Abstract
Disseminated leishmaniasis (DL) is an emerging form of Leishmania braziliensis infection characterised by multiple cutaneous lesions on different parts of the body and a high rate of mucosal involvement. Systemic production of TNFα and IFNγ in DL patients is lower than in cutaneous leishmaniasis (CL) caused by L. braziliensis, which may account for parasite dissemination due to the decreased ability to control parasite growth. In this study, the systemic and in situ immune response of DL and CL patients was characterised through evaluation of chemokine and cytokine production. In situ evaluation showed similar production of IFNγ, TNFα, IL-10, transforming growth factor-beta (TGFβ), chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL11 and chemokine (C-X-C motif) ligand 10 (CXCL10) in papular and ulcerative lesions from DL as well as in ulcerated lesions from CL. Serum levels of CXCL9, a chemokine that attracts T-cells, was higher in serum from DL than from CL. These data indicate that a decrease in the type 1 immune response in peripheral blood of DL patients is due to attraction of Leishmania antigen-activated T-cells to the multiple cutaneous lesions. This may account for the absence of or few parasites in the lesions and for the development of ulcers similar to those observed in CL.
Keywords: Disseminated leishmaniasis, Leishmania braziliensis, Cytokines, Chemokines, Immunopathogenesis, Cutaneous leishmaniasis
1. Introduction
Disseminated leishmaniasis (DL) is an emerging form of Leishmania braziliensis infection, distinguished by its unique clinical and immunological characteristics.1–3 It is characterised by the presence of a great number of cutaneous lesions spanning more than one area of the patient’s body, mixed types of skin lesions and a high frequency of mucosal involvement.2,3 The patient typically reports a single initial lesion, usually on the extremities, followed by dissemination that may involve the entire body, sometimes associated with fever and chills. The importance of DL is outlined by the severity of the disease, therapeutic challenge and an increasing prevalence. Whilst in 1986 only 0.2% of all cases of L. braziliensis infection were DL, in 2000 this figure rose to 2%.2,3
DL is distinct from cutaneous leishmaniasis (CL), mucosal leishmaniasis (ML) and diffuse cutaneous leishmaniasis (DCL).3–6 The pathogenesis of DL is not established. In a small number of previously studied cases, we observed a higher frequency of patients with a negative skin test to Leishmania antigen1 and a lower production in vitro of IFNγ and TNFα to Leishmania antigen than in patients with CL.3 Therefore, a decrease in the T-cell response has been hypothesised to play a major role in parasite dissemination, and abnormal chemokine production may be associated with this phenomena. The role of chemokines linking innate to adaptive immunity and in recruiting inflammatory cells to tissue is well established, but remains unknown in DL. For instance, chemokine (C-C motif) ligand 2 (CCL2) and CCL3 are associated with antileishmanial activity,7 and tissue expression of CCL2 is decreased in DCL.8 It is clear that the majority of fresh acneiform lesions of DL disappear during the first course of antimony therapy,2 but the majority of DL patients require more than a course of antimony therapy to cure and relapse may occur.3 It is not known why a high failure rate to antimony therapy is observed in these patients. Host and parasitic factors may account both for dissemination and response to therapy. The aim of this study was to characterise better the systemic and tissue immune response in patients with DL compared with patients with localised CL. Cytokines [IFNγ, TNFα, transforming growth factor-beta (TGFβ) and IL-10] and chemokines [CCL2, CCL3, CCL11, chemokine (C-X-C motif) ligand 9 (CXCL9) and CXCL10] were measured in peripheral blood, and tissue expression of these molecules was determined at the lesion site.
2. Materials and methods
2.1. Patients
This study included 32 DL patients recruited from 1998 to 2001 at the health post of Corte de Pedra, Bahia State, Brazil. The criteria for diagnosis were a clinical picture of DL as described below, in conjunction with parasite isolation by PCR or a positive skin test for Leishmania antigen plus histopathological features characteristic of leishmaniasis. DL was identified according to a case definition of ten or more mixed-type lesions (e.g. acneiform, papular, nodular and/or ulcerated), located on two or more body parts (head, trunk, arms and legs).3 CL patients were matched by sex and age (±5 years) with DL cases. Although not matched by occupation, all DL and CL patients lived in the same endemic area where up to 70% of these patients are farm workers. Patients with CL were characterised by a typical ulcerated lesion with raised borders and had a maximum of three cutaneous lesions. DL and CL patients had a disease duration of 1–2 months. There was no clinical evidence of HIV co-infection in CL or DL patients, and no patient had a positive HIV serology. DL patients were treated with i.v. pentavalent antimonial (Sbv) (meglumine antimony; Sanofi-Aventis, Paris, France), 20 mg/kg body weight daily for 30 days. Criteria for cure were complete involution of papular lesions and/or total scarring of ulcers 90 days after initiation of therapy. Informed consent was obtained from each participant.
