Abstract
Susceptibility to infection with Cryptococcus neoformans is tightly determined by production of IL-4. In this study, we investigated the time course of IL-4 production and its innate cellular source in mice infected intranasally with C. neoformans. We show that pulmonary IL-4 production starts surprisingly late after 6 weeks of infection. Interestingly, in the lungs of infected mice, pulmonary T helper (Th) cells and eosinophils produce significant amounts of IL-4. In eosinophil-deficient ΔdblGATA mice, IL-33 receptor–expressing Th2s are significantly reduced, albeit not absent, whereas protective Th1 and Th17 responses are enhanced. In addition, recruitment of pulmonary inflammatory cells during infection with C. neoformans is reduced in the absence of eosinophils. These data expand previous findings emphasizing an exclusively destructive effector function by eosinophilic granulocytes. Moreover, in ΔdblGATA mice, fungal control is slightly enhanced in the lung; however, dissemination of Cryptococcus is not prevented. Therefore, eosinophils play an immunoregulatory role that contributes to Th2-dependent susceptibility in allergic inflammation during bronchopulmonary mycosis.
Cryptococcus neoformans is a facultative intracellular pathogen that is acquired by inhalation of spores and/or desiccated yeasts and leads to latent pulmonary infection in immunocompetent humans.1 The development of cryptococcal meningitis occurs mainly in immunocompromised HIV-1–infected patients, most likely by reactivation of latent pulmonary C. neoformans infection.2 It is estimated that 504,000 HIV-1–infected patients die every year from cryptococcal meningitis in sub-Saharan Africa,3 which surprisingly exceeds the annual death rate of tuberculosis-associated HIV cases. Resistance against C. neoformans primarily involves monocytic effector mechanisms.4–6 In this context, T helper (Th) cells are central regulatory players with profound effects. Whereas IL-12–dependent Th1 responses are protective, with an additional contribution by IL-23–dependent Th17 responses,7–9 Th2 cells producing IL-4, IL-13, and IL-5 are detrimental.10,11 Studies12–14 that used i.v. inoculation examined the traversal of the blood-brain barrier by C. neoformans and led to the conclusion that transmigration can occur with intracellular and extracellular fungi. In case of bronchopulmonary infection, dissemination seems to rely more on Th2 cytokines. This allergic Th2-driven inflammation represents the immunopathological pathway promoting disease by allowing cryptococci to grow inside the lung and finally enabling dissemination to the brain, ultimately causing fatal meningoencephalitis.15 This sequela is accompanied by development of IL-4/IL-13–dependent alternatively activated macrophages, suggesting that those cells may be involved in dissemination. Alternatively activated macrophages are found only in susceptible mice15 and show significantly reduced control of intracellular growth.5 In addition, IL-13–dependent mucus production by goblet cells, IL-4–dependent IgE production, IL-5–dependent eosinophilia, and functional pulmonary impairment can be found; these features are also typically described in asthma.16–18 Studies10,11,19 of pulmonary and cerebral cryptococcosis in IL-4–, IL-13–, IL-4 receptor α–, and IL-4/IL-13–deficient mice or mice treated with anti-IL-5 convincingly provide a basis for future immunotherapies by targeting one or several of these Th2-associated molecules. However, it is unclear when IL-4 production starts after pulmonary infection. In addition, potential innate immune cell(s) producing IL-4 and thereby promoting Th2 initiation and/or Th2 maintenance remain to be identified. Therefore, in this study, we aimed to define the following: i) the onset and time course of IL-4 production, ii) the IL-4–producing innate cell type(s) supporting Th2 development, and iii) the immunological and phenotypic consequences of innate IL-4 production in pulmonary cryptococcosis. Our results indicate that eosinophilic granulocytes are a significant source of IL-4, with distinct regulatory consequences in murine cryptococcosis.
Materials and Methods
Mice
Female wild-type (WT) mice (Janvier, Le Genest Saint Isle, France), 4get mice20 (provided by André Gessner, Clinical Microbiology and Immunology, Erlangen, Germany), and ΔdblGATA mice21 (provided by Achim Hoerauf, Institute of Medical Microbiology, Immunology und Parasitology, Bonn, Germany), aged 6 to 10 weeks, on a BALB/c background were maintained in an individually ventilated caging system under specific pathogen-free conditions and in accordance with the guidelines approved by the Animal Care and Usage Committee of the Landesdirektion Leipzig. Sterile food and water were given ad libitum. The mice were tested periodically for pathogens, in accordance with the recommendations for health monitoring of mice provided by the Federation of European Laboratory Animal Science Associations accreditation board. All mice had negative test results for pinworms and other endoparasites and ectoparasites.
C. neoformans and Infection
Encapsulated C. neoformans, strain 1841, serotype D, was kept as a frozen stock in skim milk and was grown in Sabouraud dextrose medium (2% glucose and 1% peptone; Sigma, Deisenhofen, Germany) overnight on a shaker at 30°C. Cells were washed twice in sterile PBS, resuspended in PBS, and counted in a hematocytometer. Inocula were diluted in PBS to a concentration of 2.5 × 104/mL for intranasal (i.n.) infection. Mice were infected by i.n. application of 20-μL volumes containing 500 colony-forming units (CFUs). Before infection, mice were anesthetized i.p. with a 1:1 mixture of 10% ketamine (100 mg/mL; Ceva Tiergesundheit, Düsseldorf, Germany) and 2% xylazine (20 mg/mL; Ceva Tiergesundheit).
Leukocyte Preparation for Flow Cytometry and CFU Enumeration
Infected mice were monitored daily for survival and morbidity. After sterile removal of the brain from sacrificed mice, half was processed for histological examination and the remaining half was processed for determination of organ burden (CFU). After homogenization in 1-mL PBS with an Ultra-Turrax (T8; IKA-Werke, Staufen, Germany), serial dilutions of the homogenates were plated on Sabouraud dextrose agar plates and colonies were counted after an incubation period of 48 hours at 30°C. After sterile removal, lungs from sacrificed mice were minced and digested for 30 minutes at 37°C in RPMI 1640 medium supplemented with collagenase (Roche Diagnostics Deutschland GmbH, Mannheim, Germany), 100 μmol/L sodium pyruvate, and DNase IV (Sigma-Aldrich, Steinheim, Germany). After passage through a 100-μm nylon mesh (BD Biosciences, Heidelberg, Germany), filtrate was resuspended in 1-mL RPMI 1640 medium (PAA Laboratories, Pasching, Austria); and 50 μL was taken for CFU enumeration. Serial dilutions were plated on Sabouraud dextrose agar plates, and colonies were counted after an incubation period of 48 hours at 30°C. Remaining filtrate was resuspended in 70% Percoll (GH Healthcare Biosciences AB, Uppsala, Sweden) and layered under 26% Percoll. Leukocytes were recovered from interphase, washed with Iscove's modified Dulbecco's medium (PAA Laboratories), and counted in trypan blue (Fluka Chemie AG, Buchs, Switzerland). For surface staining, 1 × 105 to 2 × 105 cells were used; and for intracellular cytokine staining, 1 × 106 cells were acquired.
