Abstract
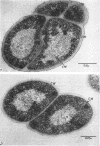
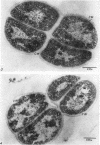
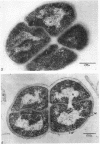
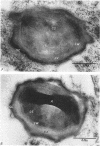
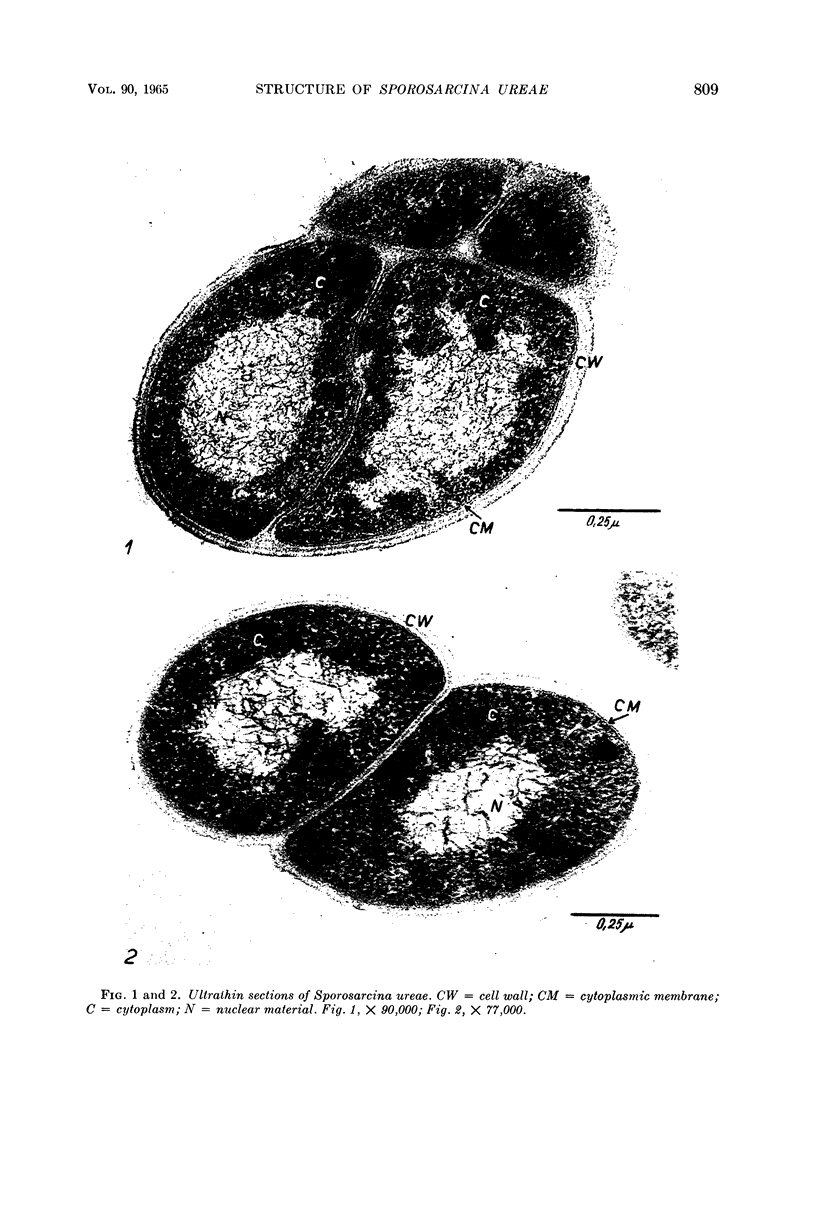
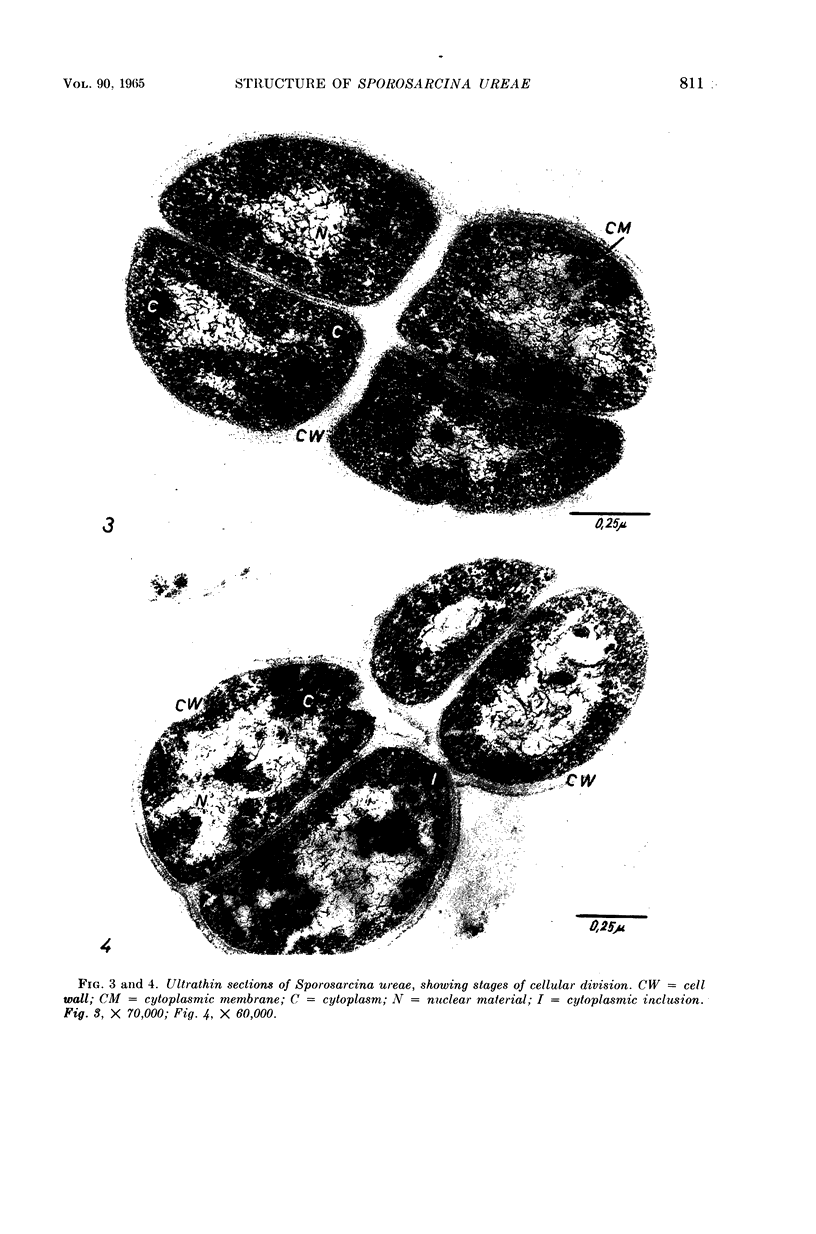
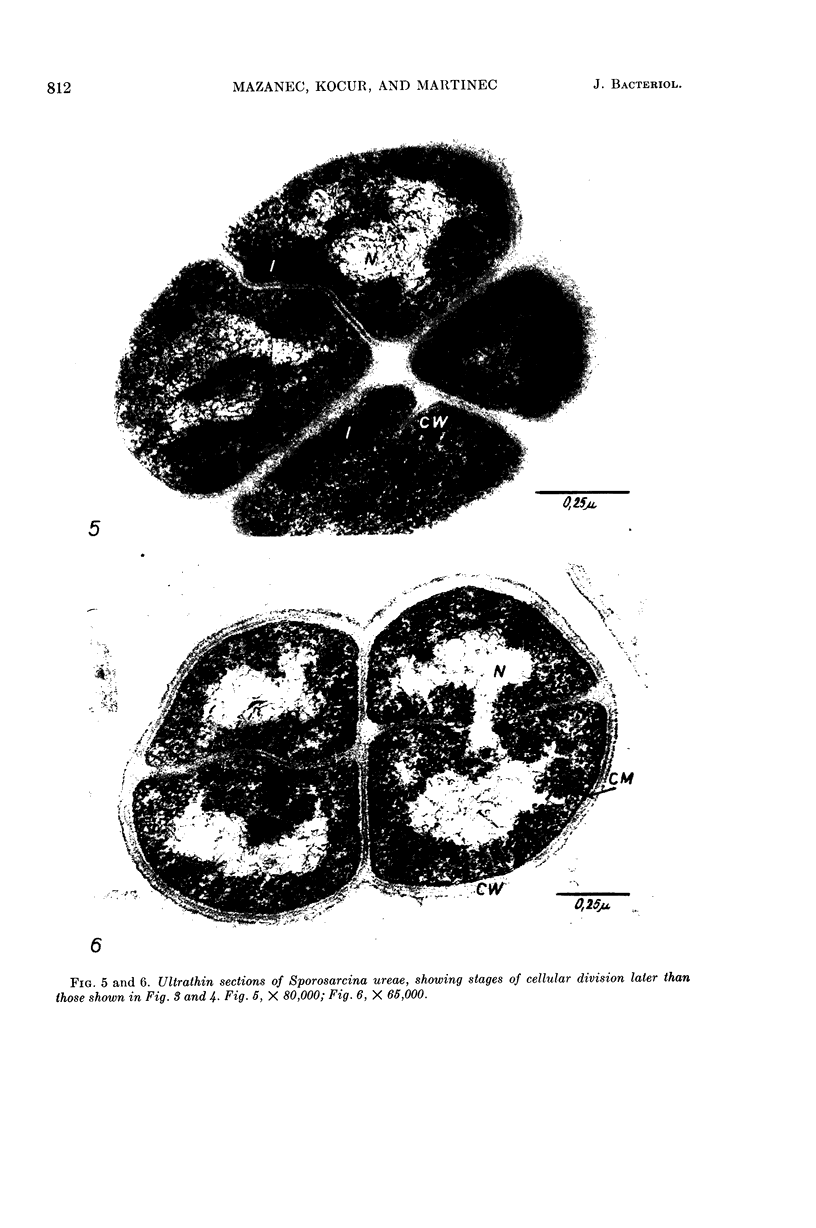
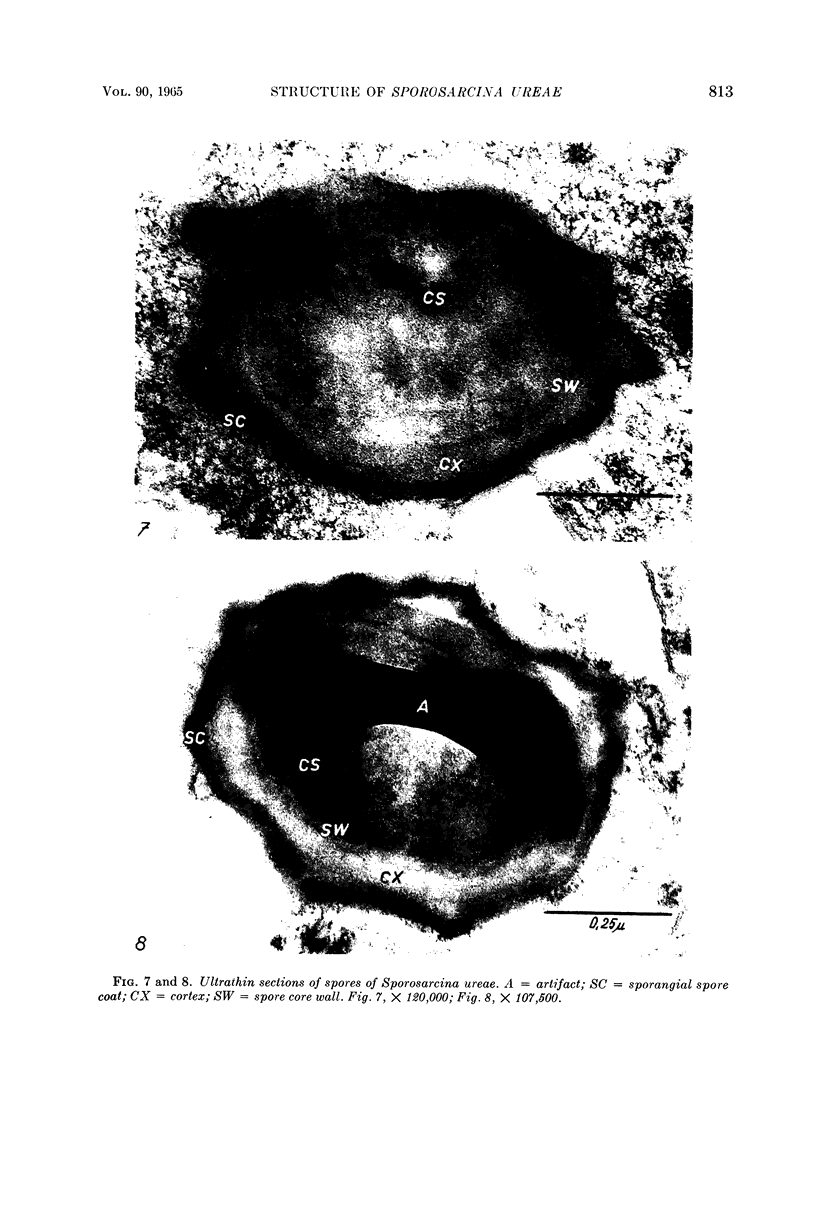
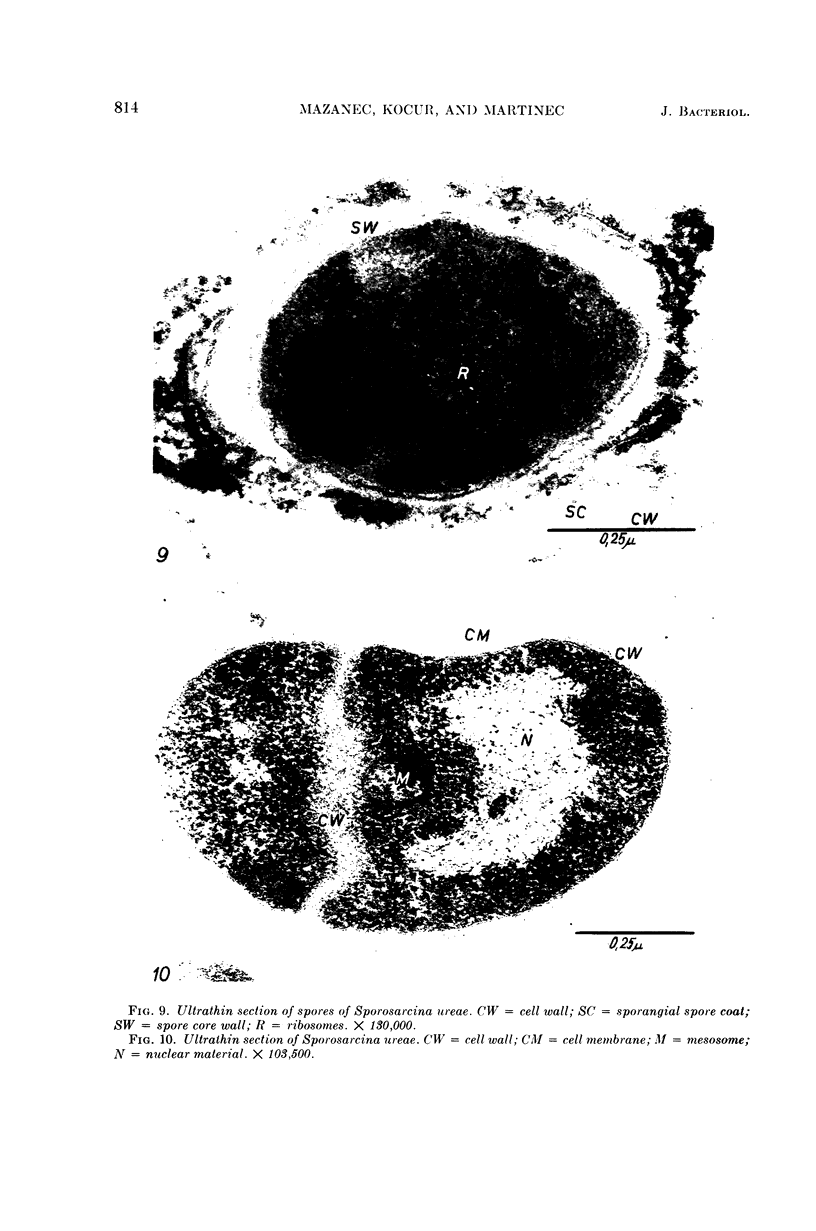
Mazanec, K. (J. E. Purkyně University, Brno, Czechoslovakia), M. Kocur, and T. Martinec. Electron microscopy of ultrathin sections of Sporosarcina ureae. J. Bacteriol. 90:808–816. 1965.—Ultrathin sections of Sporosarcina ureae cells were studied by means of electron microscopy. The cell