2.2. Soluble Leishmania antigen (SLA)
SLA used for skin test and in vitro studies was prepared with an isolate of L. braziliensis as previously described.9
2.3. PCR for Leishmania
DNA isolation was carried out from biopsy samples using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). Purified DNA was re-suspended in Tris–EDTA (TE) buffer and was stored frozen at −20 °C until use. Detection of the subgenus Viannia used the primers 5′-GGGGTTGGTGTAATATAGTGG-3′ and 5′-CTAATTGTGCACG-3′.
2.4. Evaluation of cytokine production
Levels of CCL2/monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein 1-alpha (MIP-1α)/CCL3, CCL11/eotaxin, CXCL10/IFNγ-inducible protein 10 (IP-10) and CXCL9/monokine induced by IFNγ (MIG) were measured in the serum of 21 DL patients and 21 CL patients by ELISA.7 For determination of cytokines and chemokines, peripheral blood mononuclear cells (PBMC) were obtained from heparinised venous blood layered over a Ficoll–Hypaque gradient, then washed and re-suspended in complete medium (RPMI 1640 with 10% heat-inactivated pooled human AB serum) at a concentration of 3 × 106 cells/ml. These cells were added to 24-well plates and were kept unstimulated or were stimulated with SLA (10 μg/ml) for 72 h at 37 °C in 5% CO2. Supernatants were then collected and stored at −70 °C. IFNγ, TNFα, IL-10 and IL-5 levels were measured by ELISA (R&D Systems, Minneapolis, MN, USA; and Pharmingen, San Diego, CA, USA). Serum levels of chemokines (CCL2, CCL3, CCL11, CXCL9 and CXCL10) were determined by ELISA.10
2.5. Evaluation of cytokine and chemokine expression in situ by immunohistochemistry
Skin biopsies from lesion borders of patients with localised CL and DL were frozen in O.C.T. compound (Sakura Finetek, Torrance, CA, USA). Immunohistochemistry was performed using the avidin–biotin peroxidase method with mouse anti-human MIP-1α (PeproTech, Rocky Hill, NJ, USA), IFNγ (Genzyme, Cambridge, MA, USA) and TNFα (Genzyme) and rabbit anti-human TGFβ, CXCL9 and IL-10 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), CCL11/eotaxin, CXCL10/IP-10, CCL12/MCP-1 and MIP-1α (PeproTech). After 90 min of incubation at 37 °C, slides were incubated with biotinylated goat anti-rabbit (Vector Laboratories, Burlingame, CA, USA) or goat anti-mouse (Santa Cruz Biotechnology) antibodies. Staining was developed with 3,30-diaminobenzidine (DAB) (Vector Laboratories) and was counterstained with Mayer haematoxylin. Controls were performed by incubating slides with IgG isotype control instead of primary antibodies and proceeding as described above. The whole biopsy section areas were microphotographed with a final magnification of 200 × and brown-stained areas were counted using Adobe Photoshop CS3 (Adobe Systems Inc., San José, CA, USA) and ImageJ software (National Institutes of Health, Bethesda, MD, USA). Results are expressed as percent of biopsy area stained positive.
2.6. Statistical analysis
A Mann–Whitney test was performed for all continuous non-parametric variables. Data were statistically significant if the probability of a type I error was < 0.05.