Flow Cytometry
Purified cells were adjusted to 5 × 106/mL in Iscove's modified Dulbecco's medium and stimulated either 6 hours with ionomycin (1 μg/mL; Sigma-Aldrich) and phorbol 12-myristate 13-acetate (PMA) (40 ng/mL; Alexis Corporation, Lausen, Switzerland) or 22 hours with specific antigen. For the accumulation of cytokines, brefeldin A (5 μg/mL; Sigma-Aldrich), was added for the last 4 hours. The acapsular C. neoformans serotype D strain CAP67 (provided by Dr. Bettina Fries, Albert Einstein College of Medicine, Bronx, NY) was used as a specific stimulus (1 × 107 cryptococci/mL, termed C.n. antigen) for restimulation of pulmonary leukocytes from C. neoformans–infected mice. The CAP67 strain has better restimulatory capacities than the highly virulent strain 1841. It was cultured and maintained in the same manner as strain 1841; before use, it was heat inactivated at 60°C for 1 hour.22
First, Near-IR Dead Cell Stain (Invitrogen, Darmstadt, Germany) was used to ensure discrimination and exclusion of dead cells during analysis. Second, cells were fixed with 2% paraformaldehyde (Serva, Heidelberg, Germany) for 20 minutes on ice. When intracellular staining was performed, permeabilization was included by using fluorescence-activated cell sorting buffer (ie, PBS containing 3% heat-inactivated fetal calf serum and 0.1% sodium azide) containing 0.5% saponin (w/v; Serva). Cells were incubated for 15 minutes on ice with FcR block (2 × 106 μg cells/L; purified from 2.4G2 hybridoma supernatant) and rat serum (Sigma-Aldrich) to avoid unspecific staining. Antibodies (Abs) and FcR block for intracellular staining were diluted in fluorescence-activated cell sorting buffer containing 0.5% saponin (w/v; Serva). For specific stainings, the following Abs were used: anti-CD4-PerCP-Cy5.5 (RM4-5; eBioscience, Frankfurt, Germany); anti-interferon (IFN)-γ–fluorescein isothiocyanate (XMG1.2; eBioscience); anti-IL-4–allophycocyanine (APC) (11B11; Biolegend, Fell, Germany); anti-IL-17-PE-Cy7 (eBio17B7; eBioscience); anti-Siglec-F (E50-2440; BD Biosciences) biotinylated, following standard procedures; anti-Siglec-F-PE (E50-2440; BD Biosciences); anti-F4/80-PE-Cy7 (BM8; eBioscience); anti-CD11c–APC (N418; eBioscience); anti-CD154-PE (MR1; Miltenyi Biotec, Bergisch Gladbach, Germany); and anti-T1/ST2–fluorescein isothiocyanate (MD Biosciences, Zürich, Switzerland). Appropriate isotype Abs were all from eBioscience, except for anti-IL-4 from Biolegend. Cells labeled with biotinylated Abs were further stained with streptavidin-PerCp (eBioscience). Cells were acquired on a BD FACS Calibur using CellQuest software version 3.0.1 and BD FACS CANTO II using DIVA version 6.1.1 and FlowJo version 7.6.1 (Treestar Inc., Ashland, OR) software for analysis.
IL-4 Secretion Assay
An IL-4 secretion assay (Miltenyi Biotec) was performed according to manufacturer's instructions. Percoll (GH Healthcare) purified pulmonary leukocytes were stained with anti-CD4–fluorescein isothiocyanate (RM4-5; eBioscience) and, afterward, with anti-fluorescein isothiocyanate MicroBeads (Miltenyi Biotec). Cells were then applied to an MS column (Miltenyi Biotec) to separate CD4+ and CD4− cells. Both fractions were stimulated for 2 hours with ionomycin (1 μg/mL; Sigma-Aldrich) and PMA (40 ng/mL; Alexis Corporation). After stimulation, cells were applied to an IL-4 secretion assay (Miltenyi Biotec) and were additionally stained with anti-CD11c–APC (N418; eBioscience), anti-CD3-biotin (145-2C11; eBioscience), and biotinylated anti-Siglec-F (E50-2440; BD Biosciences). Cells labeled with biotinylated Abs were further stained with streptavidin-PerCp (eBioscience).
Cytokine ELISA
Cytokine concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA) systems with unlabeled capture Abs and labeled detection Abs. To determine the concentration of IL-4, monoclonal Ab (mAb) 11B11 was used as the capture Ab and biotin-labeled BVD6-24G2 (BD Biosciences) was used as the detection Ab, followed by incubation with peroxidase-labeled streptavidin (Southern Biotechnology Associates, Birmingham, AL). IFN-γ was captured by mAb AN-18 and detected by peroxidase-labeled mAb XMG1.2. The concentration of IL-17 was detected with the R&D Systems Duoset kit (R&D Systems GmbH, Wiesbaden, Germany).
Histopathological Analysis
Lung samples were processed for histological analysis, as previously described.11
IHC
Lung samples were processed for histological analysis, as previously described.15 In brief, the accessory lobe of the lung was sterilely removed, mounted on thick filter paper with Tissue Tek optimal cutting temperature compound (Miles Scientific, Naperville, IL), snap frozen in isopentane (Fluka, Neu-Ulm, Germany) precooled on dry ice, and stored at -80°C. For immunohistochemistry (IHC), 10-μm frozen sections were prepared in a serial fashion (30 transversal sections on six consecutive levels per lung). Glucuronoxylomannan immunostaining was performed using mAb 18B7 (provided by Dr. Arturo Casadevall, Albert Einstein College of Medicine, New York, NY). The mAb 18B7 was biotinylated (Sigma-Aldrich) before use, and lung slides were incubated in a secondary step with ExtrAvidin peroxidase (Sigma-Aldrich). The peroxidase reaction product was visualized using 3,3′-diaminobenzidine (Sigma-Aldrich) as the chromogene and H2O2 as the cosubstrate.
Serum Immunoglobin Measurement
Total serum IgG1, IgG2a, and IgE levels were analyzed, as described earlier.11 For determination of C. neoformans–specific serum IgE, the following capture ELISA was developed. ELISA plates (Nunc GmbH & Co KG, Langenselbold, Germany) were coated with 5 μg/mL rat–anti-mouse IgE mAb (R35-72; BD Biosciences) in carbonate buffer (pH 9.5) overnight at 4°C. To prevent unspecific binding, plates were washed and blocked with 5% (w/v) skim milk (Sigma-Aldrich) in PBS for 3 hours at room temperature. After washing, serum samples were diluted 1:10 in blocking buffer containing 0.1% (v/v) Tween 20 (Karl Roth AG, Karlsruhe, Germany) and added in duplicate for 2 hours at room temperature. Plates were washed again and biotinylated (Sigma-Aldrich). C. neoformans 1841D homogenate (5 μg/mL) was incubated for 2 hours at room temperature. This step was followed by incubation with peroxidase-labeled streptavidin (Southern Biotechnology Associates), diluted 1:4000 in Tween 20 containing blocking buffer, for 45 minutes at room temperature for detection. Plates were washed, and the TMB Microwell peroxidase system (KPL, Gaithersburg, MD) was used as a substrate for the final colorimetric reaction. The reaction was discontinued after 2 hours by adding 1 mol/L H3PO4, and ODs were read using a Spectra-max 340 ELISA reader (Molecular Devices GmbH, Ismaning, Germany) at 450 nm, with background subtraction at 630 nm. Wells incubated without serum samples but with all other reagents were used as plate blank. To control the specificity of this ELISA, we added a monoclonal IgE isotype control (C38-2; BD Biosciences) specific for the hapten trinitrophenyl that we also used as a standard for the total IgE quantification after coating with IgE mAb (R35-72; BD Biosciences) and blocking. After another incubation with blocking buffer, plates were either incubated with anti-mouse IgE–horseradish peroxidase (23G3; Southern Biotechnology Associates) or biotinylated C. neoformans homogenate. Plates incubated with biotinylated homogenate were additionally incubated with peroxidase-labeled streptavidin (Southern Biotechnology Associates). Development was performed with the TMB Microwell peroxidase system (KPL). This confirmed that even high concentrations of anti-trinitrophenyl IgE (eg, 20 μg/mL) do not bind to biotinylated C. neoformans homogenate nonspecifically. Specificity was further confirmed by using serum samples from naïve BALB/c WT instead of anti-trinitrophenyl IgE. The median OD450 was 0.015.
