Abstract
Treatment options for inflammatory bowel disease (IBD) are incompletely helpful, and surgery is often needed. One promising class of future therapeutic agents for IBD is melanocortin-related peptides, which exhibit potent immunomodulatory effects. We investigated KdPT, a tripeptide derivative of the C-terminus of α–melanocyte-stimulating hormone, as an anti-inflammatory small molecule in vivo and in vitro. Intestinal inflammation was studied after oral administration of dextran sodium sulfate and in IL-10 gene–deficient mice. The effects of KdPT on key colonic epithelial cell functions were studied in vitro and in vivo by evaluating proliferation, wound healing, transepithelial resistance, and expression of tight junction proteins. Melanin assays were performed to determine the melanotropic effects of KdPT. KdPT-treated animals showed markedly reduced severity of inflammation in both colitis models. In colonic epithelial cells, KdPT increased proliferation, accelerated closure of wounds, and improved transepithelial electrical resistance after stimulation with interferon-γ/tumor necrosis factor-α. Moreover, treatment with KdPT also prevented the loss of tight junction protein expression and improved barrier function in vivo. KdPT acted independently of IL-1 receptor type I in vivo and did not affect melanogenesis in vitro. KdPT is capable of attenuating the course of experimental colitis in different models and maintains epithelial cell function. Furthermore, KdPT does not induce pigmentation, emphasizing the potential of this small molecule for the future treatment of IBD.
Inflammatory bowel disease (IBD) is an umbrella term comprising the two main entities Crohn's disease and ulcerative colitis. In the United States, IBD is among the five most prevalent gastrointestinal diseases, costing approximately $1.7 billion annually. An estimated 1.4 million Americans were diagnosed as having IBD in 2005.1 Commonly, IBD shows chronic, uncontrolled inflammation of the mucosa.2 Although great research efforts have been made regarding the pathogenetic understanding of IBD, its etiology is still not completely understood. However, a breakdown of epithelial barrier function seems to play a crucial role.
Most therapeutic strategies for IBD target immunosuppression to inhibit mucosal inflammation. These strategies include mesalazine, budesonide, prednisolone, azathioprine, methotrexate, and antibodies toward tumor necrosis factor-α (TNF-α).3 Despite these therapeutic options, 70% of patients with Crohn's disease will need surgery at least once in their lifetime. In contrast, ulcerative colitis can be cured by resection of the colon and rectum. However, note that the risk of complications (eg, pouchitis) rises up to 50%.4,5 In addition, many of the previously mentioned agents have adverse effects, such as bone marrow suppression, liver toxicity, and increased risk of infections or osteoporosis. Therefore, novel therapeutic approaches are needed.
KdPT is a tripeptide that is structurally similar to the three C-terminal amino acids of α–melanocyte-stimulating hormone (α-MSH) and KPV, which are thought to mediate many of the anti-inflammatory effects of α-MSH.6 α-MSH belongs to the family of melanocortins, which elicit their effects via melanocortin receptors (MC-Rs).7,8 We previously showed that MC-1R has a crucial role in intestinal inflammation.9 Among the intracellular target molecules that mediate the anti-inflammatory effects of α-MSH is the transcription factor NF-κB, whose activation by proinflammatory cytokines, such as IL-1β and TNF-α, is attenuated.10,11 Similarly, a substantial body of data demonstrates that KPV exerts anti-inflammatory effects.12 We recently showed that KPV has salutary effects in experimental colitis and acts partially independently of MC-1R.13 These data were confirmed by others, and it was also proposed that the reduction of intestinal inflammation by KPV is mediated through an H+-coupled oligopeptide transporter, called PepT1, located at the apical membrane of intestinal epithelial cells.14
In contrast to α-MSH and KPV, few data exist on KdPT. In 1988, Ferreira et al15 demonstrated that KdPT suppresses IL-1β–induced pain in the hind paws of the rat. Because the L-enantiomer of KdPT is homologous to the amino acids 193 to 195 of IL-1β, an antagonistic interaction between KdPT and IL-1β was suggested.16,17 Regarding the anti-inflammatory potential of KdPT, no further studies have been conducted to our knowledge. Preliminary data from our group, however, suggest that KdPT suppresses experimentally induced contact dermatitis in mice more effectively than does KPV.18 Herein, we provide evidence that KdPT has potent concentration-dependent anti-inflammatory effects in two different models of experimental colitis. In addition, we demonstrate various anti-inflammatory effects on colonic epithelial cells in vitro and in vivo, suggesting that KdPT might be beneficial for the treatment of intestinal inflammatory disorders by stabilizing intestinal barrier function.
Materials and Methods
Animals
C57BL/6 WT mice were purchased from Charles River Laboratories, Sulzfeld, Germany. In initial experiments, no major sex-specific differences could be detected in the course of colitis, so the experiments described herein were conducted using female mice for practical reasons only. The animals were 6 to 8 weeks old at the beginning of the experiments.
Homologous IL-10 gene–deficient mice (strain name: B6.129P2-Il10tm1Cgn/J) were purchased from The Jackson Laboratory (Bar Harbor, ME). In the experiments described herein, female mice were 8 to 9 weeks old when they received treatment with piroxicam, a nonsteroidal anti-inflammatory drug. Signaling-deficient MC-1R mice (MC1Re/e) on a C57BL/6 background were a gift from Roger D. Cone (Vollum Institute and the Center for Weight Regulation and Associated Disorders, Oregon Health and Science University, Portland, OR). Owing to a single nucleotide deletion in position 549, the MC-1R protein from this animal is out of frame, resulting in premature termination of the protein after the fourth transmembrane domain. No major sex-specific differences were reported in the course of colitis or its severity,19 so the experiments described herein were performed using female mice for practical reasons only. B6.129S7-Il1r1tm1Imx/J mice and corresponding control animals were purchased from The Jackson Laboratory. The experiments described herein were performed with 10- to 12-week-old female mice.
All the animals were kept under pathogen-free conditions at 24°C with a controlled 12-hour day-night cycle and had free access to a standard diet and drinking water. The animal studies were approved by the local animal subjects committee at the University of Münster (permit G92/2002).
DSS–Induced Colitis
Dextran sodium sulfate (DSS)–induced colitis was accomplished as described previously.9,13 In brief, mice were given 3% DSS (ICN Biomedicals Inc., Eschwege, Germany) in drinking water for 5 days, and disease activity was monitored daily by body weight measurement. Afterward, the mice received regular drinking water again to monitor the improvement in inflammation. PBS-diluted KdPT (Bachem Americas Inc., Torrance, CA) was given daily by i.p. injection, by oral gavage, or locally by rectal application in various concentrations until the end of the experiment. Control mice received PBS only.
At the end of the experiment, the mice were sacrificed and the colons removed. The colons were opened, embedded in Tissue-Tek O.C.T. (Sukura Finetek Europe, Zoeterwoude, The Netherlands), and kept frozen at −80°C until further use. Sections (5 μm) were stained with H&E and were analyzed by two blinded investigators (D.B. and C.M.).
Induction of Colitis in IL-10−/− Mice
The targeted disruption of the IL-10 gene in mice leads to spontaneous development of chronic enterocolitis.20,21 To aggravate the developing colitis, piroxicam was given on days 1 to 7 (60 mg/250 g of food) and on days 15 to 22 (80 mg/250 g of food).22 Piroxicam was mixed with rodent chow. PBS-diluted KdPT was given daily by oral administration beginning on day 0 before the start of piroxicam administration until the end of the experiment. Control mice received PBS only. Disease activity was monitored daily by body weight measurement. At the end of the experiment, mice were sacrificed and the colons removed. Colons were opened, embedded in O.C.T., and kept frozen at −80°C until further use. Sections (5 μm) were stained with H&E and were analyzed by two blinded investigators (D.B. and A.L.).
Detection of IL-1β Expression
In distal colon samples, IL-1β was measured by real-time RT-PCR. RNA was isolated by TRIzol (Invitrogen, Carlsbad, CA), followed by DNA digestion with DNase (Stratagene, La Jolla, CA) and reverse transcription using 1 μL of SuperScript II reverse transcriptase (Invitrogen) per sample. Amplification of IL-1β was performed with 40 cycles and the sense 5′-CAGGATGAGGACATGAGCACC-3′ and antisense 5′-CTCTGCAGACTCAAACTCCAC-3′ primers, generating a 455-bp product. The amplification cycles consisted of 1 minute of denaturation at 95°C, 1 minute of annealing at 60°C, and 1 minute of extension at 72°C. β-Actin was amplified with 35 cycles and the sense 5′-CAAAGACCTGTACGCCAACAC-3′ and antisense 5′CATACTCCT GCTTGCTGATCC-3′ primers. The quantitative detection of IL-1β was performed by delta CT values using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA).
Ki-67 Immunohistochemical Analysis
Five-micrometer colonic tissue sections were blocked (1% rabbit serum in PBS) for 1 hour and incubated overnight at 4°C with a diluted (1:100) primary rat anti-mouse Ki-67 monoclonal antibody (TEC-3; Dako, Copenhagen, Denmark). After washing with PBS, the sections were incubated for 30 minutes with biotinylated anti-rat IgG antibody diluted 1:100 in 1% rabbit serum for 1 hour at room temperature, rinsed, and reacted with fluorescein isothiocyanate–conjugated streptavidin (Vector Laboratories, Burlingame, CA) for 60 minutes. DAPI was used for nuclear counterstaining. To assess proliferation activity, the number of Ki-67–positive cells per total crypt epithelial cells was counted. The Ki-67 labeling index was defined as the percentage of Ki-67–positive cells per crypt.23 All the sections were analyzed by two blinded investigators (D.B. and A.L.).