3. Results
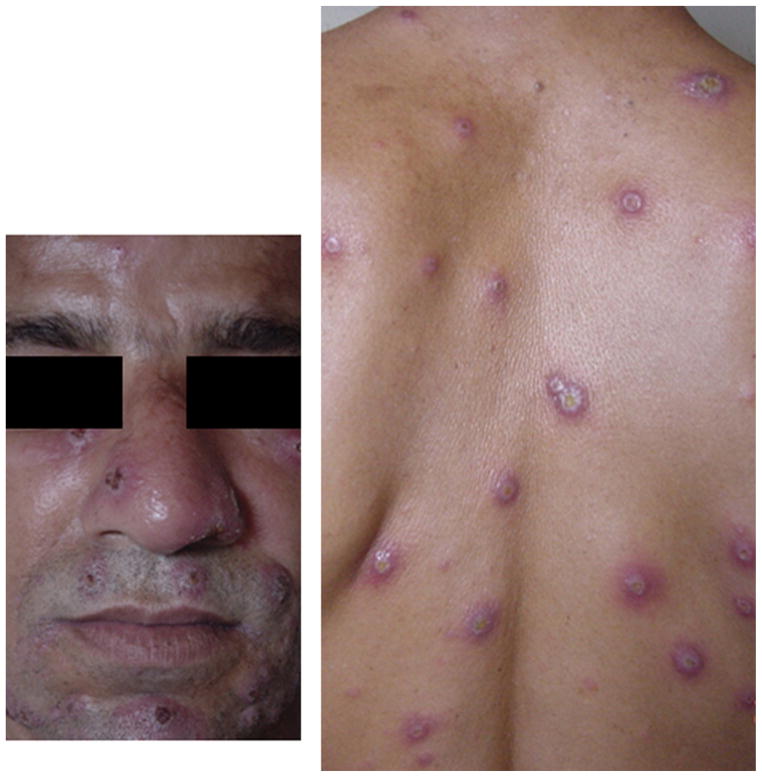
Demographic and clinical features of the 32 patients with DL are shown in Table 1. Patients were primarily adults and male, with a short duration of illness (<60 days) and had up to 412 lesions. The trunk and face were most commonly involved (Figure 1). Leishmania braziliensis was identified by PCR in 15 DL cases and in 12 CL cases. Histopathology showed absent or few parasites both in CL and DL lesions (data not shown). Failure after one course of Sbv therapy was observed in 22 (76%) of 29 DL patients.
Table 1.
Demographic and clinical findings of the 32 patients with disseminated leishmaniasis
| Characteristic | |
|---|---|
| Age (years) (mean ± SD) | 34 ± 15 |
| Gender M:F (n) | 27:5 |
| No. of lesions [median (range)] | 61 (15–412) |
| Positive skin test [n/N (%)] | 25/30 (83) |
| Mucosal disease test [n/N (%)] | 14/32 (44) |
| Failure following one course of Sbv [n/N (%)] | 22/29 (76) |
Sbv: pentavalent antimonial.
Figure 1.
A disseminated leishmaniasis patient with intense facial and trunk involvement.
3.1. Serum levels of chemokines
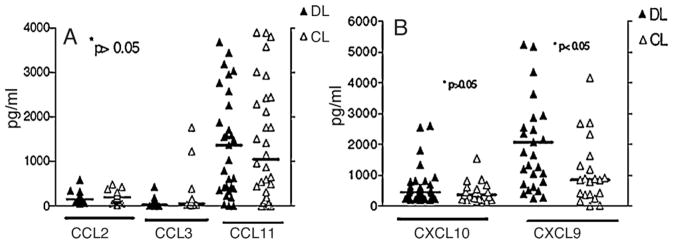
Serum levels of CCL2, MIP-1α/CCL3 and CCL11 were similar in the two groups of patients (Figure 2A). There was a trend for higher levels of CCL3 in CL (252 ± 503 pg/ml) than in DL patients (67 ± 106 pg/ml). The serum concentration of CXCL9 was higher in DL (3560 ± 4683 pg/ml) than in CL (1108 ± 1061 pg/ml) (P = 0.004) and there was a trend for higher levels of CXCL10 in DL (661 ± 634 pg/ml) than in CL (448 ± 330 pg/ml) (Figure 2B). Data are given as mean ± SD.
Figure 2.
Chemokine production in 21 disseminated leishmaniasis (DL) and 21 cutaneous leishmaniasis (CL) patients: (A) chemokine (C-C motif) ligand 2 (CCL2), macrophage inflammatory protein 1-alpha (MIP-1α/CCL3) and CCL11 production and (B) chemokine (C-X-C motif) ligand 9 (CXCL9) and CXCL10 production in serum were determined as described in Section 2.4. Chemokine levels were measured by ELISA. Symbols refer to data for individual patients and the horizontal bar indicates the median.