Statistical Analysis
The one-tailed Mann-Whitney U-test was performed to determine the significance of differences in kinetic analyses of 4get mice and between WT and ΔdblGATA mice. Data are presented as the mean ± SEM. The level of confidence for significance was P < 0.05.
Results
Pulmonary Th2 Development Occurs after 6 Weeks of Infection and Coincides with IL-4–Producing Eosinophils
Susceptibility in cryptococcosis is tightly linked with IL-4 production.7,11 BALB/c WT mice infected i.n. with only 500 CFUs of C. neoformans strain 1841 show dissemination from lung to brain beginning at approximately 6 weeks after infection (data not shown), leading to death 10 weeks after infection.7,11 This pulmonary cryptococcosis model is a long-term model compared with other published murine models.8,23–27
To monitor IL-4 production over time in this long-term model, IL-4 reporter mice, termed 4get mice, were infected, and enhanced green fluorescent protein (eGFP) expression, which is known to correlate with IL-4 transcription, was assessed.20 There were constitutively eGFP+ pulmonary leukocytes in naïve mice (Figures 1A and 2, A and B), as published by others.28,29 On infection of 4get mice, the frequency of eGFP+ leukocytes increased almost 10-fold, at 42 days postinfection (dpi), and stayed on this elevated level up to 70 dpi (Figure 1A). Because eGFP expression may indicate only IL-4 transcription,20 we wanted to assess IL-4 protein production. Indeed, we found that IL-4 production in response to antigen-specific stimulation of pulmonary leukocytes with cryptococcal antigen starts to become detectable at a similarly late time point as found for eGFP expression. IL-4 was not detectable at 35 dpi (data not shown) or earlier; instead, it started to become detectable 6 weeks after infection and increased up to 70 dpi (Figure 1B). Late IL-4 expression, monitored by eGFP expression or restimulation of pulmonary leukocytes, was further confirmed by intracellular staining of IL-4 in Th cells and revealed similar results (data not shown). Consistent with the time course of IL-4 production, total and specific IgE started to increase after 42 days of infection (Figure 1, C and D). Although the sandwich ELISA for total IgE detected a median concentration of approximately 13.65 μg/mL, starting after 42 days of infection, the ELISA for specific IgE resulted in minor signals of only up to 0.099 OD450, with a substrate development time of 2 hours. Thus, similar to parasite models,30 only a minor portion of the total IgE appears to be specific for cryptococcal antigens (Figure 1D).
Figure 1.
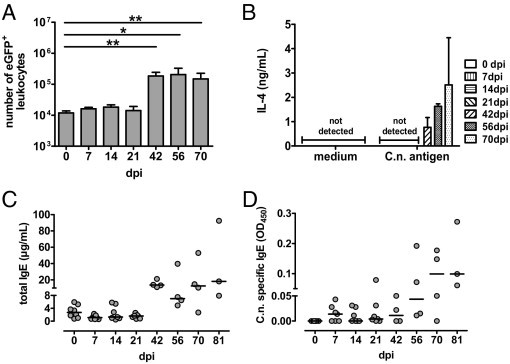
Coincidental accumulation of pulmonary leukocytes competent for IL-4 expression (indicated by eGFP), onset of IL-4 secretion, and increase of total and specific IgE. 4get mice were infected i.n. with C. neoformans 1841D. A: On the indicated dpi, leukocytes were isolated from lungs (n = 3 to 5 per time point), counted, and analyzed for eGFP-expression by flow cytometry. B: Pulmonary leukocytes were restimulated for 22 hours with C.n. antigen. IL-4 was measured by ELISA in culture supernatant. C and D: Serum samples from the same mice were examined for total IgE and Cryptococcus-specific IgE according to the Materials and Methods section. Data from two independent experiments were pooled and are expressed as the mean ± SEM. Statistical analysis was performed using the Mann-Whitney U-test. *P < 0.05 and **P < 0.01 comparison with naive 4get mice.
Figure 2.
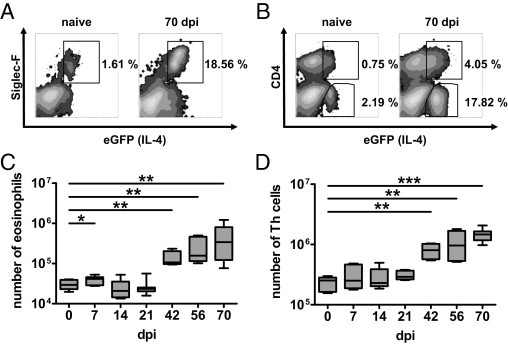
Th cells and eosinophils exclusively constitute eGFP+ cells during pulmonary cryptococcosis. Flow cytometry was performed on pulmonary leukocytes from i.n. infected 4get mice on the indicated dpi (n = 3 to 5 per time point). A: CD4−eGFP+ cells identified by Siglec-F expression as eosinophils in representative 4get mice are shown. B: eGFP expression in Th cells in the same 4get mice shown in A. C: Kinetic analysis of enumerated eosinophils. D: Th cells at the indicated dpi are shown. Kinetic data were pooled from two independent experiments. The mean ± SEM is shown for kinetic analyses. Statistics were performed with the Mann-Whitney U-test. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with naive 4get mice.
CD4+ Th cells, and innate immune cells, have been described as cellular sources of IL-4.29 To define the cell types producing IL-4 in pulmonary cryptococcosis, we characterized eGFP+ cells in the lungs of infected 4get mice. At 70 dpi, we found elevated numbers of eGFP+ pulmonary leukocytes in both CD4− and CD4+ populations (Figure 2, A and B). Interestingly, the CD4−eGFP+ population was identified to be Siglec-F+, pointing to eosinophils (Figure 2A).31 This prompted us to characterize the time course of recruitment of Th cells and eosinophilic granulocytes. The data shown in Figure 2, C and D, demonstrate that there is a similarly late time course of recruitment to the lung for both Th cells and eosinophils.
To directly define the cellular source(s) of pulmonary IL-4 production in cryptococcosis, we applied IL-4 secretion assays on magnetic cell sorting (MACS)-separated (Miltenyi) lung CD4+ and CD4− cells to avoid cross feeding between different cells (Figure 3). Th2s represent a cell type already known to be responsible for efficient IL-4 production in cryptococcosis.7,11 In addition to the Th cells as IL-4 sources (Figure 3C), we show that eosinophilic granulocytes (Figure 3A; further gated on Siglec-F+/CD11cdim) produce significant amounts of IL-4 on Cryptococcus infection (Figure 3B). Interestingly, a major portion of these eosinophils (ie, 17.49%) produced IL-4 constitutively after pulmonary infection with C. neoformans (Figure 3B, top; mean fluorescence intensity of isotype control (not shown) versus medium, 133.59 versus 330.43). This could be further enhanced (41.63% of all eosinophils) by ex vivo stimulation with a combination of PMA and ionomycin (mean fluorescence intensity, 784.66; Figure 3B, bottom). Together, these data demonstrate that, during pulmonary cryptococcosis, Th2 cells and eosinophils contribute to late IL-4 production at a point when IgE production is significantly increased and C. neoformans disseminates from the lung to the brain.