Cell Culture
Normal human melanocytes (PromoCell, Heidelberg, Germany) were maintained in MGM-M2 plus all supplements (Cascade Biologics, Portland, OR). HT-29 (passage 21), Caco-2, and T84 cells (passages 22 to 28) were cultured in Dulbecco's modified Eagle's medium (Gibco-Invitrogen, Cergy-Pontoise, France) supplemented with 20% fetal bovine serum (Gibco-Invitrogen), 1% nonessential amino acids, and 1% penicillin/streptomycin in a 5% CO2, 95% humidity environment at 37°C.
Cells were seeded on Costar Transwell membrane inserts with 0.4-μm pores (Corning, New York, NY) at a density of 5 × 104 cells/cm2 for transport measurements or on 24-well plastic culture dishes for uptake experiments. Three days after seeding, the medium was changed daily. The experiments were conducted on day 17 of culture if not specified otherwise.
In Vitro KdPT Uptake Studies
On the day of the uptake experiment, the medium was removed and monolayers were washed three times with a Krebs' modified buffer at pH 5, 6, or 7.4. Cells were then incubated for 5 minutes with 0.5 mL of a 20 μmol/L Gly-Sar solution containing 0.4 μCi/mL 3H–Gly-Sar (Isobio, Fleurus, Belgium) (specific activity, 0.5 Ci/mmol) or a 20 μmol/L KdPT solution containing 0.4 μCi/3H-KdPT (Isobio) as a tracer with or without Gly-Gly (Sigma-Aldrich, St Louis, MO), KPdT, and KPV (Bachem) or Gly-Sar (Sigma-Aldrich), Gly-Pro (Sigma-Aldrich) as potential competitors. At the end of incubation, cells were washed three times in ice-cold buffer. Radioactivity was determined by liquid scintillation counting, and the results are expressed as disintegrations per minute per square centimeter per 5 minutes or as disintegrations per minute per gram per milliliter.
Transport Experiments
On the day of the transport experiment, the medium was removed and apical and basolateral compartments were washed three times with a Krebs' modified buffer at pH 6 or 7.4, respectively, at 37°C or 4°C. Transepithelial electrical resistance (TER) was measured in each well using an EVOM epithelial voltohmmeter (World Precision Instruments, Saratosa, FL). No monolayer was used if the TER value was <150 Ω/cm2. Cells were then incubated for 15 minutes with gentle circular shaking. After incubation, the apical buffer was removed and filled with 0.5 mL of 20 μmol/L KdPT solution containing 0.4 μCi/mL 3H-KdPT (Isobio) (specific activity, 0.4 Ci/mmol) as a tracer with or without 50 mmol/L of Gly-Gly (Sigma-Aldrich), KPdT (Bachem), or Gly-Sar (Sigma-Aldrich) or Gly-Pro (Sigma-Aldrich) as potential competitors. Basolateral compartments were sampled at 0, 5, 10, 15, 20, 25, and 30 minutes, and 3H-KdPT concentration was calculated after measuring radioactivity using a β-counter (Beckman LS 6000 TA liquid scintillation counter; Beckman Coulter, Fullerton, CA). Apparent permeability (Papp) was used to describe Gly-Sar or KdPT transport across the monolayer following the equation Papp = (dC/dt).(V/C0.A), where V is the volume of the basolateral compartment, C0 is the initial drug concentration in the apical compartment chamber, A is the surface of the membrane, and dC/dt is the permeability rate (plot slope of the concentration in the receiver chamber versus time).
Everted Intestinal Rings Uptake Assays
Uptake of (3H)KdPT or (3H)Gly-Sar by everted intestinal rings was determined as previously described.24 Briefly, animals were anesthetized, and the entire intestine from the duodenum to the colon was quickly excised, rinsed with ice-cold saline solution, everted, and cut into 1-cm pieces. Rings from the duodenum, the jejunum, the ileum, and the colon were incubated for 5 minutes in MES buffer, pH 6, in a shaking water bath at 37°C, with continuous bubbling with 95% oxygen. The buffer contained 1 μCi/(3H)KdPT ± 170 mmol/L of a mixture of Gly-Gly and Gly-Pro(94:6) or 0.5 μCi/mL (3H)Gly-Sar ± 170 mmol/L of a mixture of Gly-Gly and Gly-Pro. After the incubation period, rings were washed three times in ice-cold saline solution and weighed. Radioactivity incorporated into the tissue was determined by liquid scintillation counting after solubilization of the tissues in Soluene (Beckman) overnight at 56°C. Results are expressed as disintegration per minute per gram (dpm g−1) tissue wet weight.
Mechanically Induced Cell Layer Wound Assays
In vitro wound assays were performed using a modified method first described by Burk.25 Confluent monolayers of Caco-2 cells grown in 60-mm plastic dishes (Falcon; Becton Dickinson, Mount View, CA) were cut using a razor blade. Two wounds (20 to 25 mm) at a distance of 10 mm were established per dish. Cells were washed twice with PBS to remove residual cell debris. Wounded monolayers were then cultured for 24 hours in fresh serum-deprived medium (0.1% fetal bovine serum) with or without KdPT at various concentrations. After methylene blue staining, the number of cells across the wound border was expressed as the mean number of cells that crossed the wound border in a standardized wound area. Wound areas were standardized by taking photographs at 200-fold magnification using a DM LB microscope with a DC 300F Leica camera (Leica Microsystems Schweiz, Heerbrugg, Switzerland). Experiments were performed in triplicate, and several wound areas per plate were used to quantify migration.
MTS Cell Proliferation/Viability Assay
The CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega, Mannheim, Germany) was used to determine the number of viable cells in culture. Caco-2 cells were seeded into 96-well microplates containing routine growth medium. The plates were preincubated with or without KdPT at concentrations from 10−5 to 10−11 mol/L for 24 hours at 37°C. MTS reagent was added, and cell cultures were incubated at 37°C for 4 hours. Absorbance was recorded at 490 nm.
TER Assays after Cytokine Stimulation
T84 epithelial cells (American Type Culture Collection, Manassas, VA) were grown in 1:1 Dulbecco's modified Eagle's medium and Ham's F-12 medium supplemented with 15 mmol/L HEPES (pH 7.5), 14 mmol/L NaHCO3, antibiotics, and 6% NCS.26 Cells were then seeded on collagen-coated, permeable polycarbonate filters (5-μm pore size) with surface areas of 5 cm2 (Costar, Cambridge, MA), as described previously. TNF-α (10 ng/mL; Genzyme, Cambridge, MA) and interferon-γ (IFN-γ) (100 U/mL; Genentech, San Francisco, CA) were added basolaterally to monolayers. T84 cells were co-stimulated with KdPT at various concentrations. All the monolayers were checked for high TER every 24 hours using an epithelial voltohmmeter.
Immunofluorescence of Tight Junction Proteins
On day 10 after the start of DSS application and on day 31 after the start of KdPT application in IL-10–deficient mice, frozen sections (5 μm) with histologically intact epithelium were collected on coated slides, dried overnight, fixed in acetone for 3 minutes, and dried for 30 minutes. Fixed sections were washed in PBS and blocked in PBS with 2% bovine serum albumin (Sigma-Aldrich) for 30 minutes. Slides were incubated with the primary antibody for 30 to 60 minutes at room temperature (rabbit anti–ZO-1, rabbit anti-occludin, rabbit anti–claudin-1, rabbit anti–claudin-3, and rabbit anti–claudin-5; Zytomed Systems GmbH, Berlin, Germany). Slides were washed three times with PBS and were incubated for 1 hour with the appropriate secondary antibody [Alexa Fluor 488–conjugated goat anti-rabbit Ig (Invitrogen)]. Slides were washed, and nuclei were counterstained with DAPI (Sigma-Aldrich). Stained sections were mounted using ProLong Gold antifade (Invitrogen). Samples were imaged using a Nikon epifluorescence microscope (Eclipse E800; Nikon Instruments, Melville, NY) connected to a Nikon FDX-35 camera (Nikon Instruments).
In Vivo Permeability to Evans Blue
An isoflurane-N2O anesthesia laparotomy was performed, and a small polyethylene tube (G22) was inserted into the proximal colon ascendens (immediately adjacent to the cecum) and secured by a ligature. Via this tube, the colon was gently flushed until all stool was rinsed out. Evans blue (Sigma-Aldrich) was instilled into the colon and was left in place for 15 minutes. The colon was rinsed with PBS until the perianal washout was clear. The animals were euthanized, and the colon was rapidly taken out. It was rinsed again, followed by 1 mL of 6 mmol/L N-acetylcysteine to eliminate dye sticking to the colonic mucus. The colon was opened and rinsed once more with PBS. The whole colon was placed in 2 mL of N, N-dimethylformamide for 12 hours to extract the Evans blue dye. Dye concentration in the supernatant was measured spectrophotometrically at 610 nm and was given as extinction per gram colonic tissue.27,28
Melanin Assays
Normal human melanocytes (1 to 1.5 × 105 cells) were seeded into 3.5-cm tissue culture plates. After deprivation from bovine pituitary extract for 48 hours, cells were treated with KdPT at 10−6 to 10−10 mol/L. As a positive control, bovine pituitary extract was used. After 5 days, cells were detached by trypsinization and centrifuged. Cell pellets were dissolved in 1 N of NaOH, and melanin concentration was determined by measuring the OD at 405 nm in relation to a standard curve generated by synthetic melanin (Sigma-Aldrich).