3.2. Analysis of cytokine production in peripheral blood mononuclear cells from disseminated leishmaniasis and cutaneous leishmaniasis patients
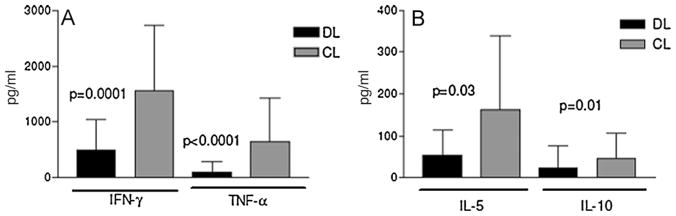
As shown in Figure 3A, the concentration of TNFα (112 ± 188 pg/ml, range 0–884 pg/ml) and IFNγ (490 ± 530 pg/ml, range 0–1782 pg/ml) in supernatants of antigen-stimulated PBMCs from DL patients was lower (P < 0.0001) than in supernatants of PBMC from CL patients (641 ± 775 pg/ml and 1561 ± 1166 pg/ml, respectively). Undetectable concentrations of IFNγ expressed as 0 pg/ml were only observed in 3 (9.4%) of 32 cases of DL. IL-5 and IL-10 levels were significantly lower (P < 0.05) in DL (54 ± 61 pg/ml and 22 ± 53 pg/ml, respectively) than in CL patients (161 ± 117 pg/ml and 44 ± 62 pg/ml) (Figure 3B). In all cases, the IFNγ level was higher than IL-5 and IL-10 levels. Data are given as mean ± SD.
Figure 3.
Cytokine profiles in 12 disseminated leishmaniasis (DL) and 8 cutaneous leishmaniasis (CL) patients: (A) IFNγ and TNFα and (B) IL-5 and IL-10 production in peripheral blood mononuclear cell (PBMC) supernatants stimulated with Leishmania antigen were determined as described in Section 2.4. Levels of cytokines were measured by ELISA. Data are given as mean (blocks) ± SD (bars).
In the present series, 44% of the patients with DL had mucosal involvement. However, different from the deep ulcers and nasal septum perforation observed in classical ML patients, the patients presented nodular and superficial ulcers. Previous studies comparing the immunological response in patients with ML and CL have shown that ML is characterised by high production of IFNγ and TNFα and low production of IL-10.11–13 Therefore, levels of these cytokines in patients with DL and mucosal lesions were compared with those without mucosal disease. Whilst there was no difference in IFNγ production between these two groups of patients (665 ± 538 pg/ml vs. 460 ± 538 pg/ml), production of TNFα by PBMCs from patients with DL with mucosal lesions was higher (191 ± 275 pg/ml) than in those without mucosal disease (93 ± 211 pg/ml) (P = 0.02).
3.3. In situ chemokine and cytokine production in disseminated leishmaniasis and cutaneous leishmaniasis
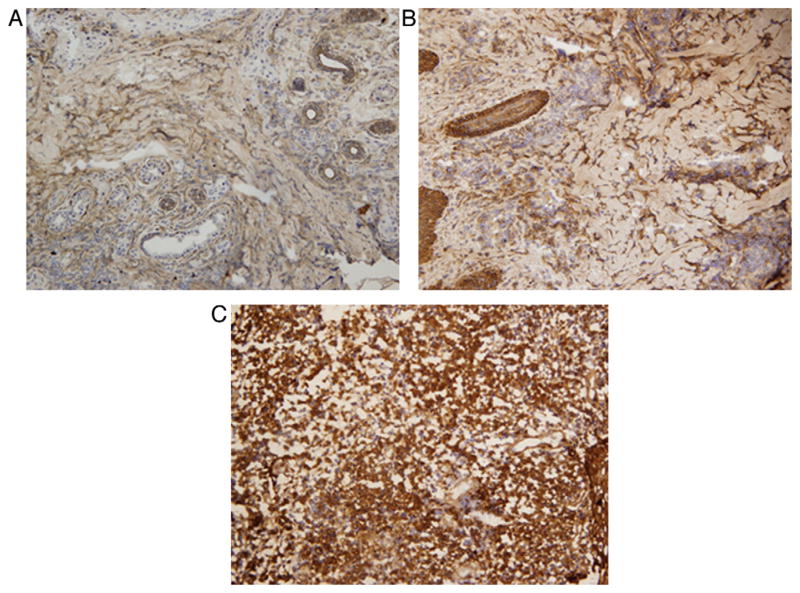
Tissue expression of CCL2, CCL3, CCL11, CXCL9, CXCL10, IFNγ, TNFα, IL-10 and TGFβ was analysed in the ulcerated lesions of CL patients and was compared with papular and ulcerated lesions from matched DL patients (Table 2). Among the chemokines, similar tissue expression was found both in DL and CL lesions, although there was a trend for a generally lower production in papular DL compared with ulcerated CL and DL. Expression of CXCL10 was higher in ulcerated DL lesions, although this was not significant (Figure 4). Analysis of CXCL9 expression in the tissue was not possible due to cross-reaction of the antibodies leading to high background levels.
