Abstract
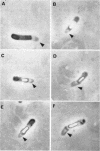
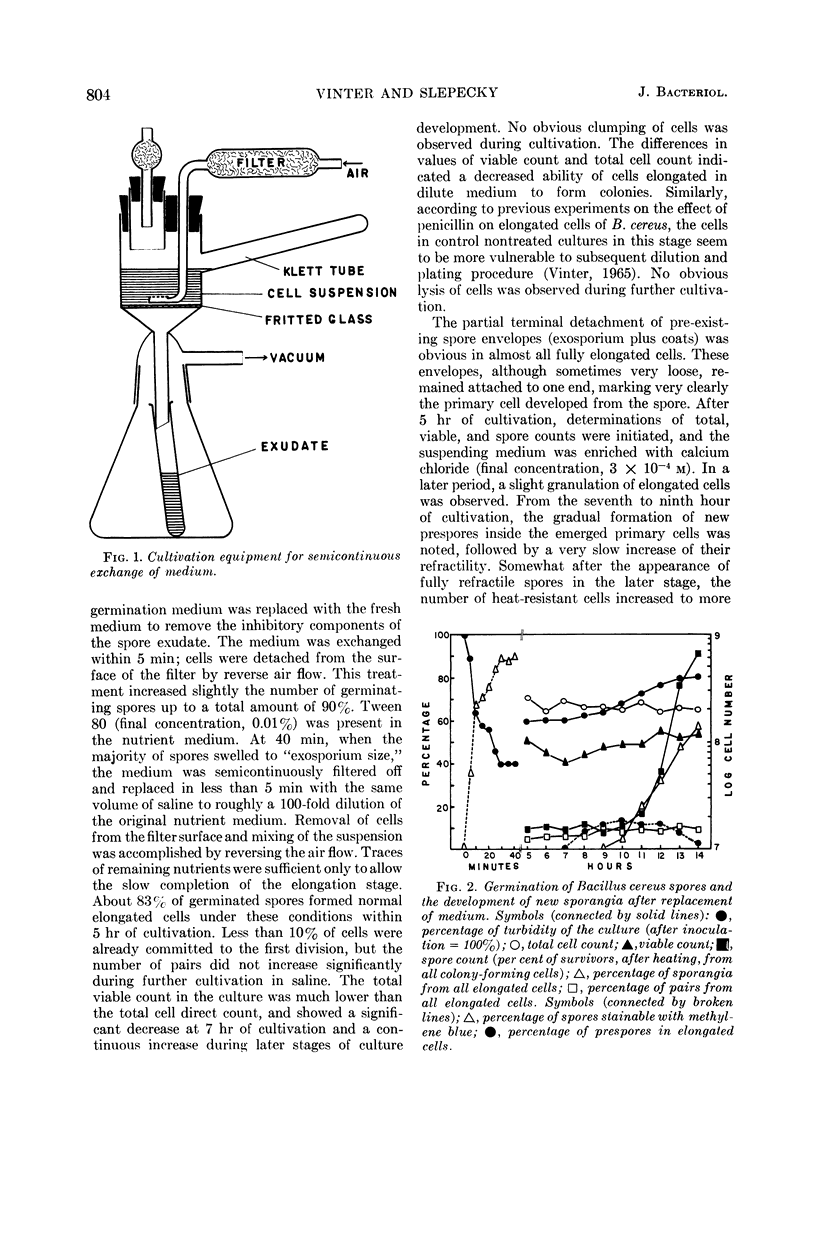
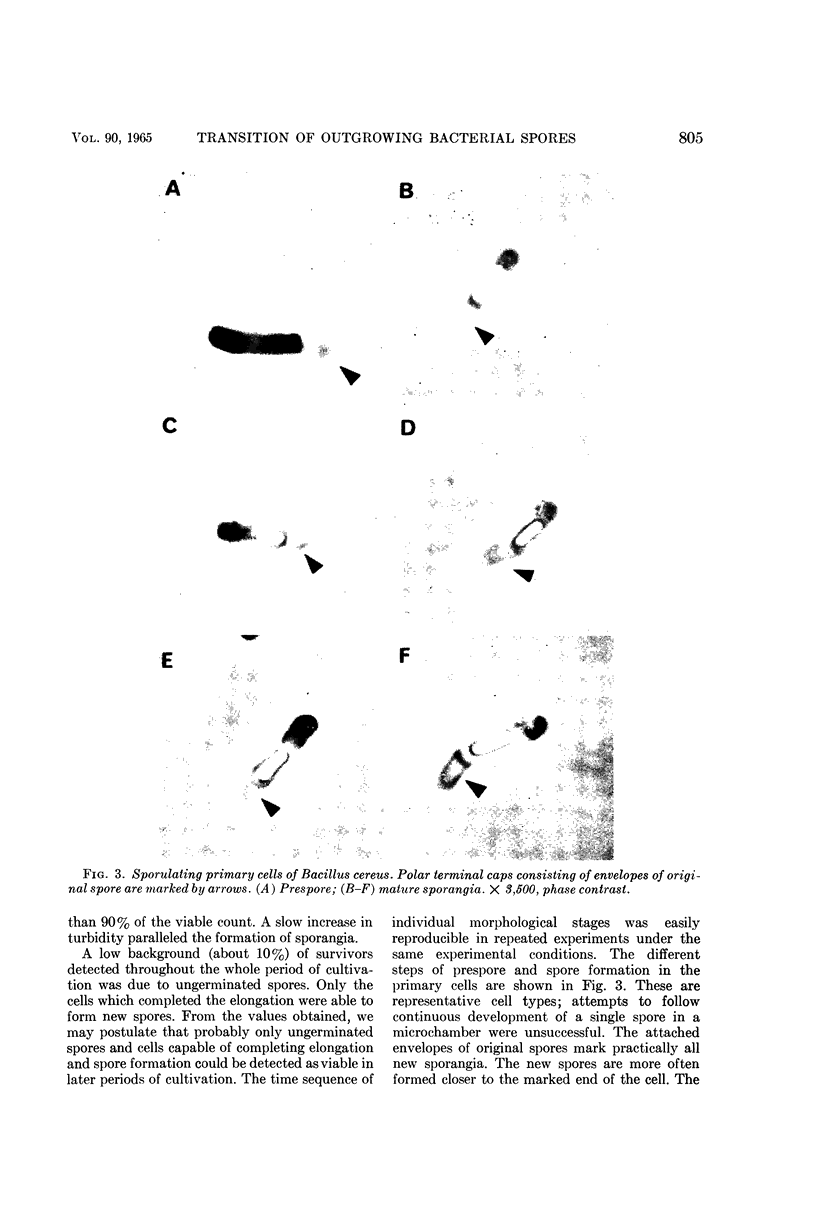
Vinter, Vladimir (Syracuse University, Syracuse, N.Y.), and Ralph A. Slepecky. Direct transition of outgrowing bacterial spores to new sporangia without intermediate cell division. J. Bacteriol. 90:803–807. 1965.—A direct transition was observed of the primary cell developed after germination of Bacillus cereus spores into new sporangia without intermediate division stages. Two simple methods were used for replacement of outgrowing spores into diluted medium or saline. Elongated primary cells prevented from division by limitation of nutrients in the suspending medium were able to form new forespores in 8 hr and sporangia in 12 hr. These new sporangia were still marked by attached envelopes of the original spore. Under the same conditions, cells replaced during the first divisions quickly lysed. Spores formed in the elongated primary cell during “microcycle sporogenesis” possessed normal heat resistance and refractility and were later released from sporangia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FITZ-JAMES P. C. The phosphorus fractions of Bacillus cereus and Bacillus megaterium. II. A correlation of the chemical with the cytological changes occurring during spore germination. Can J Microbiol. 1955 Aug;1(7):525–548. doi: 10.1139/m55-066. [DOI] [PubMed] [Google Scholar]