Statistical Analysis
For statistical analysis of body weight, mean ± SE body weights were determined each day. To test the results for significance, a two-way analysis of variance test was applied where suitable. A P < 0.05 was considered significant.
Results
KdPT Attenuates DSS-Induced Colitis
In the first set of experiments, the effect of i.p.-administered KdPT was investigated. For evaluation of the prophylactic potency, mice received 10 μg of KdPT i.p. beginning 2 days before DSS application for 13 days until the end of the experiment, and control animals received PBS. At the beginning of the experiment, the mean ± SE body weight of all the animals was 20.4 ± 0.53 g. Progressive weight loss was observed in both groups starting on day 6 after KdPT application (Figure 1A). KdPT-treated animals, however, lost significantly less body weight (mean ± SE maximum: 17.5% ± 1.78%) compared with PBS-treated mice (mean ± SE maximum: 27.9% ± 1.41%; P < 0.05). All the animals survived DSS treatment. Histologic examination on day 13 after starting KdPT application showed less epithelial disintegration and denudation, ulceration, edema, and muscular thickening and fewer immune cell infiltrates and lymphatic follicles in KdPT-treated mice than in controls (Figure 1B). Histologic scoring according to the method of Dieleman et al29 confirmed a significantly higher mean ± SE score in control mice (25.8 ± 2.25 AU) versus KdPT-treated animals (9.0 ± 3.29 AU; P < 0.003; Figure 1C).
Figure 1.
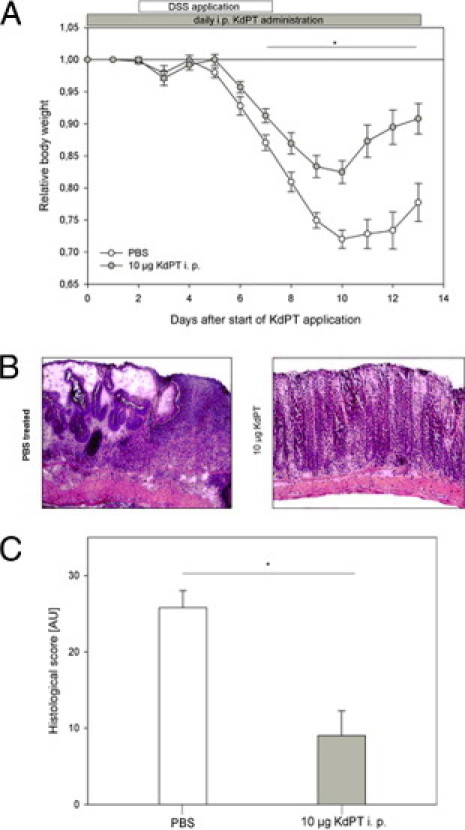
KdPT ameliorates DSS-induced colitis. C57BL/6 WT mice received 3% DSS in their drinking water for 5 days, and inflammation was monitored by daily measurement of individual weights. A: From day 2 onward, one group was treated with 10 μg of KdPT i.p. daily, and control animals received an equivalent volume of PBS i.p. Data are mean ± SE; n = 5 for each group. *P < 0.05. B: Representative histologic images of control mice and KdPT-treated animals 13 days after induction of colitis. C: Histologic scores of colonic tissue according to the method of Dieleman et al29 of control mice and KdPT-treated animals 13 days after induction of colitis. Data are mean ± SE; n = 5 for each group. *P < 0.003.
Next, the effect of KdPT on colonic inflammation was tested in a therapeutic setting and at various concentrations. Mice received 0.5, 5, 10, 25, or 50 μg of KdPT i.p. daily from day 2 after starting DSS for 9 days until the end of the experiment, and control animals received PBS (Figure 2A). Animals treated with 5, 10, or 25 μg of KdPT i.p. daily showed a significantly reduced loss of body weight and recovered significantly earlier from colitis than did controls. For example, the mean ± SE body weight of all animals treated with 10 μg of KdPT i.p. daily was 17.1 ± 0.46 g at the beginning of the experiment. Body weight dropped in both groups starting on day 6 after DSS application. KdPT-treated animals again exhibited significantly less reduction in body weight (mean ± SE maximum: 11.6% ± 3.41%) compared with that of PBS-treated mice (mean ± SE maximum: 22.6% ± 2.88%; P < 0.05). None of the treated animals died, and histologic analysis was performed on sacrificed mice on day 10 after DSS. In accordance with the reduced body weight drop, KdPT-treated mice had less epithelial disintegration with ulcerations, edema, and muscular thickening; fewer immune cell infiltrates and lymphatic follicles; and smaller areas of epithelial denudation (mean ± SE score: 13.5 ± 1.8 AU) than did PBS controls (mean ± SE score: 25.5 ± 2.3 AU; Figure 2B). Moreover, IL-1β mRNA expression was reduced approximately threefold in mucosal samples of KdPT-treated mice (P < 0.01; data not shown). Treatment with 0.5 μg of KdPT i.p. daily did not attenuate the course of DSS-induced colitis, whereas the group treated with 50 μg of KdPT i.p. daily even developed significantly higher loss of body weight than did control animals (data not shown).
Figure 2.
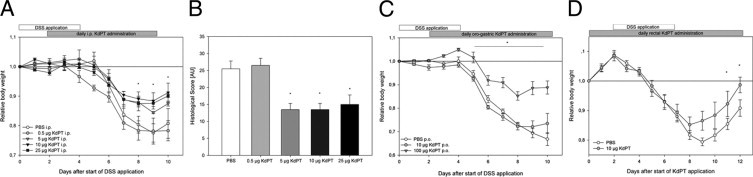
Effect of KdPT at various concentrations after i.p. administration and after oral and rectal application in DSS-induced colitis. C57BL/6 mice received various amounts of KdPT by daily i.p. injections starting 2 days after induction of DSS colitis. Inflammation was monitored by individual body weight. A: Overall weight loss was significantly less intense in mice receiving 5 and 25 μg of KdPT daily, whereas lower concentrations were not effective. Data are mean ± SE; n = 5 for each group. *P < 0.05. B: Histologic scores of colonic tissue according to the method of Dieleman et al29 in control mice and animals treated with different doses of KdPT i.p. Note that KdPT doses of <5 μg of KdPT per day did not ameliorate DSS-induced colitis. Data are mean ± SE; n = 5 to 7 for each group. *P < 0.05. C: Impact of oral administration of KdPT on DSS-induced colitis. C57BL/6 mice received 10 or 100 μg of KdPT by oral gavage 2 days after starting DSS treatment. Note that only 100 μg was effective. Data are mean ± SE; n = 5 for each group. *P < 0.05. D: Rectal application of KdPT (10 μg) improves DSS-induced colitis. Data are mean ± SE; n = 5 for each group. *P < 0.05.
In another set of experiments, we investigated whether administration of KdPT by oral gavage might have anti-inflammatory effects on DSS-induced colitis (Figure 2C). For this purpose, mice were treated with 10, 100, and 500 μg of KdPT p.o. daily beginning on day 2 after the start of DSS application. Treatment with 10 μg of KdPT did not alter the course of colitis. In contrast, mice treated with 100 μg of KdPT lost significantly less body weight than did controls (P < 0.05). Treatment with higher dosages of KdPT, eg, 500 μg daily, aggravated the course of colitis, as indicated by an increased loss of body weight (data not shown).
Finally, we checked whether local intraluminal application of KdPT had anti-inflammatory effects in DSS-induced colitis (Figure 2D). Mice received 10 μg of KdPT rectally by gavage needle on day 2 before starting DSS until day 12. The initial mean ± SE body weight of all the animals was 18.5 ± 0.37 g, and it dropped in KdPT- and PBS-treated mice. KdPT-treated mice lost a mean ± SE maximum of 14.8% ± 2.62% of their initial weight, whereas controls lost 20.7% ± 1.31% at most (P < 0.05; Figure 2D). In accordance with the weight data, histologic scoring of colonic tissue of sacrificed mice (day 12) revealed significantly less inflammation in KdPT-treated animals (mean ± SE: 2.4 ± 1.83 AU) than in controls (mean ± SE: 20.8 ± 2.8 AU; P < 0.04).
Taken together, these findings highlight a significant anti-inflammatory action of KdPT in experimentally induced colitis. When applied i.p., doses of 10 to 25 μg of KdPT per day are sufficient to attenuate the course of DSS-induced colitis (Figure 2A). To elicit beneficial effects of KdPT on DSS-induced colitis by oral administration, higher doses, ie, beginning at 100 μg, are needed.
KdPT Attenuates Colitis in Nonsteroidal Anti-Inflammatory Drug–Treated IL-10–Deficient Mice
To extend the previous findings, we used another model of experimental colitis. IL-10–deficient mice are known to develop a spontaneous colitis that can be aggravated by oral administration of a nonsteroidal drug, such as piroxicam.22 Accordingly, IL-10–deficient mice received an effective oral dose of KdPT (100 μg daily) beginning on day 0 after administration of piroxicam until the end of the experiment on day 31, and control animals received PBS. Immediately after starting the first piroxicam treatment, all the placebo-treated animals showed progressive weight loss up to a mean ± SE of 19.9% ± 3.8% on day 27. However, the KdPT-treated group exhibited a significantly less pronounced loss of body weight of a mean ± SE of 8.4% ± 2.9% (P < 0.05) on day 27, confirming the protective effect of KdPT regarding intestinal inflammation (Figure 3A).
Figure 3.