Table 2.
In situ production of cytokines and chemokines in 8 cutaneous leishmaniasis (CL) and 12 disseminated leishmaniasis (DL) patients
| Cytokine/chemokine (% of staining area) (median ± SD) | CL | DL papule | DL ulcer a |
|---|---|---|---|
| IFNγ | 19.2 ± 10.5 | 18.7 ± 6.1 | 18.9 ± 11.9 |
| TNFα | 14.2 ± 6.5 | 8.0 ± 10.4 | 13.3 ± 16.5 |
| IL-10 | 21.5 ± 12.6 | 17.6 ± 4.2 | 22.7 ± 15.4 |
| TGFβ | 16.8 ± 16.3 | 21.7 ± 8.8 | 35.9 ± 7.1 |
| CCL2 | 27.9 ± 10.4 | 16.7 ± 3.9 | 22.8 ± 10.9 |
| CCL3/MIP-1α | 12.4 ± 7.9 | 19.5 ± 7.5 | 19.1 ± 15 |
| CXCL10 | 17.6 ± 16.8 | 23.8 ± 3.4 | 23.1 ± 18.1 |
| Eotaxin | 25.8 ± 4.5 | 17.6 ± 5.9 | 23.8 ± 13 |
TGFβ: transforming growth factor-beta; CCL: chemokine (C-C motif) ligand; MIP-1α: macrophage inflammatory protein 1-alpha; CXCL: chemokine (C-X-C motif) ligand.
The P-value was > 0.05 when comparing the three groups.
Figure 4.
Tissue expression (% of stained area) of chemokine (C-X-C motif) ligand 10 (CXCL10) in cutaneous leishmaniasis (CL) and disseminated leishmaniasis (DL): biopsy section showing production of CXCL10 in (A) a CL ulcerated lesion, (B) a DL papule and (C) a DL ulcer (final magnification, 200 ×).
In situ expression of IFNγ, TNFα, IL-10 and TGFβ also showed a similar pattern, with a higher (non-significant) TGFβ expression in ulcerated DL (Table 2).
4. Discussion
The host immunological response plays a pivotal role in the pathogenesis of tegumentary leishmaniasis. Whilst in CL and ML an exaggerated type 1 immune response is associated with tissue damage,12,13 in patients with DCL an absence of cell-mediated immune response allows parasite multiplication and dissemination.14,15 DL patients may present a hundred lesions and, compared with CL and ML, have a lower production of IFNγ and TNFα.3 Therefore, it has been suggested that a decrease in the peripheral production of Th1 cytokines may favour dissemination of the lesions in DL. Here we compared the systemic and tissue immune response in CL and DL. These data indicate that the tissue immune response observed in DL is similar to that detected in CL. As these patients present multiple lesions, it is likely that the majority of Leishmania-reactive T-cells are in the lesion, leading to a decrease in parasite antigen-specific T-cells in the blood and consequently decreased production of TNFα and IFNγ observed in PBMC cultures of DL compared with patients with CL.