Figure 3.
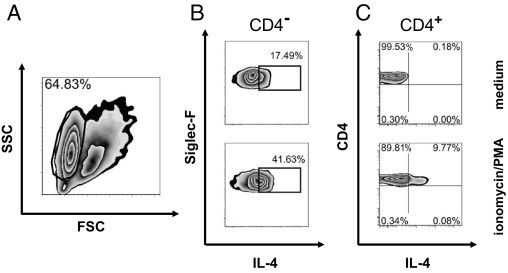
Both Th cells and eosinophils are the main producers of IL-4 in the lungs of infected mice. Isolated pulmonary leukocytes from i.n. infected BALB/c WT mice were positively enriched for CD4+ Th cells by MACS. A: The remaining CD4− cells include eosinophils [gated on a side scatter (SSC)high/forward scatter (FSC)low plot]. Both CD4− cells (B) and CD4+ Th cells (C) were either stimulated with ionomycin and PMA (bottom) or left untreated (top) before performing an IL-4 secretion assay. One of two independent experiments is shown.
In the Absence of Eosinophils, Th2 Responses Are Reduced and Th1/Th17 Responses Are Enhanced
IL-4 is not essential for Th2 differentiation but plays a nonredundant role in the maintenance of Th2 responses.32 We were interested in whether eosinophils can contribute to Th2 responses in cryptococcosis. Thus, we infected WT and eosinophil-deficient ΔdblGATA mice21 i.n. with C. neoformans. The i.n. infection of WT mice led to accumulation of Siglec-F+/CD11cdim eosinophils in the lung, which was not the case for ΔdblGATA mice, as expected (Figure 4A). We then assessed the frequency of pulmonary Th2 cells in infected WT versus ΔdblGATA mice. In the absence of eosinophils, pulmonary Th2 cells, characterized by the expression of IL-33 receptor (IL-33R), are greatly reduced, but not completely lacking, in ΔdblGATA mice (Figure 4B). Earlier data demonstrated that the IL-33R, also termed T1/ST2, is specifically expressed on differentiated but not on naïve Th cells.33
Figure 4.
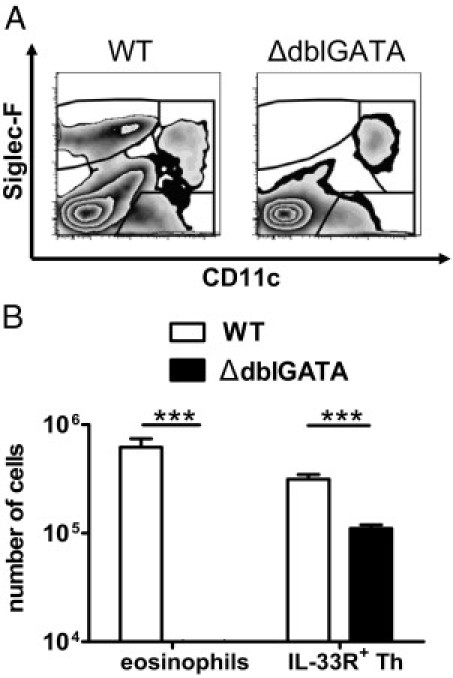
Mice devoid of eosinophils harbor fewer Th cells, indicated by IL-33R expression. Flow cytometry was performed on pulmonary leukocytes from i.n. infected BALB/c WT and ΔdblGATA mice at 60 dpi. A: The absence of eosinophils is confirmed by plots because no CD11cdim/Siglec-Fhigh can be detected in the ΔdblGATA mice (right). A representative WT mouse is shown (left). B: Enumeration of total IL-33R+ CD4+ Th cells is shown, together with eosinophils. One of three independent experiments is shown (n = 6 to 7 per genotype). Values are given as the mean ± SEM. Statistical analysis was performed by using the Mann-Whitney U-test. ***P < 0.001.
Th cells are central regulators of anticryptococcal immune responses.25,34,35 Although Th2 responses are detrimental,11,15 Th1 and Th17 responses are protective.7,9,25,35,36 To gain a deeper insight into the Th cytokine profile in the absence of eosinophils, we analyzed IL-4, IFN-γ, and IL-17A production by pulmonary Th cells on infection of WT and eosinophil-deficient ΔdblGATA mice. Analysis of IL-4, IFN-γ, and IL-17A in the supernatants of pulmonary leukocytes stimulated with cryptococcal antigen revealed reduced IL-4 and enhanced IFN-γ and IL-17 production by restimulated pulmonary leukocytes of ΔdblGATA versus WT mice (Figure 5, A–C). IL-4 production is substantially reduced, but not completely lacking, in pulmonary CD4+ T cells from ΔdblGATA mice. This indicates a shift from Th2 to Th1/Th17 responses in the absence of eosinophils.
Figure 5.
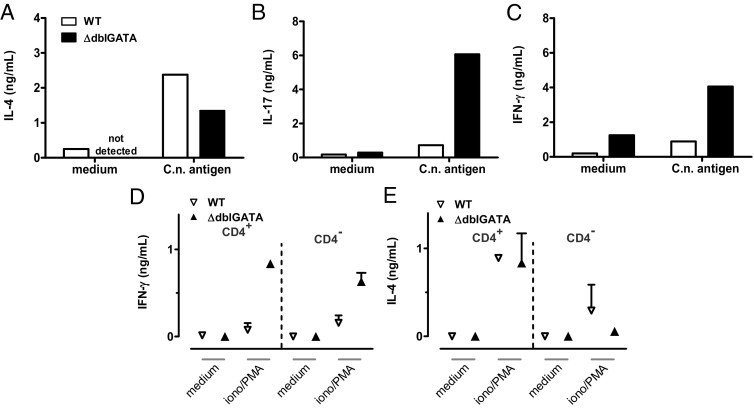
The absence of eosinophils promotes pronounced secretion of Th1- and Th17-associated cytokines and reduces the Th2 response. Pulmonary leukocytes were isolated from i.n. infected BALB/c WT and ΔdblGATA mice at 60 dpi. Cells were pooled per group and stimulated with C.n. antigen for 22 hours. A through C: IL-4, IL-17, and IFN-γ were measured by ELISA in culture supernatant, according to the Materials and Methods section. One representative of three independent experiments is shown (n = 6 to 7 per genotype). In addition, IFN-γ (D) and IL-4 (E) in the supernatant from MACS-enriched pulmonary CD4+ Th cells and remaining cells either stimulated with ionomycin/PMA or left untreated for 2 hours from BALB/c WT and ΔdblGATA mice at 60 dpi are shown. Data were pooled from two independent experiments (n = 3 pooled mice per genotype and experiment).