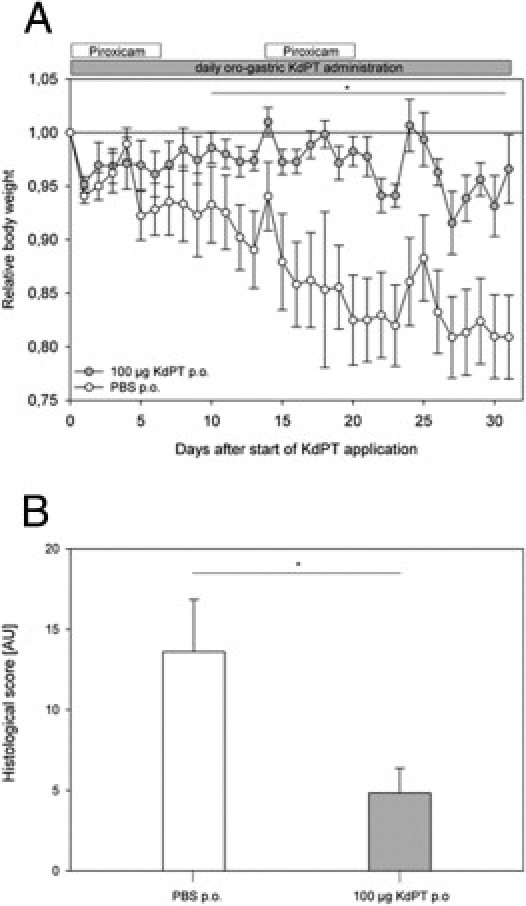
KdPT attenuates colitis in nonsteroidal anti-inflammatory drug–treated IL-10–deficient mice. A: IL-10–deficient mice orally received 100 μg of KdPT per day beginning on day 0 after administration of piroxicam until the end of the experiment on day 31, and control animals received PBS. Note the progressive weight loss in PBS-treated animals compared with KdPT-treated mice. Data are mean ± SE; n = 7 mice. *P < 0.05. B: Histologic scores of colonic tissue in PBS- and KdPT-treated animals after 31 days. Data are mean ± SE; n = 7 per group. *P < 0.01.
Histologic examination on day 31 after starting KdPT application revealed less severe inflammation with reduced involvement of the submucosa and a lower number of crypt abscesses in KdPT-treated animals than in controls (Figure 3B). Histologic scoring according to Berg et al30 confirmed a significantly higher mean ± SE score in control mice (13.6 ± 3.2 AU) versus KdPT-treated animals (4.8 ± 1.5 AU; P < 0.01).
KdPT Stabilizes Intestinal Barrier Function
Intestinal epithelial cells provide an effective barrier that is crucially involved in the pathogenesis of intestinal inflammation. Because regeneration of the epithelial cell lining is important for recovery from intestinal inflammation, we first studied the effect of KdPT on epithelial cell proliferation in vivo and in vitro. Therefore, the number of Ki-67–immunoreactive cells in colonic tissue was assessed as a proliferative cell marker, determined after KdPT administration in DSS-treated and IL-10–deficient mice. KdPT, given either i.p. or p.o., dramatically increased the number of Ki-67–immunoreactive cells compared with control (P < 0.05; Figure 4) in both models tested, whereas no effects of KdPT were determined in control mice (data not shown). We also performed MTS assays to determine directly the impact of KdPT on cell proliferation and viability in vitro: stimulation of Caco-2 cells with 10−7 and 10−9 mol/L KdPT for 24 hours increased MTS activity by approximately mean ± SE 43% ± 6.3% and 64% ± 19%, respectively (P < 0.05; Figure 5A).
Figure 4.
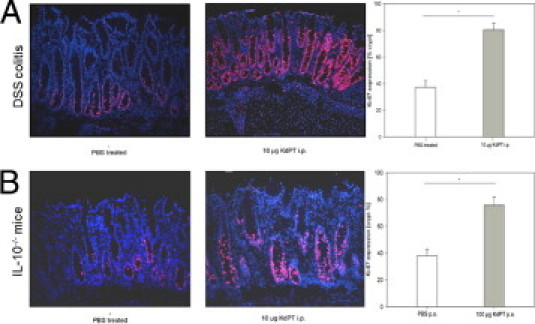
Induction of cell proliferation after stimulation with KdPT in DSS-treated and IL-10−/− mice. Immunohistochemical analysis of the cell proliferation marker Ki-67 in colonic tissue of PBS-treated mice and KdPT-treated animals 13 days after induction of DSS colitis (A) and in IL-10−/− mice 31 days after piroxicam treatment (B). Data are mean ± SE; n = 5 for each group. *P < 0.05.
Figure 5.
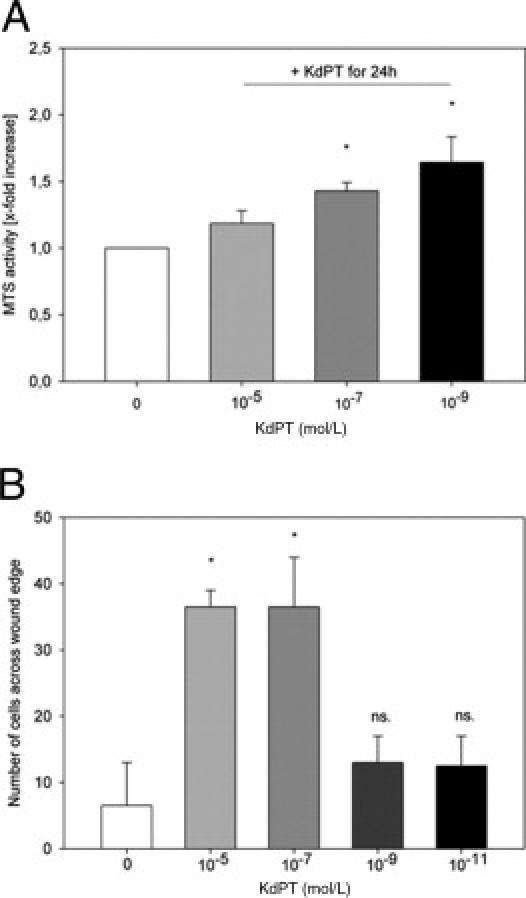
Induction of cell proliferation after stimulation with KdPT in vitro. A: MTS activity in Caco-2 cells after stimulation with KdPT. Cells were stimulated with KdPT at the doses indicated for 24 hours. The number of viable Caco-2 cells after treatment with 10−7 and 10−9 mol/L KdPT was significantly increased compared with cells incubated with medium alone (mean ± SE relative increase: 1.43 ± 0.06 and 1.64 ± 0.19, respectively) Data are mean ± SE; n = 6, *P < 0.05. B: Increased wound healing in Caco-2 monolayers after stimulation with KdPT. A defined wound was set, and cells were stimulated with medium or medium plus KdPT at various concentrations. Migration of Caco-2 cells after in vitro wounding and KdPT treatment as determined by cell counting in a standardized viewing field. Data are mean ± SE. Independent experiments (n = 5) were performed in quadruplicate. *P < 0.05.
To further characterize the effect of KdPT on epithelial cell injury, we analyzed the effect of KdPT on mechanically induced cell monolayer wounds. After setting a defined wound to a monolayer of Caco-2 cells, KdPT at 10−5 and 10−7 mol/L significantly accelerated wound healing within 24 hours compared with control (*P < 0.05; Figure 5B).
KdPT Stabilizes Tight Junction Proteins in Vitro and in Vivo
Because the proinflammatory cytokines IFN-γ and TNF-α have been reported to break down the epithelial barrier function by affecting tight junction protein localization, we next analyzed the effects of KdPT on IFN-γ/TNF-α–treated T84 epithelial monolayers.26 Confluent T84 monolayers grown on permeable supports were incubated with KdPT after IFN-γ/TNF-α stimulation, and TER was measured every 24 hours for 96 hours. At all time points tested, KdPT-treated T84 cells maintained significantly better resistance after IFN-γ/TNF-α stimulation, suggesting that KdPT also targets tight junction protein localization in epithelial cells (Figure 6A).
Figure 6.
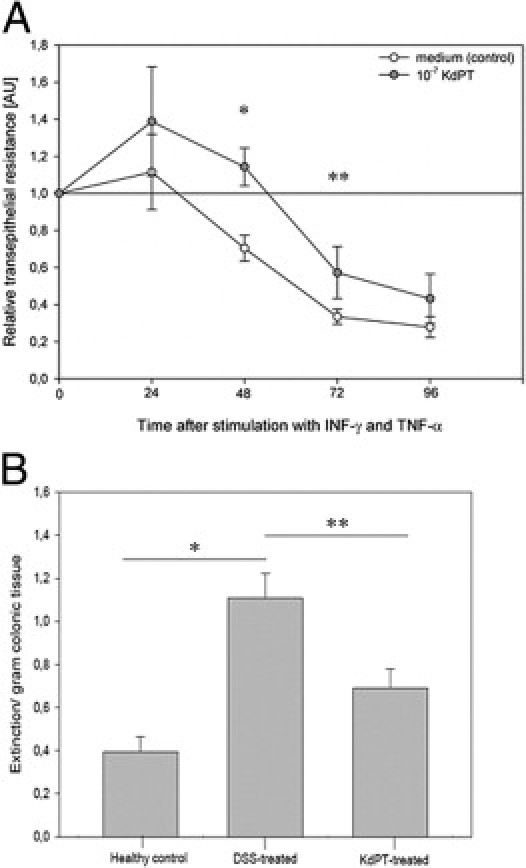
KdPT increases TER in colonic epithelial cells. A: TER in epithelial cells (T84) decreases after stimulation with IFN-γ/TNF-α. After 48 hours, mean ± SE TER was 70.4% ± 7%, and co-stimulation with KdPT significantly increased TER (110% ± 10%). Data are mean ± SE; n = 6. *P = 0.01, 48 hours; **P = 0.04, 72 hours. B: KdPT prevents increased colonic epithelial permeability in acute DSS-induced colitis. To assess the permeability of the colonic epithelium, the colon was perfused with Evans blue in vivo for 15 minutes, and its uptake into the mucosa was quantified spectrophotometrically. Compared with controls (mean ± SE extinction/gram colon tissue: 0.39 ± 0.07), there was a massively increased uptake of Evans blue in the DSS-treated group (mean ± SE extinction/gram colon tissue: 1.11 ± 0.13), indicating a disrupted epithelial barrier. Concomitant treatment with KdPT completely prevented this increase in permeability (mean ± SE: 0.69 ± 0.09). Values are given as mean ± SE; n = 5 per group. *P = 0.026, healthy control versus DSS-treated; **P = 0.48, DSS-treated versus KdPT-treated.