The hypothesis that parasite dissemination in DL is associated with a decrease in type 1 immune response comes from studies that show a decreased production of IFNγ and TNFα in supernatants of PBMCs of DL in comparison with CL and also an absence of delayed-type hypersensitivity response to Leishmania antigens in some DL patients.3 This hypothesis was supported by the role of the absence of type 1 immune response in DCL, the anergic form of CL, which is also associated with multiple cutaneous lesions.6 However, DCL differs from DL in clinical, histopathological and immunological features. Specifically, in DCL the lesions usually do not ulcerate and histopathology shows an increase in macrophages with a high number of amastigotes inside the cells.6,14 In DL the papular and acneiform lesions progress to ulcer, lymphocyte infiltration and granulomas are found, and an absence or low numbers of parasites are documented.2 These histopathological findings are similar to those observed in CL. We confirmed here that IFNγ and TNFα systemic levels are significantly lower in DL compared with CL. However, it is clear that the majority of these patients produce IFNγ and some of them produce as much IFNγ as patients with CL.
Chemokines play a relevant role in the migration of activated T-cells and are themselves induced by T-cell-derived cytokines. For example, CXCL9 and CXL10 act on chemokine (C-X-C motif) receptor 3 (CXCR3), which are preferentially expressed on Th1 lymphocytes and are themselves induced by IFNγ.7 CXCL11 can induce production of IL-12 by dendritic cells, whereas CCL2 and CCL3 can attract monocytic cells and induce antileishmanial activity.7,16 It has been shown that tissue expression of CCL2 is decreased in DCL in comparison with CL.8 We found no differences regarding the in situ expression of cytokines (IFNγ, TNFα, TGFβ) and chemokines (CCL2, CCL3, CCL11, CXCL10) comparing papular and ulcerated lesions of DL with the classical ulcerated lesion of CL. In the current series, CXCL9 levels were higher in DL compared with CL. Elevated levels of CXCL9 in the serum of DL patients contrast with the lower production of IFNγ and TNFα by PBMCs of these same patients. However, it could indicate that Leishmania-specific T-cells were being recruited to the lesion site and were participating in the pathogenesis of the lesion, as pro-inflammatory cytokines are associated with tissue damage in L. braziliensis infection.12,13
Mucosal lesions were found in 44% of the DL patients in this study. ML is characterised by an increased secretion of TNFα,13 and treatment with pentoxifylline, a TNFα inhibitor, associated with antimony accelerates healing of ML.17 Although DL patients produce lower levels of TNFα than CL and ML patients, it was shown here that systemic TNFα production in DL patients with mucosal lesions was higher than that observed in patients with DL without mucosal involvement. These data further support a role for TNFα in the pathogenesis of mucosal disease.
These data show that the tissue immune response in DL is similar to that observed in CL. It is possible that a transitory impairment of the T-cell response early in Leishmania infection may play a role in parasite dissemination. However, as the infection progresses, the local immune response is able to control parasite growth and only a few amastigotes remain in the lesion site. Later on, as a result of the local inflammatory response, the lesion becomes ulcerated.2 The documentation that the histopathology of DL is similar to CL and characterised by lymphocyte and macrophage infiltration2 supports this sequence of events not yet demonstrated. This study pointed out that other possibilities should be evaluated to explain the peculiar manifestation observed in this clinical form of leishmaniasis. First, because a down-modulation of the immune response is observed in the early phase of CL.18 Second, because we recently showed that isolates of L. braziliensis from DL patients have genotypic and phenotypic differences compared with isolates from CL.19,20 Therefore, the possibility that dissemination is due predominantly to parasite factors of L. braziliensis should be evaluated. Regarding the decreased levels of type 1 cytokines detected in supernatants of PBMCs from patients with DL, the current data argue in favour that it may be due to a low number of Leishmania-antigen specific T-cells in peripheral blood as these cells have been attracted to tissue in an attempt to kill parasites present in the multiple lesions observed in these patients. The presence of parasite antigen-specific T-cells at the lesion site explains the low parasite burden observed in DL as well as the in situ cytokine and chemokine profile similar to CL described here, which may explain the development of ulcerative lesions resembling what is found in CL.
Acknowledgments
The authors acknowledge Elbe Silva for secretarial assistance in the preparation of this manuscript as well as Ednaldo Lima do Lago, Maria Neuza Lago and Dr Luiz Henrique Guimarães for patient assistance in the endemic area.
