesis, and autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Am J Pathol. 2001;159:1567–1579. doi: 10.1016/S0002-9440(10)62541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivisaari A.K., Kallajoki M., Ala-aho R., McGrath J.A., Bauer JW Königová R., Medvecz M., Beckert W., Grénman R., Kähäri V.M. Matrix metalloproteinase-7 activates heparin-binding epidermal growth factor-like growth factor in cutaneous squamous cell carcinoma. Br J Dermatol. 2010;163:726–735. doi: 10.1111/j.1365-2133.2010.09924.x. [DOI] [PubMed] [Google Scholar]
- 26.Brideau G., Mäkinen M.J., Elamaa H., Tu H., Nilsson G., Alitalo K., Pihlajaniemi T., Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67:11528–11535. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- 27.Ala-aho R., Johansson N., Grenman R., Fusenig N.E., Lopez-Otin C., Kähäri V.M. Inhibition of collagenase-3 (MMP-13) expression in transformed human keratinocytes by interferon-γ is associated with activation of extracellular signal-regulated kinase-1,2 and STAT1. Oncogene. 2000;19:248–257. doi: 10.1038/sj.onc.1203306. [DOI] [PubMed] [Google Scholar]
- 28.Kononen J., Bubendorf L., Kallioniemi A., Bärlund M., Schraml P., Leighton S., Torhorst J., Mihatsch M.J., Sauter G., Kallioniemi O.P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 29.Poblete M.T., Nualart F., del Pozo M., Perez J.A., Figueroa C.D. Alpha 1-antitrypsin expression in human thyroid papillary carcinoma. Am J Surg Pathol. 1996;20:956–963. doi: 10.1097/00000478-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Jarzab B., Wiench M., Fujarewicz K., Simek K., Jarzab M., Oczko-Wojciechowska M., Wloch J., Czarniecka A., Chmielik E., Lange D., Pawlaczek A., Szpak S., Gubala E., Swierniak A. Gene expression profile of papillary thyroid cancer: sources of variability and diagnostic implications. Cancer Res. 2005;65:1587–1597. doi: 10.1158/0008-5472.CAN-04-3078. [DOI] [PubMed] [Google Scholar]
- 31.El-Akawi Z.J., Al-Hindawi F.K., Bashir N.A. Alpha-1 antitrypsin (alpha1-AT) plasma levels in lung, prostate and breast cancer patients. Neuro Endocrinol Lett. 2008;29:482–484. [PubMed] [Google Scholar]
- 32.Joshi N., Johnson L.L., Wei W.Q., Abnet C.C., Dong Z.W., Taylor P.R., Limburg P.J., Dawsey S.M., Hawk E.T., Qiao Y.L., Kirsch I.R. Gene expression differences in normal esophageal mucosa associated with regression and progression of mild and moderate squamous dysplasia in a high-risk Chinese population. Cancer Res. 2006;66:6851–6860. doi: 10.1158/0008-5472.CAN-06-0662. [DOI] [PubMed] [Google Scholar]
- 33.Shirasuna K., Sugiyama M., Watatani K., Morioka S., Hayashido Y. Serum alpha-1-antitrypsin in patients with malignant tumors occurring in the oral region. Int J Oral Maxillofac Surg. 1987;16:516–520. doi: 10.1016/s0901-5027(87)80096-6. [DOI] [PubMed] [Google Scholar]
- 34.Higashiyama M., Doi O., Yokouchi H., Kodama K., Nakamori S., Tateishi R. Alpha-1-antichymotrypsin expression in lung adenocarcinoma and its possible association with tumor progression. Cancer. 1995;76:1368–1376. doi: 10.1002/1097-0142(19951015)76:8<1368::aid-cncr2820760812>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Kloth J.N., Gorter A., Fleuren G.J., Oosting J., Uljee S., ter Haar N., Dreef E.J., Kenter G.G., Jordanova E.S. Elevated expression of SerpinA1 and SerpinA3 in HLA-positive cervical carcinoma. J Pathol. 2008;215:222–230. doi: 10.1002/path.2347. [DOI] [PubMed] [Google Scholar]