To test whether KdPT is also functionally capable of maintaining intestinal barrier function after DSS challenge in vivo, we measured Evans blue uptake into the colonic mucosa (Figure 6B). Compared with controls, a strong and significant increase of Evans blue uptake into the colonic mucosa of DSS-treated mice was observed. This increase of Evans blue uptake could be significantly reduced by concomitant oral administration of KdPT (P < 0.05), demonstrating that KdPT therapy ameliorates the leakiness of the colonic epithelium.
To further investigate the effect of KdPT treatment on tight junction protein expression in vivo, we examined the effect of the tripeptide on protein expression and organization of candidate tight junction proteins by immunofluorescence microscopy after DSS treatment and in IL-10–deficient mice. In the colon of control mice, the tight junction proteins ZO-1, occludin, claudin-1, claudin-3, and claudin-5 were strongly expressed at the epithelial apical cell-cell contacts, at the surface, and in the crypts (Figure 7). For claudin-5, a typical additional staining of the basolateral plasma membrane was observed. After treatment with DSS, a substantial loss of ZO-1, occludin, and claudin-5 was detected, as reported earlier.31 This loss was manifested by a reduction in staining intensity and complete discontinuations in membrane staining in multiple areas. This was most dramatically observed for occludin and ZO-1 at the apical membrane. In contrast, in the colon of KdPT-treated animals, the expression of ZO-1, occludin, and claudin-3 was essentially maintained at the epithelial surface and in the crypts. Only a few areas showed reduced expression of these proteins. However, no effects on tight junction protein expression could be observed in the early phase after the induction of colitis (days 0 to 2; data not shown). In addition, staining for ZO-1, occludin, claudin-1, claudin-3, and claudin-5 in KdPT-treated IL-10 gene–deficient mice indicates that the expression of these tight junction proteins was maintained.
Figure 7.
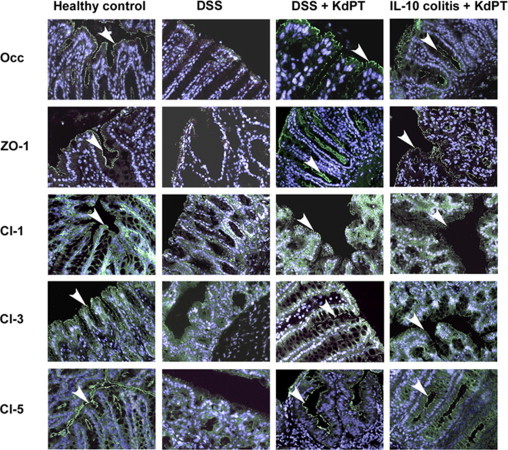
KdPT maintains tight junction protein localization in colonic epithelium in situ. The tight junction proteins ZO-1, occludin (Occ), claudin-1 (Cl-1), Cl-3, and Cl-5 were stained after DSS challenge and in IL-10–deficient mice. In control animals, ZO-1, Occ, Cl-1, and Cl-3 are mostly localized at the apical membrane and are displayed by an intense apical fluorescence band; Cl-5 was additionally expressed at the basolateral membrane. In IL-10 gene–deficient mice (day 31 after the start of KdPT application) and after treatment with DSS (day 10 after the start of DSS application), the apical staining of ZO-1, Occ, Cl-1, Cl-3, and Cl-5 is strongly reduced, whereas in KdPT-treated animals, the junction proteins stain markedly longer. Images are representative of five animals in each group. Arrowheads represent the positive membrane staining of indicated tight junction proteins.
KdPT Attenuates Colitis in IL-1 Receptor–Deficient Mice
Owing to the sequence similarity between KdPT and amino acids 193 to 195 of human IL-1β, KdPT may act as an IL-1 antagonist.15 Indeed, we recently found potent attenuating effects of KdPT on IL-1β–induced IL-6 and IL-8 expression in human sebocytes, presumably by interaction between the small peptides and the IL-1 receptor (IL-1R).32 In addition, recent findings revealed increased susceptibility of DSS-induced colitis in mice with deficient IL-1R type I.33 Therefore, we tested the effect of KdPT in DSS-induced colitis of IL-1R type I–deficient mice. IL-1R–deficient mice received an effective oral dose of KdPT (100 μg/d) beginning on day 2 after administration of DSS until the end of the experiment on day 9, and control animals received PBS. Progressive weight loss was observed in both groups starting on day 6 after DSS application (Figure 8A). KdPT-treated animals, however, again lost significantly less body weight (mean ± SE maximum: 7.4% ± 2.2%) compared with that observed in PBS-treated mice (mean ± SE maximum: 14.3% ± 1.9%; P < 0.05). All the animals survived DSS treatment. On day 9 after DSS application, histologic examination according to the method of Dieleman et al29 confirmed a significantly higher score in control mice (mean ± SE: 11.8 ± 2.8 AU) than in KdPT-treated animals (mean ± SE: 6.7 ± 1.7 AU; P < 0.05; Figure 8B).
Figure 8.
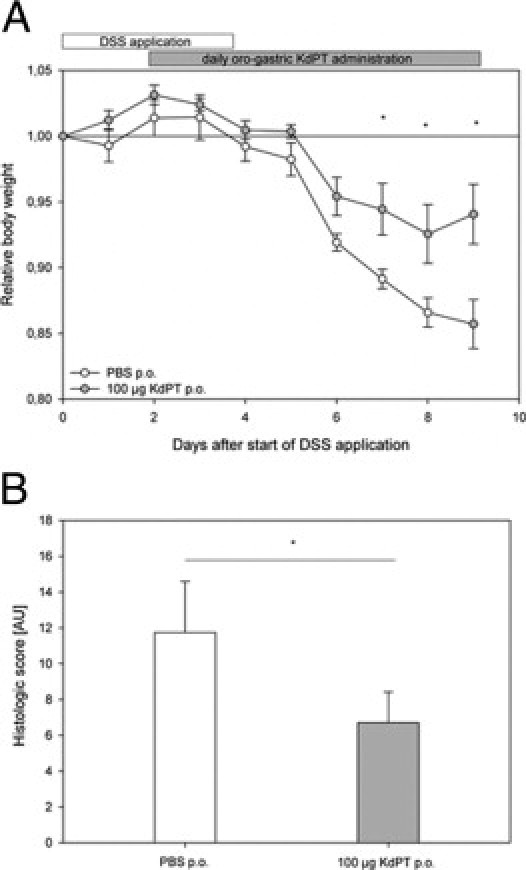
KdPT ameliorates DSS-induced colitis in IL-1R type I–deficient mice. A: IL-1R type I–deficient mice received 3% DSS in their drinking water for 5 days, and inflammation was monitored by daily measurement of individual weights. From day 2 after the start of DSS application, one group was treated with 100 μg of KdPT p.o. daily, and control animals received an equivalent volume of PBS i.p. Data are mean ± SE; n = 7 for each group. *P < 0.05. B: Histologic scores of colonic tissue according to the method of Dieleman et al29 of control mice and KdPT-treated animals 9 days after induction of colitis. Data are mean ± SE; n = 7 for each group. *P < 0.05.
Uptake of KdPT Is Transporter Mediated
Previous data have shown that the effect of KPV, another anti-inflammatory tripeptide sharing 100% identity with the last three amino acids of the C-terminal domain of α-MSH, involves the oligopeptide transporter PepT1.14 To analyze the characteristics of KdPT transport into epithelial cells, we first studied the impact of extracellular pH on KdPT absorption in Caco-2 cells. This human colonic cell line expresses PepT1.34 KdPT uptake increased with the proton gradient, and it was lowest when the proton gradient was abolished (intracellular and extracellular pH fixed at 7.4). Second, KdPT transport was shown to be energy dependent because it was drastically reduced at 4°C. Finally, KdPT transport was saturable, suggesting that KdPT uptake is transporter mediated (Figure 9).
Figure 9.