wall consists of several layers and is 340 A thick. The cytoplasm is of globular structure and includes ribosomelike structures, occasional mesosomes, and inclusions not precisely identifiable. The nuclear area has various shapes and is formed by filaments 10 to 20 A thick which proceed in various directions. Cell division occurs similarly to that of sarcinate. Both synchronic and asynchronic cell division was observed. The spores of S. ureae consist of an outer coat having several layers, a cortex, a spore wall, and cytoplasm. The results of the present investigation substantiate our previous suggestion that S. ureae should be transferred from the family Micrococcaceae to the family Bacillaceae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPMAN G. B. Electron microscopy of cellular division in Sarcina lutea. J Bacteriol. 1960 Jan;79:132–136. doi: 10.1128/jb.79.1.132-136.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscopy of ultrathin sections of bacteria. III. Cell wall, cytoplasmic membrane, and nuclear material. J Bacteriol. 1959 Jul;78(1):96–104. doi: 10.1128/jb.78.1.96-104.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., RIBI E. ULTRASTRUCTURE OF THE EXOSPORIUM ENVELOPING SPORES OF BACILLUS CEREUS. J Bacteriol. 1964 Dec;88:1774–1789. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALE C. M., BISSET K. A. The pattern of growth and flagellar development in motile gram-positive cocci. J Gen Microbiol. 1958 Jun;18(3):688–691. doi: 10.1099/00221287-18-3-688. [DOI] [PubMed] [Google Scholar]
- HOLBERT P. E. An effective method of preparing sections of Bacillus polymyxa sporangia and spores for electron microscopy. J Biophys Biochem Cytol. 1960 Apr;7:373–376. doi: 10.1083/jcb.7.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIJPER A., CROCKER C. G., SAVAGE N. Sarcinae: motility, kind of flagella, and specific agglutination. J Bacteriol. 1955 Feb;69(2):151–158. doi: 10.1128/jb.69.2.151-158.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]