Funding: This study was supported by the Brazilian National Research Council (CNPq), Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and NIH grant # AI-30639. OB and PRM are investigators of the CNPq, and EMC is a senior investigator of the CNPq.
Footnotes
Authors’ contributions: PRM, OB, MEAR, AS, JSS and EMC designed the study protocol; PRM and MEAR carried out the clinical assessment; OB carried out the immunoassays and the systemic cytokine/chemokine determination; DC and MM carried out the in situ cytokine/chemokine determinations; AS carried out Leishmania isolation and identification; PRM, OB, DC, JSS, MM and MMT analyzed and interpreted the data; PRM, MEAR and OB drafted the manuscript; MMT, DC, JSS, AS, MM and EMC revised the manuscript. All authors read and approved the final manuscript. EMC and PRM are guarantors of the paper.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Ethics Committee of the Hospital Universitário Prof. Edgard Santos (Salvador, Brazil).
References
- 1.Costa JM, Marsden PM, Llanos-Cuentas EA, Netto EM, Carvalho EM, Barral A, et al. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J Trop Med Hyg. 1986;89:319–23. [PubMed] [Google Scholar]
- 2.Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–25. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 3.Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186:1829–34. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- 4.Marsden PD. Mucosal leishmaniasis (“espundia” Escomel, 1911) Trans R Soc Trop Med Hyg. 1986;80:859–76. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 5.Jones TC, Johnson WDJ, Barretto AC, Lago E, Marsden PD. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis. J Infect Dis. 1987;156:73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- 6.Convit J, Pinardi ME, Rondon AJ. Diffuse cutaneous leishmaniasis: a disease due to an immunological defect of the host. Trans R Soc Trop Med Hyg. 1972;66:603–10. doi: 10.1016/0035-9203(72)90306-9. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira MJ, Teixeira CR, Andrade BB, Barral-Netto M, Barral A. Chemokines in host–parasite interactions in leishmaniasis. Trends Parasitol. 2006;22:32–40. doi: 10.1016/j.pt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Ritter U, Körner H. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 2002;24:295–301. doi: 10.1046/j.1365-3024.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 9.Reed SG, Badaró R, Masur H, Carvalho EM, Lorenco R, Lisboa A, et al. Selection of a specific skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira AL, Jr, Cardoso F, Souza AL, Teixeira MM. Increased serum concentrations of monokine induced by interferon-gamma/CXCL9 and interferon-gamma-inducible protein 10/CXCL-10 in Sydenham’s chorea patients. J Neuroimmunol. 2004;150:157–62. doi: 10.1016/j.jneuroim.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, et al. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985;135:4144–8. [PubMed] [Google Scholar]
- 12.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–8. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 13.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barral A, Costa JM, Bittencourt AL, Barral-Netto M, Carvalho EM. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Int J Dermatol. 1995;34:474–9. doi: 10.1111/j.1365-4362.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 15.Bomfim G, Nascimento C, Costa J, Carvalho EM, Barral-Netto M, Barral A. Variation of cytokine patterns related to therapeutic response in diffuse cutaneous leishmaniasis. Exp Parasitol. 1996;84:188–94. doi: 10.1006/expr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 16.Oghumu S, Lezama-Dávila CM, Isaac-Márquez AP, Satoskar AR. Role of chemokines in regulation of immunity against leishmaniasis. Exp Parasitol. 2010;126:389–96. doi: 10.1016/j.exppara.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado PR, Lessa H, Lessa M, Guimarães LH, Bang H, Ho JL, et al. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–93. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 18.Rocha PN, Almeida RP, Bacellar O, de Jesus AR, Filho DC, Filho AC, et al. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J Infect Dis. 1999;180:1731–4. doi: 10.1086/315071. [DOI] [PubMed] [Google Scholar]
- 19.Schriefer A, Schriefer AL, Goes-Neto A, Guimaraes LH, Carvalho LP, Almeida RP, et al. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72:508–14. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leopoldo PT, Machado PR, Almeida RP, Schriefer A, Giudice A, Jesus AR, et al. Differential effects of antigens from L. braziliensis isolates from disseminated and cutaneous leishmaniasis on in vitro cytokine production. BMC Infect Dis. 2006;6:75. doi: 10.1186/1471-2334-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]