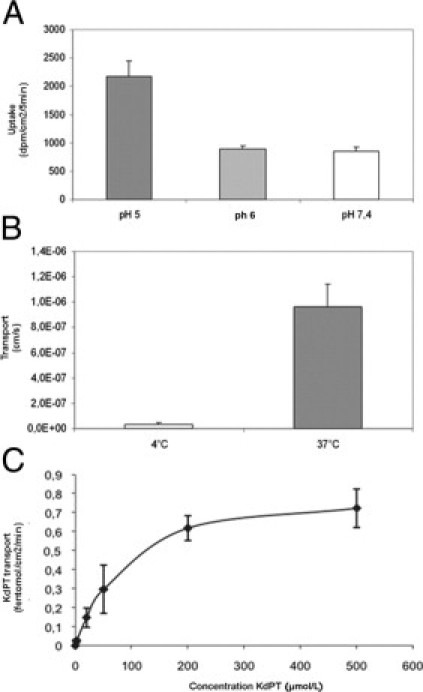
Saturable uptake of KdPT is dependent on pH and energy. Uptake or transport of KdPT by intestinal epithelial cells was measured in vitro in Caco-2 cells. With increased proton gradient, uptake of KdPT was significantly enhanced approximately threefold (P < 0.05) (A), whereas a temperature of 4°C almost completely abolished its transport (B). C: Saturable uptake of KdPT by Caco-2 cells was shown by increasing the concentration of KdPT up to 500 μmol/L. Data are mean ± SE. All the experiments were performed in triplicate.
However, in Caco-2 cells, KdPT was absorbed only at very low efficacy compared with Gly-Sar, a classical and specific PepT1 substrate (mean ± SE: 561 ± 29 versus 7946 ± 336 dpm/cm2/5 minutes). In HT-29 cells lacking PepT1,14 Gly-Sar uptake was drastically reduced, whereas KdPT uptake was decreased by only approximately 50% compared with KdPT uptake in Caco-2 cells (mean ± SE: 561 ± 29 versus 286 ± 47 dpm/cm2/5 minutes; Figure 10A). PepT1 protein expression in Caco-2 cells increases with confluence and cell differentiation, as does Gly-Sar uptake (day 4: 469 ± 66 dpm/g/mL versus day 17: 2194 ± 92 dpm/g/mL). However, KdPT uptake actually decreased with increasing cell differentiation in Caco-2 cells (day 4: 417 ± 98 dpm/g/mL versus day 17: 155 ± 8 dpm/g/mL; Figure 10B). If KdPT were a substrate of PepT1, it would competitively inhibit Gly-Sar uptake. We, therefore, examined the inhibitory effect of increased concentrations of KdPT, KPV, or Gly-Pro, a well-characterized dipeptide PepT1 substrate, on PepT1-mediated uptake of Gly-Sar (Figure 10C). As already described, Gly-Pro induced a dose-dependent inhibition of Gly-Sar uptake (40% inhibition at 0.1 mmol/L, 80% at 1 mmol/L, and 92% at 10 mmol/L). On the other hand, KPV decreased Gly-Sar uptake only at a high concentration (92% inhibition at 10 mmol/L) without any effect at lower concentrations. KdPT (10 mmol/L) induced a 60% maximum decrease in Gly-Sar uptake. Consequently, at comparable concentrations, KdPT seemed less potent than did Gly-Pro or KPV in inhibiting Gly-Sar uptake. Conversely, excess Gly-Sar or Gly-Pro (50 mmol/L) induced a decrease in KdPT transport across Caco-2 monolayers (Figure 10D).
Figure 10.
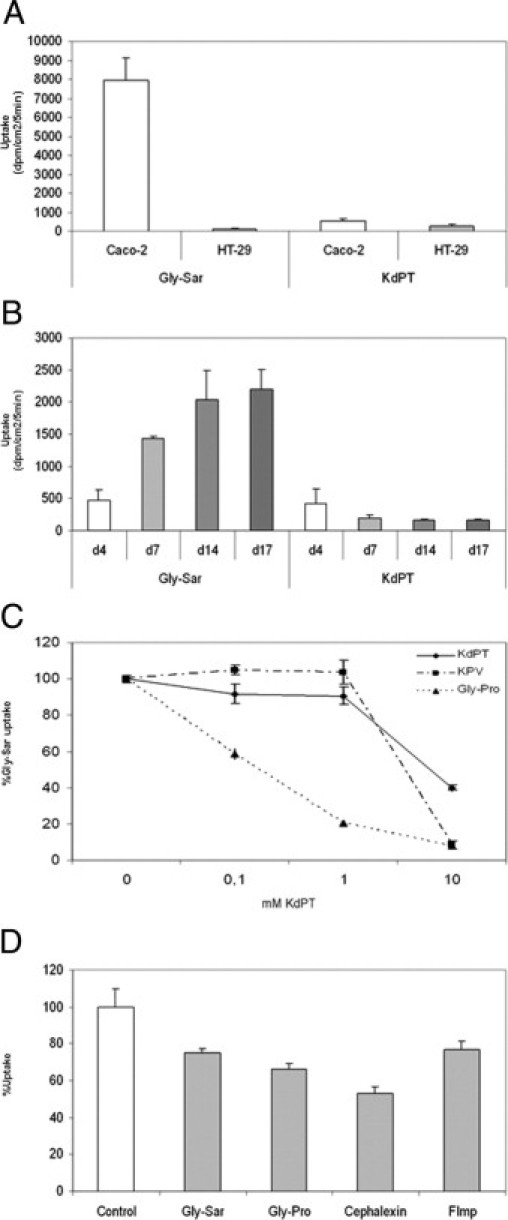
PepT1 mediated transport of KdPT in vitro. A: Caco-2 cells expressing the oligopeptide transporter PepT1 effectively transport the PepT1 substrate Gly-Sar, whereas HT-29 cells lack PepT1 expression. In contrast, the transport of radiolabeled KdPT was significantly less effective in Caco-2 cells compared with Gly-Sar, whereas HT-29 cells did not exhibit a significant difference. B: Uptake of Gly-Sar increased from day 4 to day 17, whereas uptake of KdPT significantly dropped. C: Competition assays showed that Gly-Sar uptake was only partially inhibited by high concentrations of KdPT. D: Conversely, excessive amounts of various PepT1 substrates induced a decrease in KdPT uptake in Caco-2 cells. Data are mean ± SE. All the experiments were performed in triplicate.
In in vivo experiments, uptake of Gly-Sar and KdPT was analyzed in control and DSS-treated mice in different intestinal segments (ie, duodenum, jejunum, ileum, and colon) in the presence or absence of large amounts of cold competitive substrates (Figure 11A). The uptake of Gly-Sar followed the PepT1 gradient of protein expression along the digestive tract [mean ± SE: (179 ± 32) × 103 dpm/g/5 minutes in the duodenum versus (34 ± 8) × 103 dpm/g/5 minutes in the colon]. Moreover, the competitors (Gly-Gly + Gly-Pro) completely blocked Gly-Sar uptake. Gly-Sar uptake was greater than KdPT uptake in all intestinal segments except the colon. In contrast, KdPT uptake was maximal in the distal part of the digestive tract (where PepT1 is absent) compared with other intestinal segments, and the presence of PepT1 competitors did not affect KdPT uptake. DSS treatment did not modify Gly-Sar uptake regardless of the intestinal segment studied (Figure 11B). DSS treatment induced a significant increase in KdPT absorption in the inflamed colon [(20 ± 2) × 103 dpm/g/5 minutes versus (33 ± 3) × 103 dpm/g/5 minutes; P < 0.05] but did not alter KdPT uptake in the small intestine, suggesting increased availability under inflammatory conditions (Figure 11C). Taken together, these data propose that the uptake of KdPT in vitro and in vivo is transporter mediated but is not solely dependent on the oligopeptide transporter PepT1.
Figure 11.
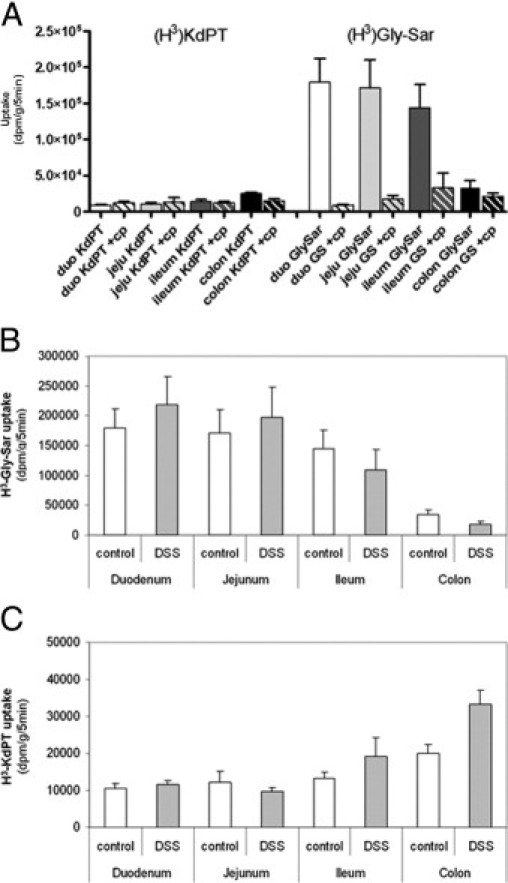
Intestinal uptake of KdPT in vivo. Whereas absorption of KdPT was maximal in the colon (A, left), PepT1-specific Gly-Sar was more effectively absorbed in the small intestinal tract and followed the PepT1 gradient of protein expression (A, right). PepT1 competitors (cp) totally blocked Gly-Sar uptake, whereas KdPT uptake was unaffected (A, hatched bars). DSS treatment did not modify Gly-Sar uptake (B) but induced significantly increased KdPT absorption in the inflamed colon (C). duo, duodenum; jeju, jejunum.
KdPT Does Not Affect Melanin Synthesis in Normal Human Melanocytes
Although several melanocortin peptides, including α-MSH, have potent immunomodulatory effects in vitro and in vivo, a major disadvantage limiting their clinical use in humans is pigment induction. We, therefore, performed melanin assays of normal human melanocytes exposed to KdPT at various doses. In contrast to bovine pituitary extracts (containing natural melanocortins) and used as a positive control, none of the examined KdPT concentrations led to a significant change in melanin synthesis (Figure 12).