By direct intracellular staining of IL-4 in CD4+ T cells (Figure 6), we corroborate the data shown in Figure 5, A–C, clearly demonstrating a pronounced Th2 response in Cryptococcus-infected WT mice. We were also able to demonstrate that only CD154+ Th cells (ie, antigen-specific Th cells37,38) from infected mice responded with IL-4 production on stimulation with cryptococcal antigen (data not shown). Pulmonary Th cells from ΔdblGATA mice have similar proportions of Th1 cells, while they generate higher proportions of Th17 cells (Figure 6, antigen panel). A similar relative frequency of IFN-γ+ Th1 cells (Figure 6) but elevated IFN-γ levels in supernatants of antigen specifically restimulated pulmonary leukocytes (Figure 5C), suggests higher IFN-γ production on a per-cell basis in Th1 cells from ΔdblGATA mice (Figure 6, C.n. antigen; IFN-γ mean fluorescence intensity, 529.30 for WT and 713.26 for ΔdblGATA). The hypothesis of a greater IFN-γ potency of ΔdblGATA Th cells on a single-cell basis is further supported by the results shown in Figure 5D (CD4+ ionomycin/PMA; mean concentration of WT versus ΔdblGATA, 0.078 versus 0.835 pg/mL) because purified pulmonary Th cells were restimulated at an equal cell concentration when using this approach. Analysis of IL-4 from the same Th cells revealed comparable potency in IL-4 secretion (Figure 5E; CD4+ ionomycin/PMA). Therefore, the reduced amounts of IL-4 shown in Figure 5A may result from fewer Th cells in the lungs of eosinophil-deficient mice (Figure 7B). More important, we detected a substantial amount of IL-4 in the Th-depleted fraction after ionomycin/PMA stimulation (Figure 5E; CD4− ionomycin/PMA; mean concentration of WT versus ΔdblGATA, 294 versus 0.056 pg/mL). Because ΔdblGATA mice are devoid of eosinophils (Figure 4A) and no other potential source(s) of IL-4 could be identified in this infection model, the detected IL-4 appears to depend on eosinophils in WT mice and reaches approximately one third of the Th-derived IL-4 (Figure 5E; CD4+ ionomycin/PMA; mean concentration of WT versus ΔdblGATA, 890 versus 832 pg/mL). Consistent with lower IL-4 production in ΔdblGATA mice (Figure 5A), we observed substantially reduced IgE and elevated IgG2a (a marker for a Th1 response) serum levels in the absence of eosinophils (data not shown). Therefore, the presence of eosinophils contributes to enhanced fatal Th2 and reduced protective Th1 and Th17 responses.
Figure 6.
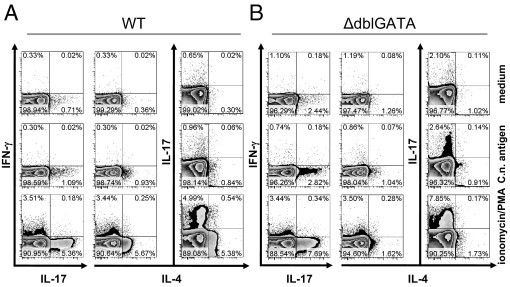
The Th cytokine profile is dependent on eosinophils on infection. Pulmonary leukocytes were isolated from i.n. infected BALB/c WT (A) and ΔdblGATA (B) mice at 60 dpi. Cells were pooled per group and allowed to rest for 22 hours as the negative control (top), stimulated with C.n. antigen for 22 hours (middle), or stimulated with ionomycin/PMA for 6 hours (bottom). Intracellular cytokine staining was performed according to the Materials and Methods section, and plots are gated on living CD4+ Th cells. Appropriate isotype controls for staining Abs were used (data not shown), confirming the specific staining. One representative of three independent experiments is shown (n = 6 to 7 per genotype).
Figure 7.
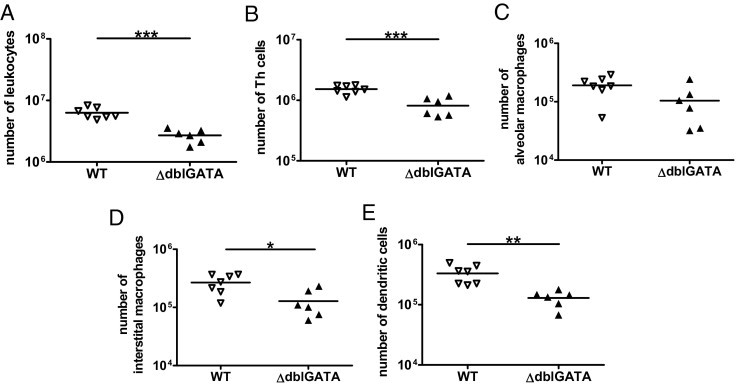
Recruitment of leukocytes to lung parenchyma after cryptococcal infection in the absence and presence of eosinophils. Pulmonary leukocytes were isolated from i.n. infected BALB/c WT and ΔdblGATA mice at 60 dpi. The numbers of total leukocytes (A), CD4+ Th cells (B), alveolar macrophages (C), interstitial macrophages (D), and dendritic cells (E) are shown. One representative of three independent experiments is shown as the mean ± SEM (n = 6 to 7 per genotype). Statistical analysis was performed by using the Mann-Whitney U-test. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with BALB/c WT and ΔdblGATA mice.
Elevated Recruitment of Leukocytes in the Presence of Eosinophils
To study the pulmonary inflammatory response in the presence and absence of eosinophils, infected WT versus ΔdblGATA mice were analyzed at 60 dpi when significant eosinophils were present in the lungs of C. neoformans–infected 4get mice (Figure 2C). The pulmonary inflammatory response of infected 4get mice closely resembles WT mice (data not shown). Interestingly, more lung leukocytes were found in WT compared with ΔdblGATA mice (Figure 7A). An elevated frequency of total leukocytes in the presence of eosinophils corresponded with elevated numbers of Th cells in the lung (Figure 7B). We wanted to characterize the composition of other pulmonary leukocytes important in cryptococcosis. Macrophages are central effector cells that are able to direct the outcome of C. neoformans infection.4,5,39,40 Alveolar and interstitial macrophages, and pulmonary dendritic cells were reduced in the absence of eosinophils (Figure 7, C–E). In summary, the data demonstrate a significant role of eosinophils in the recruitment of inflammatory cells on pulmonary infection with C. neoformans.
In the Absence of Eosinophils, Fungal Replication Is Reduced, but This Does Not Prevent Dissemination of C. neoformans to the Brain
Histopathological analysis of lungs from infected WT and ΔdblGATA mice revealed fewer and smaller foci of cryptococci in the absence of eosinophils (Figure 8, A and B). Infected WT mice developed large aggregates of fungi in their lungs (Figure 8, C–F), which was also corroborated by detection of the cryptococcal capsular component glucuronoxylomannan (Figure 8, G and H). When we analyzed the total number of viable cryptococci contained in the lung, we observed substantially fewer (approximately 84-fold reduction of median fungal load) in ΔdblGATA mice compared with WT mice; however, this difference did not reach statistical significance (Figure 9A, P = 0.0734). In line with this finding, dissemination of Cryptococcus to the brain was not prevented in ΔdblGATA mice (Figure 9B, P = 0.1375). This indicates that the absence of eosinophils has a limited impact on protective pulmonary immunity against C. neoformans and does not suffice to prevent fungal dissemination.
Figure 8.