- KEYNAN A., EVANCHIK Z., HALVORSON H. O., HASTINGS J. W. ACTIVATION OF BACTERIAL ENDOSPORES. J Bacteriol. 1964 Aug;88:313–318. doi: 10.1128/jb.88.2.313-318.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Ordal Z. J. REVERSIBLE ACTIVATION FOR GERMINATION AND SUBSEQUENT CHANGES IN BACTERIAL SPORES. J Bacteriol. 1963 Jan;85(1):207–217. doi: 10.1128/jb.85.1.207-217.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIEMANN H., ORDAL Z. J. Germination of bacterial endospores with calcium and dipicolinic acid. Science. 1961 May 26;133(3465):1703–1704. doi: 10.1126/science.133.3465.1703. [DOI] [PubMed] [Google Scholar]
- Rogolsky M., Slepecky R. A. Elimination of a genetic determinant for sporulation of Bacillus subtilis with acriflavin. Biochem Biophys Res Commun. 1964 Jun 15;16(3):204–208. doi: 10.1016/0006-291x(64)90326-2. [DOI] [PubMed] [Google Scholar]
- VINTER V., VECHET B. SPORS OF MICROORGANISMS. XV. THE ALTERATION OF HEAT SENSITIVITY AND ITS RELATION TO RADIATION RESISTANCE OF BACTERIAL SPORES. Folia Microbiol (Praha) 1964 Jul;35:238–248. doi: 10.1007/BF02875843. [DOI] [PubMed] [Google Scholar]
- WOESE C. R., FORRO J. R. Correlations between ribonucleic acid and deoxyribonucleic acid metabolism during spore germination. J Bacteriol. 1960 Dec;80:811–817. doi: 10.1128/jb.80.6.811-817.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]