Figure 12.
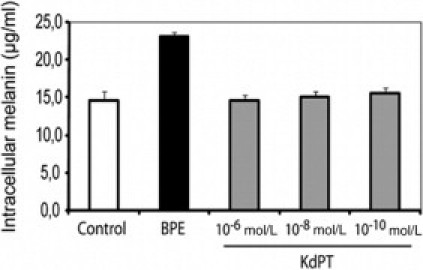
KdPT does not alter melanin synthesis. Normal human melanocytes were exposed to various doses of KdPT for 5 days. Bovine pituitary extracts (BPE) (containing natural melanocortins) were used as positive control. Melanin amounts in the lysed cells were determined photometrically using a synthetic melanin standard. Melanin measurements were performed in triplicate and represent mean ± SD. Data depict one of three individual experiments with identical results.
Discussion
In this study, we demonstrate potent anti-inflammatory efficacy of the MSH-related tripeptide KdPT in two well-established models of experimental colitis. Systemic and local administration of KdPT attenuated the course of acute DSS-induced colitis in a concentration-dependent manner, and similar efficacy was demonstrated in IL-10 knockout mice. Furthermore, the compound is able to facilitate the regeneration of epithelial wounds, and it also protects the epithelial barrier by reducing the inflammation-induced breakdown of paracellular permeability in vitro and in vivo.
In light of the melanocortin peptides tested so far, the present results introduce a novel anti-inflammatory agent with future potential for the treatment of IBD. Rajora et al35 originally reported that α-MSH reduces colonic inflammation in the DSS model. These authors found decreased colonic expression of TNF-α and nitric oxide after α-MSH treatment. In support of the anti-inflammatory effect of α-MSH in experimental colitis, it was shown that α-MSH attenuates trinitrobenzene sulfonic acid–induced colitis.36 Recently, Lactobacillus casei–secreting α-MSH was also reported to ameliorate DSS-induced colitis,37 and we could show that the α-MSH fragment KPV has anti-inflammatory effects in the DSS and transfer colitis model.13 We do not know which of the previously mentioned peptides is most efficient in experimental colitis because direct comparative pharmacologic studies are not yet available. However, preliminary data indicate a more robust inhibition of the murine contact hypersensitivity reaction by KdPT compared with KPV.18 In addition, KdPT does not induce melanogenesis, which represents a significant advantage compared with α-MSH.
Not only pretreatment with KdPT but also therapeutic application after induction of colitis leads to reduced disease activity in DSS-induced colitis. Moreover, the tripeptide also mediates potent therapeutic effects in colitis of IL-10 gene–deficient mice, demonstrating a robust anti-inflammatory capacity regarding intestinal inflammation. Targeted disruption of the IL-10 gene leads to spontaneous development of chronic enterocolitis, with massive infiltration of lymphocytes, activated macrophages, and neutrophils,21,30 that is primarily immune mediated but is also dependent on luminal bacteria. In contrast, KdPT does not alter the development of infection with Citrobacter rodentium, an enteropathogenic Escherichia coli infection in mice (data not shown).
Regarding the potential molecular mechanism of KdPT as an anti-inflammatory agent, it has already been speculated that the tripeptide may compete with IL-1β for binding to the IL-1R.16,17 This hypothesis was based on the antagonistic effects of KdPT on IL-1β–induced pain in the rat hind-paw assay and on the sequence homology between amino acids 193 to 195 of IL-1β (KPT) and the tripeptide as a stereoisomer. Recent studies investigating the direct antagonistic action of KdPT and IL-1β in vitro in fact further suggested an interference of KdPT with IL-1β at the IL-1R type I level.32 These studies were performed in vitro and on human cells. Using IL-1R type I–deficient mice, however, we could not find an essential role of the IL-1R type I as a mediator of the in vivo efficacy of KdPT. Accordingly, KdPT exerts its anti-inflammatory and protective actions in chemically induced colitis of mice by alternative mechanisms, eg, by acting on the epithelial barrier.
Because KdPT has structural similarity with the last three amino acids of the C-terminal domain of α-MSH, an interaction with MC-Rs seemed possible a priori. Using α-MSH radioligand binding assays and the natural MC-1R antagonist Agouti signaling protein, we recently demonstrated that KdPT does not bind to MC-1R in vitro.32 In addition, we investigated whether KdPT has a protective effect on the course of acute DSS-induced colitis in MC-1R signaling–deficient mice. The effect of KdPT was abolished in the previously mentioned mice strain (see Supplemental Figure S1 at http://ajp.amjpathol.org). Indeed, DSS-induced colitis is aggravated in MC-1R–deficient mice,9 possibly reducing the in vivo capacity of KdPT to control intestinal inflammation. On the other hand, this finding may point to a complex role of MC-1R in experimentally induced colitis and the molecular effector mechanism of KdPT, which warrants further investigation.
It has recently been shown that the KdPT-related and truncated α-MSH tripeptide KPV is transported via an H+-coupled oligopeptide transporter, PepT1, located at the apical membrane of intestinal epithelial cells.14 In this study, nanomolar concentrations of KPV inhibited the activation of NF-κB and mitogen-activated protein kinases, thereby reducing proinflammatory cytokine secretion by epithelial cells and by Jurkat T cells. Owing to the structural similarity of KPV and KdPT, it seems likely that the compound is also transported by PepT1; it was, therefore, important to check whether this transporter might be involved in KdPT action on DSS-induced colitis. The present experiments revealed that the presence of competitive and noncompetitive inhibitors of PepT1 reduces the intracellular uptake of KdPT. On the other hand, compared with well-known substrates of PepT1, KdPT is transported less efficiently into Caco-2 cells and in different gastrointestinal segments, suggesting that the efficacy of KdPT is not solely dependent on PepT1 and that other transporters might also be involved. Considering the temperature- and pH-dependent uptake characteristics combined with the saturation kinetics, we assume that PepT1, at least in part, mediates cellular uptake of KdPT. Intriguingly, KdPT uptake in the inflamed colon was significantly higher (50%), which may reflect up-regulation of PepT1 or other transporters in chronically inflamed colons. This might be of substantial advantage in the treatment of IBD with such small anti-inflammatory oligopeptides.14,38,39
We further believe that nonspecific, non–receptor-mediated effects of KdPT are rather unlikely for several reasons. First, in vitro data suggest receptor-mediated uptake into epithelial cells. Second, the concentrations of KdPT used in both colitis models are low, and abundant miscellaneous tripeptides are part of the general intestinal contents. Furthermore, a saturating effect of KdPT on intestinal transporters can also be excluded by the present in vitro data, showing that quite high concentrations of KdPT have to be used to inhibit the transport of other peptides specific for PepT1. Finally, we demonstrated that KdPT is still effective even in the presence of excessive oligopeptides (data not shown).
In the present experiments, the tripeptide stimulated proliferation of intestinal epithelial cells in vivo and in vitro, enhanced closure of mechanically induced wounds of Caco-2 monolayers, attenuated the TNF-α/IFN-γ–induced breakdown of TER, and also prevented the redistribution of tight junction proteins in DSS colitis. All of them are readouts of intestinal barrier function. Previous findings could demonstrate that tight junction proteins play a crucial role in experimental and human IBD.31,40 Tight junction proteins are internalized by macropinocytosis into colonic epithelial monolayers when incubated with proinflammatory cytokines and in DSS colitis.30 In addition, occludin and JAM-A were found to be localized in subapical vesicle-like structures in actively inflamed human ulcerative colitis mucosa.41 The present in vitro and in vivo data strongly suggest that KdPT prevents the inflammation-associated changes in tight junction protein distribution, thereby inducing a significantly improved barrier function in experimental colitis.
The present results identifying epithelial integrity as a mechanism of action for MSH-related peptides are supported by a study by Bonfiglio et al,42 who showed that KPV facilitated corneal wound healing in rabbits. In addition, it is known that α-MSH has beneficial effects on gut ischemia injury that may relate to the acceleration of epithelial restitution.32 In addition, we did not show a significant alteration in cytokine expression by lamina propria lymphocytes after KdPT treatment in vivo, and significantly higher doses of KdPT were needed to down-regulate cytokine expression by RAW macrophages in vitro (data not shown), suggesting that epithelial cells are the primary target of KdPT.
In conclusion, we suggest KdPT to be a new nonmelanotropic and potent anti-inflammatory tripeptide addressing epithelial integrity as a key mechanism involved in the pathogenesis of IBD and, therefore, representing an appealing new therapeutic option for clinical trials in IBD.
Acknowledgments
We thank Sonja Dufentester, Elke Weber, and Britta Ringelkamp for their expert technical assistance.
Footnotes
Supported by an interdisciplinary grant from the Deutsche Forschungsgemeinschaft (MA 2247/3-1 to M.Bö. and C.M.) and by a grant from Wolff Arzneimittel, Bielefeld, Germany (A.L.).
C.M. and A.L. contributed equally to this work.
CME Disclosure: The authors did not disclose any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.05.013.
Current address of T.K. and C.M., Department of Internal Medicine and Gastroenterology, Klinikum Lueneburg, Lueneburg, Germany.
Supplementary data
KdPT did not ameliorate DSS-induced colitis in MC-1R signaling–deficient mice. A: Signaling-deficient MC-1R mice (MC1Re/e) received 2% DSS in their drinking water for 5 days, and inflammation was monitored by daily measurement of individual weights. Starting 2 days after induction of DSS colitis, MC1Re/e mice received 100 μg of KdPT orally per day, and control animals received an equivalent volume of PBS. Data are mean ± SE; n = 5 for each group. B: Histologic scores of colonic tissue according to the method of Dieleman et al28 of control mice and KdPT-treated animals 13 days after induction of colitis. Data are means ± SE; n = 5 for each group.