- 36.Abel E.L., Angel J.M., Kiguchi K., DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin. fundamentals and applications. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.H., Jang J.J., Lee J.Y., Kim S.Y., Park W.S., Shin M.S., Dong S.M., Na E.Y., Kim K.M., Kim C.S., Kim S.H., Yoo N.J. Fas ligand is expressed in normal skin and in some cutaneous malignancies. Br J Dermatol. 1998;139:186–191. doi: 10.1046/j.1365-2133.1998.02353.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim J., Modlin R.L., Moy R.L., Dubinett S.M., McHugh T., Nickoloff B.J., Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas: a mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 39.Johansson N., Ala-aho R., Uitto V., Grénman R., Fusenig N.E., Lopez-Otin C., Kähäri V.M. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000;113:227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 40.Petrache I., Fijalkowska I., Medler T.R., Skirball J., Cruz P., Zhen L., Petrache H.I., Flotte T.R., Tuder R.M. α-1 Antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laine A., Leroy A., Hachulla E., Davril M., Dessaint J.P. Comparison of the effects of purified human alpha 1-antichymotrypsin and alpha 1-proteinase inhibitor on NK cytotoxicity: only alpha 1-proteinase inhibitor inhibits natural killing. Clin Chim Acta. 1990;190:163–173. doi: 10.1016/0009-8981(90)90170-w. [DOI] [PubMed] [Google Scholar]
- 42.Zelvyte I., Stevens T., Westin U., Janciauskiene S. α1-Antitrypsin and its C-terminal fragment attenuate effects of degranulated neutrophil-conditioned medium on lung cancer HCC cells, in vitro. Cancer Cell Int. 2004;4:7. doi: 10.1186/1475-2867-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Congote L.F., Temmel N. The C-terminal 26-residue peptide of serpin A1 stimulates proliferation of breast and liver cancer cells: role of protein kinase C and CD47. FEBS Lett. 2004;576:343–347. doi: 10.1016/j.febslet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 44.Suiqing C., Min Z., Lirong C. Overexpression of phosphorylated-STAT3 correlated with the invasion and metastasis of cutaneous squamous cell carcinoma. J Dermatol. 2005;32:354–360. doi: 10.1111/j.1346-8138.2005.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 45.Koseki S., Aoki T., Ansai S., Hozumi Y., Mitsuhashi Y., Kondo S. An immunohistochemical study of E-cadherin expression in human squamous cell carcinoma of the skin: relationship between decreased expression of E-cadherin in the primary lesion and regional lymph node metastasis. J Dermatol. 1999;26:416–422. doi: 10.1111/j.1346-8138.1999.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 46.Airola K., Johansson N., Kariniemi A.L., Kähäri V.M., Saarialho-Kere U.K. Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J Invest Dermatol. 1997;109:225–231. doi: 10.1111/1523-1747.ep12319441. [DOI] [PubMed] [Google Scholar]
- 47.Kerkelä E., Ala-aho R., Jeskanen L., Rechardt O., Grénman R., Shapiro S.D., Kähäri V.-M., Saarialho-Kere U. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114:1113–1119. doi: 10.1046/j.1523-1747.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 48.Impola U., Jeskanen L., Ravanti L., Syrjänen S., Baldursson B., Kähäri V.M., Saarialho-Kere U. Expression of matrix metalloproteinase (MMP)-7 and MMP-13 and loss of MMP-19 and p16 are associated with malignant progression in chronic wounds. Br J Dermatol. 2005;152:720–726. doi: 10.1111/j.1365-2133.2005.06447.x. [DOI] [PubMed] [Google Scholar]
- 49.de Sá S.V., Corrëa-Giannella M.L., Machado M.C., Krogh K., de Almeida M.Q., Albergaria Pereira M.A., Coelho Siqueira S.A., Patzina R.A., Ibuki F.S., Sogayar M.C., Giannella-Neto D. Serpin peptidase inhibitor clade A member 1 as a potential marker for malignancy in insulinomas. Clin Cancer Res. 2007;13:5322–5330. doi: 10.1158/1078-0432.CCR-06-1477. [DOI] [PubMed] [Google Scholar]
- 50.Griffith O.L., Melck A., Jones S.J., Wiseman S.M. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24:5043–5051. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]