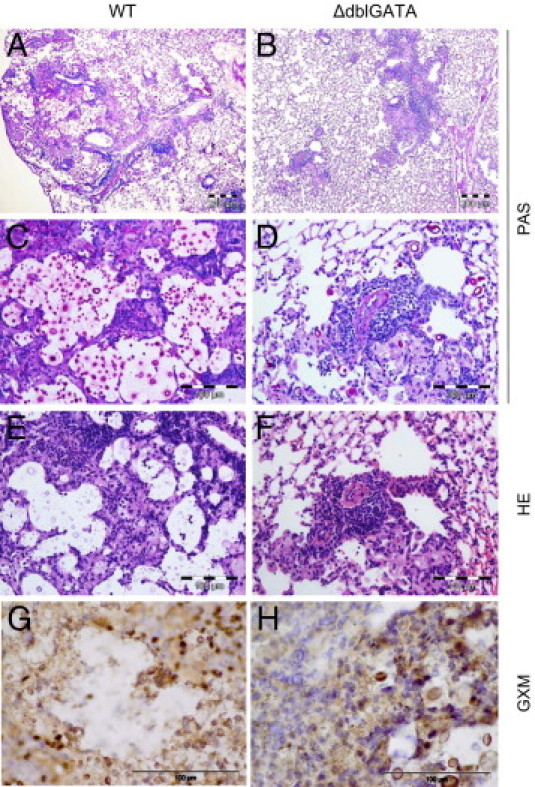
Lung sections from infected WT and ΔdblGATA mice indicate better fungal control in the absence of eosinophils at 60 dpi. A through D: PAS staining. Scale bars: 200 μm (A and B); 100 μm (C and D). E and F: H&E staining. Scale bar = 100 μm. G and H: IHC was performed on sections from the same mice, and glucuronoxylomannan (GXM)–containing foci are brown. Scale bar = 100 μm. ΔdblGATA mice show reduced numbers of cryptococci in the lungs (A–F) and formation of smaller foci of accumulating cryptococci and GXM compared with WT mice (G and H). There was pronounced influx of inflammatory cells in WT compared with ΔdblGATA mice.
Figure 9.
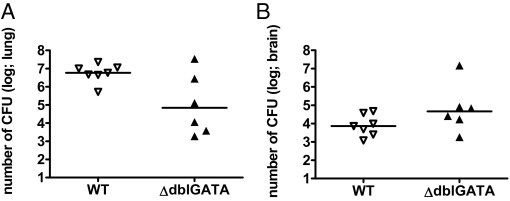
Organ burden in the presence and absence of eosinophils. Lung (A) and brain (B) fungal burden of WT and ΔdblGATA mice at 60 dpi was evaluated according to the Materials and Methods section. One representative of three independent experiments is shown as the median (n = 6 to 7 per genotype). Statistical analysis was performed by using the Mann-Whitney U-test, indicating P = 0.0734 for lung burden and P = 0.1375 for brain burden.
Discussion
In this study, we report a regulatory role of eosinophilic granulocytes in cryptococcosis. Eosinophils have been mentioned before in murine models of cryptococcosis,10,23,41–43 observed in human cryptococcosis,44–46 and described with an emphasis on tissue damage.41 Although in vitro eosinophils have phagocytosed C. neoformans23 and presented cryptococcal antigens,47 in vivo, no evidence for uptake of C. neoformans by eosinophils has been found by others4 and in this study (data not shown). Herein, we highlight an immunoregulatory role of eosinophils that contribute to IL-4–dependent immunopathological features during murine pulmonary C. neoformans infection. We provide evidence for previously unrecognized features of eosinophils during bronchopulmonary infection. The protective immune response against C. neoformans relies on Th1-biased cellular immunity.7,48 However, even in the presence of IFN-γ, IL-4 production has been detrimental in pulmonary cryptococcosis.11 An exquisite role of IL-4 signaling strength has been demonstrated in our pulmonary cryptococcosis model, with a gene dosage effect of the IL-4 receptor α alleles.18 Thus, in this report, we focus on nonprotective IL-4 production by Th cells and innate immune cells. In brief, the IL-4 competence of Th cells and eosinophils was determined by eGFP expression during 10 weeks of infection. Both cell populations show a concomitant late increase in lung parenchyma. The quantitative data obtained for IL-4 derived from Th cells and eosinophils indicate that Th2 cells are a major cellular source, followed by eosinophils as an innate cellular source of IL-4 (Figure 5E). Moreover, in cryptococcosis, eosinophils promote Th2 responses but are not essential for Th2 differentiation because we found a considerable residual frequency of IL-33R+ Th2 cells in eosinophil-deficient mice (Figure 4B). Although the absence of eosinophils favors the development of a more Th1/Th17 pronounced response by modulating the Th cell cytokine secretory capacity (Figures 5, D and E, and 6), this cannot prevent dissemination of fungi, as shown by the brain cryptococcal burden (Figure 9B). Dissemination of cryptococci to the brain is only abrogated when IL-4, IL-13, or IL-4/IL-13 signaling is completely abolished.15
The Th2 promoting property of eosinophils has also been shown recently in a murine asthma model induced by an Aspergillus fumigatus extract.49 Similar to pulmonary cryptococcosis, eosinophil-deficient ΔdblGATA mice showed reduced levels of pulmonary Th2-related cytokines and mononuclear cell recruitment.49 The contribution of other innate immune cells (eg, basophils) potentially involved in fatal Th2 initiation in cryptococcosis remains to be tested, because basophils have been shown recently in models of parasitic disease and a protease allergen model to play an essential role in Th2 differentiation.50–52
In addition to well-described developmental requirements for Th1 and Th17 responses,53,54 mechanisms of Th2 initiation are less unraveled and controversy on Th2-inducing molecules and cells continues.32,33,54–58 Since the introduction of the Th1/Th2 paradigm, IL-4 has been tightly associated with Th2 responses59–61; there is clear evidence that IL-4 is indispensable for Th2 maintenance.62 For Th2 initiation, current studies point to nonhematopoietic cells that appear to be able to support innate immune cells by secretion of chemokines63 and novel cytokines, such as IL-33,33 IL-25,32,58 and thymic stromal lymphopoietin (TSLP).64 In pulmonary cryptococcosis, airway epithelial cells and eosinophils would be candidates for cross talk between resident tissue cells and leukocytes.65 Interestingly, we found expression of IL-33R on eosinophils in this study of pulmonary cryptococcosis (data not shown). Thus, eosinophils could be cellular targets of IL-33 produced by epithelial cells66 and, thereby, could contribute to Th2 initiation. The definitive roles of IL-33, its cellular sources, and targets in anticryptococcal immunity remain to be defined.
Eosinophils were recognized for a long time as effector cells acting by degranulation in helminth/parasitic infections with Trichinella spiralis67,68 or Schistosoma mansoni.69 Eosinophils demonstrate protective mechanisms that rely on degranulation in bacterial infections70 and release mitochondrial DNA in a unique way that clumps bacteria together.71 In addition, antiviral effects have been reported.72 Investigations of the role of eosinophils in fungal infection with Candida albicans,73–75 Alternaria alternata,76 or C. neoformans10,23,41,42 were made, with a focus on their effector function. Similarly, a study10 using anti-IL-5 treatment showed an association of eosinophil frequency with susceptibility during cryptococcosis. During the past decade, several reports77,78 extended the function of eosinophils beyond the sole defense against nonphagotizable pathogens. Eosinophils produced various chemokines and cytokines modulating immune responses in different models.29,49,79,80 Lee and colleagues81 recently introduced the term LIAR (regulators of local immunity and/or remodeling/repair) for eosinophils; this term summarizes more recently discovered regulatory properties of eosinophilic granulocytes. Our data from a chronic fungal infection support the regulators of local immunity and/or remodeling/repair concept of eosinophil function. Other eosinophil-dependent factors, in addition to IL-4, that are involved in regulation of the Th cytokine profile and leukocyte recruitment require further investigation to enlighten the pathophysiological role that eosinophils play in cryptococcosis.