References
- 1.Bousvaros A., Sylvester F., Kugathasan S., Szigethy E., Fiocchi C., Colletti R., Otley A., Amre D., Ferry G., Czinn S.J., Splawski J.B., Oliva-Hemker M., Hyams J.S., Faubion W.A., Kirschner B.S., Dubinsky M.C. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. doi: 10.1097/01.mib.0000228358.25364.8b. [DOI] [PubMed] [Google Scholar]
- 2.Papadakis K.A., Targan S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann J.C., Preiss J.C., Autschbach F., Buhr H.J., Hauser W., Herrlinger K., Hohne W., Koletzko S., Krieglstein C.F., Kruis W., Matthes H., Moser G., Reinshagen M., Rogler G., Schreiber S., Schreyer A.G., Sido B., Siegmund B., Stallmach A., Bokemeyer B., Stange E.F., Zeitz M. [Clinical practice guideline on diagnosis and treatment of Crohn's disease]: German. Z Gastroenterol. 2008;46:1094–1146. doi: 10.1055/s-2008-1027796. [DOI] [PubMed] [Google Scholar]
- 4.Leowardi C., Heuschen G., Kienle P., Heuschen U., Schmidt J. Surgical treatment of severe inflammatory bowel diseases. Dig Dis. 2003;21:54–62. doi: 10.1159/000071340. [DOI] [PubMed] [Google Scholar]
- 5.Gionchetti P., Rizzello F., Helwig U., Venturi A., Lammers K.M., Brigidi P., Vitali B., Poggioli G., Miglioli M., Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 6.Hiltz M.E., Lipton J.M. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide α-MSH. FASEB J. 1989;3:2282–2284. [PubMed] [Google Scholar]
- 7.Tatro J.B. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation. 1996;3:259–284. doi: 10.1159/000097281. [DOI] [PubMed] [Google Scholar]
- 8.Catania A., Gatti S., Colombo G., Lipton J.M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Maaser C., Kannengiesser K., Kucharzik T. Role of the melanocortin system in inflammation. Ann N Y Acad Sci. 2006;1072:123–134. doi: 10.1196/annals.1326.016. [DOI] [PubMed] [Google Scholar]
- 10.Ichiyama T., Sakai T., Catania A., Barsh G.S., Furukawa S., Lipton J.M. Inhibition of peripheral NF-κB activation by central action of α-melanocyte-stimulating hormone. J Neuroimmunol. 1999;99:211–217. doi: 10.1016/s0165-5728(99)00122-8. [DOI] [PubMed] [Google Scholar]
- 11.Lipton J.M., Catania A. Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 12.Brzoska T., Luger T.A., Maaser C., Abels C., Bohm M. α-Melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- 13.Kannengiesser K., Maaser C., Heidemann J., Luegering A., Ross M., Brzoska T., Bohm M., Luger T.A., Domschke W., Kucharzik T. Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:324–331. doi: 10.1002/ibd.20334. [DOI] [PubMed] [Google Scholar]
- 14.Dalmasso G., Charrier-Hisamuddin L., Nguyen H.T., Yan Y., Sitaraman S., Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–178. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira S.H., Lorenzetti B.B., Bristow A.F., Poole S. Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 16.Follenfant R.L., Nakamura-Craig M., Henderson B., Higgs G.A. Inhibition by neuropeptides of interleukin-1 β-induced, prostaglandin-independent hyperalgesia. Br J Pharmacol. 1989;98:41–43. doi: 10.1111/j.1476-5381.1989.tb16860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugridge K.G., Perretti M., Ghiara P., Parente L. α-Melanocyte-stimulating hormone reduces interleukin-1β effects on rat stomach preparations possibly through interference with a type I receptor. Eur J Pharmacol. 1991;197:151–155. doi: 10.1016/0014-2999(91)90514-q. [DOI] [PubMed] [Google Scholar]
- 18.Luger T.A., Brzoska T. α-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann Rheum Dis. 2007;66(Suppl 3):iii52–iii55. doi: 10.1136/ard.2007.079780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maaser C., Kannengiesser K., Specht C., Lugering A., Brzoska T., Luger T.A., Domschke W., Kucharzik T. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415–1422. doi: 10.1136/gut.2005.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg D.J., Zhang J., Weinstock J.V., Ismail H.F., Earle K.A., Alila H., Pamukcu R., Moore S., Lynch R.G. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn R., Lohler J., Rennick D., Rajewsky K., Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 22.Blum A.M., Metwali A., Elliott D.E., Berg D.J., Weinstock J.V. CD4+ T cells from IL-10-deficient mice transfer susceptibility to NSAID-induced Rag colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G320–G325. doi: 10.1152/ajpgi.00527.2003. [DOI] [PubMed] [Google Scholar]
- 23.de Silva H.J., Gatter K.C., Millard P.R., Kettlewell M., Mortensen N.J., Jewell D.P. Crypt cell proliferation and HLA-DR expression in pelvic ileal pouches. J Clin Pathol. 1990;43:824–828. doi: 10.1136/jcp.43.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lostao M.P., Urdaneta E., Martinez-Anso E., Barber A., Martinez J.A. Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett. 1998;423:302–306. doi: 10.1016/s0014-5793(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 25.Burk R.R. A factor from a transformed cell line that affects cell migration. Proc Natl Acad Sci U S A. 1973;70:369–372. doi: 10.1073/pnas.70.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruewer M., Luegering A., Kucharzik T., Parkos C.A., Madara J.L., Hopkins A.M., Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 27.Kitajima S., Takuma S., Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 28.Ukena S.N., Singh A., Dringenberg U., Engelhardt R., Seidler U., Hansen W., Bleich A., Bruder D., Franzke A., Rogler G., Suerbaum S., Buer J., Gunzer F., Westendorf A.M. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieleman L.A., Palmen M.J., Akol H., Bloemena E., Pena A.S., Meuwissen S.G., Van Rees E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg D.J., Davidson N., Kuhn R., Muller W., Menon S., Holland G., Thompson-Snipes L., Leach M.W., Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mennigen R., Nolte K., Rijcken E., Utech M., Loeffler B., Senninger N., Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 32.Mastrofrancesco A., Kokot A., Eberle A., Gibbons N.C., Schallreuter K.U., Strozyk E., Picardo M., Zouboulis C.C., Luger T.A., Bohm M. KdPT, a tripeptide derivative of α-melanocyte-stimulating hormone, suppresses IL-1β–mediated cytokine expression and signaling in human sebocytes. J Immunol. 2010;185:1903–1911. doi: 10.4049/jimmunol.0902298. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Navajas J.M., Law J., Nguyen K.P., Bhargava M., Corr M.P., Varki N., Eckmann L., Hoffman H.M., Lee J., Raz E. Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J Exp Med. 2010;207:2799–2807. doi: 10.1084/jem.20101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adibi S.A. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113:332–340. doi: 10.1016/s0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- 35.Rajora N., Boccoli G., Catania A., Lipton J.M. α-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381–385. doi: 10.1016/s0196-9781(96)00345-2. [DOI] [PubMed] [Google Scholar]
- 36.Oktar B.K., Ercan F., Yegen B.C., Alican I. The effect of α-melanocyte stimulating hormone on colonic inflammation in the rat. Peptides. 2000;21:1271–1277. doi: 10.1016/s0196-9781(00)00269-2. [DOI] [PubMed] [Google Scholar]
- 37.Yoon S.W., Lee C.H., Kim J.Y., Kim J.Y., Sung M.H., Poo H. Lactobacillus casei secreting α-MSH induces the therapeutic effect on DSS-induced acute colitis in Balb/c mice. J Microbiol Biotechnol. 2008;18:1975–1983. [PubMed] [Google Scholar]
- 38.Bai J.P., Amidon G.L. Structural specificity of mucosal-cell transport and metabolism of peptide drugs: implication for oral peptide drug delivery. Pharm Res. 1992;9:969–978. doi: 10.1023/a:1015885823793. [DOI] [PubMed] [Google Scholar]
- 39.Thwaites D.T., Cavet M., Hirst B.H., Simmons N.L. Angiotensin-converting enzyme (ACE) inhibitor transport in human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1995;114:981–986. doi: 10.1111/j.1476-5381.1995.tb13301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laukoetter M.G., Nava P., Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruewer M., Utech M., Ivanov A.I., Hopkins A.M., Parkos C.A., Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 42.Bonfiglio V., Camillieri G., Avitabile T., Leggio G.M., Drago F. Effects of the COOH-terminal tripeptide α-MSH(11–13) on corneal epithelial wound healing: role of nitric oxide. Exp Eye Res. 2006;83:1366–1372. doi: 10.1016/j.exer.2006.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
KdPT did not ameliorate DSS-induced colitis in MC-1R signaling–deficient mice. A: Signaling-deficient MC-1R mice (MC1Re/e) received 2% DSS in their drinking water for 5 days, and inflammation was monitored by daily measurement of individual weights. Starting 2 days after induction of DSS colitis, MC1Re/e mice received 100 μg of KdPT orally per day, and control animals received an equivalent volume of PBS. Data are mean ± SE; n = 5 for each group. B: Histologic scores of colonic tissue according to the method of Dieleman et al28 of control mice and KdPT-treated animals 13 days after induction of colitis. Data are means ± SE; n = 5 for each group.