In conclusion, IL-4 production by both eosinophils and antigen-specific Th2 cells is a relatively late event in pulmonary cryptococcosis. A late and as of yet unidentified process appears to promote the onset of IL-4 production that dominates the production of otherwise protective cytokines IL-17 and IFN-γ. This suggests a cytokine hierarchy, with IL-4 on top of IFN-γ/IL-17 underlining the exquisite role of IL-4 in cryptococcosis. Therefore, it is intriguing to develop therapies antagonizing IL-4 or its receptor. Certainly, the late onset of IL-4 production by Th cells and eosinophils (shown herein) and functional studies in IL-4– or IL-4 receptor α–deficient mice, reported earlier by us,18 make IL-4 or its receptor attractive drug targets in allergic bronchopulmonary mycosis and possibly in asthma.
Acknowledgments
We thank our colleagues in Leipzig (Anett Grohs, Petra Krumbholz, and the animal caretaker team, headed by Rowina Voigtländer), Münster (Petra Meier and Cordula Westermann), and Berlin (Alexandra Döser).
Footnotes
Supported by the Doktorandenförderprogramm (graduation program) of the University of Leipzig (D.P.) and a grant from the Deutsche Forschungsgemeinschaft (German Research Foundation) (MU 2283/2-1 to U.M.).
G.A. and U.M. contributed equally to this work.
CME Disclosure: None of the authors disclosed any relevant financial relationships.
References
- 1.Lin X., Heitman J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell T.G., Perfect J.R. Cryptococcosis in the era of AIDS: 100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 4.Feldmesser M., Tucker S., Casadevall A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001;9:273–278. doi: 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 5.Voelz K., Lammas D.A., May R.C. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voelz K., May R.C. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9:835–846. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decken K., Kohler G., Palmer-Lehmann K., Wunderlin A., Mattner F., Magram J., Gately M.K., Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoag K.A., Lipscomb M.F., Izzo A.A., Street N.E. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 9.Kleinschek M.A., Muller U., Schutze N., Sabat R., Straubinger R.K., Blumenschein W.M., McClanahan T., Kastelein R.A., Alber G. Administration of IL-23 engages innate and adaptive immune mechanisms during fungal infection. Int Immunol. 2010;22:81–90. doi: 10.1093/intimm/dxp117. [DOI] [PubMed] [Google Scholar]
- 10.Huffnagle G.B., Boyd M.B., Street N.E., Lipscomb M.F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- 11.Muller U., Stenzel W., Kohler G., Werner C., Polte T., Hansen G., Schutze N., Straubinger R.K., Blessing M., McKenzie A.N., Brombacher F., Alber G. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y.C., Stins M.F., McCaffery M.J., Miller G.F., Pare D.R., Dam T., Paul-Satyaseela M., Kim K.S., Kwon-Chung K.J. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlier C., Chretien F., Baudrimont M., Mordelet E., Lortholary O., Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol. 2005;166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlier C., Nielsen K., Daou S., Brigitte M., Chretien F., Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenzel W., Muller U., Kohler G., Heppner F.L., Blessing M., McKenzie A.N., Brombacher F., Alber G. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol. 2009;174:486–496. doi: 10.2353/ajpath.2009.080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman D.L., Khine H., Abadi J., Lindenberg D.J., Pirofski L., Niang R., Casadevall A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 17.Goldman D.L., Davis J., Bommarito F., Shao X., Casadevall A. Enhanced allergic inflammation and airway responsiveness in rats with chronic Cryptococcus neoformans infection: potential role for fungal pulmonary infection in the pathogenesis of asthma. J Infect Dis. 2006;193:1178–1186. doi: 10.1086/501363. [DOI] [PubMed] [Google Scholar]
- 18.Muller U., Stenzel W., Kohler G., Polte T., Blessing M., Mann A., Piehler D., Brombacher F., Alber G. A gene-dosage effect for interleukin-4 receptor alpha-chain expression has an impact on Th2-mediated allergic inflammation during bronchopulmonary mycosis. J Infect Dis. 2008;198:1714–1721. doi: 10.1086/593068. [DOI] [PubMed] [Google Scholar]
- 19.Osterholzer J.J., Surana R., Milam J.E., Montano G.T., Chen G.H., Sonstein J., Curtis J.L., Huffnagle G.B., Toews G.B., Olszewski M.A. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol. 2009;174:932–943. doi: 10.2353/ajpath.2009.080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohrs M., Shinkai K., Mohrs K., Locksley R.M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 21.Yu C., Cantor A.B., Yang H., Browne C., Wells R.A., Fujiwara Y., Orkin S.H. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinschek M.A., Muller U., Brodie S.J., Stenzel W., Kohler G., Blumenschein W.M., Straubinger R.K., McClanahan T., Kastelein R.A., Alber G. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 23.Feldmesser M., Casadevall A., Kress Y., Spira G., Orlofsky A. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infect Immun. 1997;65:1899–1907. doi: 10.1128/iai.65.5.1899-1907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez Y., Arora S., Erb-Downward J.R., McDonald R.A., Toews G.B., Huffnagle G.B. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol. 2005;174:1027–1036. doi: 10.4049/jimmunol.174.2.1027. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami K., Kohno S., Morikawa N., Kadota J., Saito A., Hara K. Activation of macrophages and expansion of specific T lymphocytes in the lungs of mice intratracheally inoculated with Cryptococcus neoformans. Clin Exp Immunol. 1994;96:230–237. doi: 10.1111/j.1365-2249.1994.tb06547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami K., Koguchi Y., Qureshi M.H., Miyazato A., Yara S., Kinjo Y., Iwakura Y., Takeda K., Akira S., Kurimoto M., Saito A. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-gamma production by NK cells. J Immunol. 2000;165:941–947. doi: 10.4049/jimmunol.165.2.941. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K., Qureshi M.H., Zhang T., Koguchi Y., Yara S., Takeda K., Akira S., Kurimoto M., Saito A. Involvement of endogenously synthesized interleukin (IL)-18 in the protective effects of IL-12 against pulmonary infection with Cryptococcus neoformans in mice. FEMS Immunol Med Microbiol. 2000;27:191–200. doi: 10.1111/j.1574-695X.2000.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 28.Gessner A., Mohrs K., Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 29.Voehringer D., Shinkai K., Locksley R.M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 30.Pochanke V., Koller S., Dayer R., Hatak S., Ludewig B., Zinkernagel R.M., Hengartner H., McCoy K.D. Identification and characterization of a novel antigen from the nematode Nippostrongylus brasiliensis recognized by specific IgE. Eur J Immunol. 2007;37:1275–1284. doi: 10.1002/eji.200737135. [DOI] [PubMed] [Google Scholar]
- 31.Shinkai K., Mohrs M., Locksley R.M. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 32.Paul W.E. What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol. 2010;88:236–239. doi: 10.1038/icb.2010.2. [DOI] [PubMed] [Google Scholar]
- 33.Lohning M., Stroehmann A., Coyle A.J., Grogan J.L., Lin S., Gutierrez-Ramos J.C., Levinson D., Radbruch A., Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huffnagle G.B., Yates J.L., Lipscomb M.F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami K., Kohno S., Kadota J., Tohyama M., Teruya K., Kudeken N., Saito A., Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-gamma and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–143. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen G.H., McDonald R.A., Wells J.C., Huffnagle G.B., Lukacs N.W., Toews G.B. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 2005;73:1788–1796. doi: 10.1128/IAI.73.3.1788-1796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chattopadhyay P.K., Yu J., Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 38.Frentsch M., Arbach O., Kirchhoff D., Moewes B., Worm M., Rothe M., Scheffold A., Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 39.Arora S., Hernandez Y., Erb-Downward J.R., McDonald R.A., Toews G.B., Huffnagle G.B. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174:6346–6356. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y., Wang F., Bhan U., Huffnagle G.B., Toews G.B., Standiford T.J., Olszewski M.A. TLR9 signaling is required for generation of the adaptive immune protection in Cryptococcus neoformans-infected lungs. Am J Pathol. 2010;177:754–765. doi: 10.2353/ajpath.2010.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldmesser M., Kress Y., Casadevall A. Intracellular crystal formation as a mechanism of cytotoxicity in murine pulmonary Cryptococcus neoformans infection. Infect Immun. 2001;69:2723–2727. doi: 10.1128/IAI.69.4.2723-2727.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain A.V., Zhang Y., Fields W.B., McNamara D.A., Choe M.Y., Chen G.H., Erb-Downward J., Osterholzer J.J., Toews G.B., Huffnagle G.B., Olszewski M.A. Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infect Immun. 2009;77:5389–5399. doi: 10.1128/IAI.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera J., Casadevall A. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J Immunol. 2005;174:8017–8026. doi: 10.4049/jimmunol.174.12.8017. [DOI] [PubMed] [Google Scholar]
- 44.Marwaha R.K., Trehan A., Jayashree K., Vasishta R.K. Hypereosinophilia in disseminated cryptococcal disease. Pediatr Infect Dis J. 1995;14:1102–1103. [PubMed] [Google Scholar]
- 45.Sun H.Y., Alexander B.D., Lortholary O., Dromer F., Forrest G.N., Lyon G.M., Somani J., Gupta K.L., Del B.R., Pruett T.L., Sifri C.D., Limaye A.P., John G.T., Klintmalm G.B., Pursell K., Stosor V., Morris M.I., Dowdy L.A., Munoz P., Kalil A.C., Garcia-Diaz J., Orloff S.L., House A.A., Houston S.H., Wray D., Huprikar S., Johnson L.B., Humar A., Razonable R.R., Fisher R.A., Husain S., Wagener M.M., Singh N. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785–791. doi: 10.3109/13693780903496617. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi H., Komase Y., Ikehara M., Yamamoto T., Shinagawa T. Disseminated cryptococcal infection with eosinophilia in a healthy person. J Infect Chemother. 2008;14:319–324. doi: 10.1007/s10156-008-0618-z. [DOI] [PubMed] [Google Scholar]
- 47.Garro A.P., Chiapello L.S., Baronetti J.L., Masih D.T. Rat eosinophils stimulate the expansion of Cryptococcus neoformans-specific CD4(+) and CD8(+) T cells with a T-helper 1 profile. Immunology. 2011;132:174–187. doi: 10.1111/j.1365-2567.2010.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy J.W. Protective cell-mediated immunity against Cryptococcus neoformans. Res Immunol. 1998;149:373–386. doi: 10.1016/s0923-2494(98)80761-x. [DOI] [PubMed] [Google Scholar]
- 49.Fulkerson P.C., Fischetti C.A., McBride M.L., Hassman L.M., Hogan S.P., Rothenberg M.E. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R., Nair M.G., Du Y., Zaph C., Van R.N., Comeau M.R., Pearce E.J., Laufer T.M., Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimoto T., Yasuda K., Tanaka H., Nakahira M., Imai Y., Fujimori Y., Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 52.Sokol C.L., Chu N.Q., Yu S., Nish S.A., Laufer T.M., Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy K.M., Reiner S.L. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J., Paul W.E. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comeau M.R., Ziegler S.F. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–147. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- 56.Nagata Y., Kamijuku H., Taniguchi M., Ziegler S., Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 57.Omori M., Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y.H., Liu Y.J. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boom W.H., Liano D., Abbas A.K. Heterogeneity of helper/inducer T lymphocytes, II: effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988;167:1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurt-Jones E.A., Hamberg S., Ohara J., Paul W.E., Abbas A.K. Heterogeneity of helper/inducer T lymphocytes, I: lymphokine production and lymphokine responsiveness. J Exp Med. 1987;166:1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherwinski H.M., Schumacher J.H., Brown K.D., Mosmann T.R. Two types of mouse helper T cell clone, III: further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankovic D., Kullberg M.C., Noben-Trauth N., Caspar P., Paul W.E., Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 63.Ochkur S.I., Jacobsen E.A., Protheroe C.A., Biechele T.L., Pero R.S., McGarry M.P., Wang H., O'Neill K.R., Colbert D.C., Colby T.V., Shen H., Blackburn M.R., Irvin C.C., Lee J.J., Lee N.A. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 64.Barrett N.A., Austen K.F. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Headley M.B., Zhou B., Shih W.X., Aye T., Comeau M.R., Ziegler S.F. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gleich G.J., Frigas E., Loegering D.A., Wassom D.L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925–2927. [PubMed] [Google Scholar]
- 68.Wassom D.L., Gleich G.J. Damage to Trichinella spiralis newborn larvae by eosinophil major basic protein. Am J Trop Med Hyg. 1979;28:860–863. [PubMed] [Google Scholar]
- 69.Butterworth A.E., Vadas M.A., Wassom D.L., Dessein A., Hogan M., Sherry B., Gleich G.J., David J.R. Interactions between human eosinophils and schistosomula of Schistosoma mansoni, II: the mechanism of irreversible eosinophil adherence. J Exp Med. 1979;150:1456–1471. doi: 10.1084/jem.150.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linch S.N., Kelly A.M., Danielson E.T., Pero R., Lee J.J., Gold J.A. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77:4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., Schmid I., Straumann A., Reichenbach J., Gleich G.J., Simon H.U. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 72.Domachowske J.B., Bonville C.A., Dyer K.D., Easton A.J., Rosenberg H.F. Pulmonary eosinophilia and production of MIP-1alpha are prominent responses to infection with pneumonia virus of mice. Cell Immunol. 2000;200:98–104. doi: 10.1006/cimm.2000.1620. [DOI] [PubMed] [Google Scholar]
- 73.Inoue Y., Matsuwaki Y., Shin S.H., Ponikau J.U., Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 74.Ishikawa T., Yu M.C., Arbesman C.E. Electron microscopic demonstration of phagocytosis of Candida albicans by human eosinophilic leukocytes. J Allergy Clin Immunol. 1972;50:183–187. doi: 10.1016/0091-6749(72)90049-8. [DOI] [PubMed] [Google Scholar]
- 75.Witkin S.S., Jeremias J., Ledger W.J. Vaginal eosinophils and IgE antibodies to Candida albicans in women with recurrent vaginitis. J Med Vet Mycol. 1989;27:57–58. [PubMed] [Google Scholar]
- 76.Yoon J., Ponikau J.U., Lawrence C.B., Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobsen E.A., Taranova A.G., Lee N.A., Lee J.J. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 78.Rothenberg M.E., Hogan S.P. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 79.Blanchard C., Rothenberg M.E. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobsen E.A., Ochkur S.I., Pero R.S., Taranova A.G., Protheroe C.A., Colbert D.C., Lee N.A., Lee J.J. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J.J., Jacobsen E.A., McGarry M.P., Schleimer R.P., Lee N.A. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


