Abstract
Understanding human cortical maturation is a central goal for developmental neuroscience. Significant advances toward this goal have come from two recent strands of in vivo structural magnetic resonance imaging research: (1) longitudinal study designs have revealed that factors such as sex, cognitive ability, and disease are often better related to variations in the tempo of anatomical change than to variations in anatomy at any one time point; (2) largely cross-sectional applications of new surface-based morphometry (SBM) methods have shown how the traditional focus on cortical volume (CV) can obscure information about the two evolutionarily and genetically distinct determinants of CV: cortical thickness (CT) and surface area (SA). Here, by combining these two strategies for the first time and applying SBM in >1250 longitudinally acquired brain scans from 647 healthy individuals aged 3–30 years, we deconstruct cortical development to reveal that distinct trajectories of anatomical change are hidden within, and give rise to, a curvilinear pattern of CV maturation. Developmental changes in CV emerge through the sexually dimorphic and age-dependent interaction of changes in CT and SA. Moreover, SA change itself actually reflects complex interactions between brain size-related changes in exposed cortical convex hull area, and changes in the degree of cortical gyrification, which again vary by age and sex. Knowing of these developmental dissociations, and further specifying their timing and sex-biases, provides potent new research targets for basic and clinical neuroscience.
Introduction
Since the advent of in vivo structural neuroimaging, the anatomy of the cortex has come under especially intense scrutiny, with cortical volume (CV) being the principal morphometric index of interest. Initially, cross-sectional studies established robust relationships between CV and factors such as age, sex, cognitive ability, and disease (Mechelli et al., 2005). Then longitudinal studies capable of directly measuring anatomical change showed that normal CV development follows a curvilinear inverted-U trajectory (Giedd et al., 1999) and, in this context, CV variation at any one point in time is often a poorer marker of brain maturation, sexual dimorphism, and pathology than variation in how CV changes over time (Giedd and Rapoport, 2010). Alongside these advances, however, several lines of evidence have converged to demonstrate that CV is very much a composite measure of cortical anatomy, which integrates (and thus potentially obscures information about) several biologically distinct morphometric properties of the brain.
Cortical volume is determined by two different dimensions of the cortical sheet: surface area (SA) and cortical thickness (CT). Surface area is in turn comprised of the area of exposed cortical surface or convex hull area (CHA) and the area of cortex hidden in sulci. Degree of sulcation can be quantified by calculating a gyrification index (GI)—the ratio between total SA and CHA (Van Essen and Drury, 1997). Like CV (Schmitt et al., 2007) and CV change (Brans et al., 2008), these diverse neuroanatomical phenotypes, which together determine CV, are all highly heritable (Panizzon et al., 2009; Rogers et al., 2010). However, different determinants of CV capture distinct evolutionary (Rakic, 1995), genetic (Panizzon et al., 2009; Raznahan et al., 2010b), and cellular (Chenn and Walsh, 2002) processes, which are all conflated in the single measure of CV. For example, disease states (Raznahan et al., 2010a), common genetic variants (Joyner et al., 2009), and environmental modifications (Park et al., 2009) can all have distinct consequences for different CV subcomponents.
Despite CHA, GI, SA, and CT each carrying unique biological information, only CT has been studied longitudinally (Shaw et al., 2008). Resultantly, little is known about how changes in CHA, GI, SA, and CT interact over time to shape typical CV development (Giedd et al., 1999), or about differences in CV development between groups defined by sex or disease status (Giedd and Rapoport, 2010). Cross-sectional attempts to fractionate CV development have produced inconsistent results (Salat et al., 2004; Ostby et al., 2009).
If changes in CT and SA (and CHA and GI within SA) make contributions to CV change that differ as a function of developmental stage and sex, then fractionating CV change becomes a crucial step toward better understanding the biological underpinning of cortical development in health and disease (Geschwind and Konopka, 2009). We therefore used the largest known longitudinal database of typical brain development to characterize reference maturational trajectories for the diverse brain properties that define CV, and to determine whether changes in these brain properties contribute to CV in a manner that varies with age and sex.
Materials and Methods
We included a total of 1274 magnetic resonance imaging brain scans, taken from 647 healthy people (319 females, 607 scans; 328 males, 667 scans) between the ages of 3 and 30 years. Participant characteristics are summarized in Table 1. All structural magnetic resonance imaging (sMRI) scans were T-1 weighted images with contiguous 1.5 mm axial slices and 2.0 mm coronal slices, obtained on the same 1.5-T General Electric Sigma scanner using a three-dimensional (3D) spoiled gradient recalled echo sequence. Each scan was processed using a well validated and fully automated technique for MRI analysis (Im et al., 2008) that estimates total CV and creates a 3D reconstruction of the convoluted cortical sheet from which four metrics are derived: mean CT across ∼80,0000 points (vertices) on the cortical surface, total SA, total CHA, and GI.
Table 1.
Participant characteristics
| Characteristic | Group |
Difference | |
|---|---|---|---|
| Male | Female | ||
| Number of individuals | 328 | 319 | |
| Handedness, n | n.s | ||
| Right | 282 | 275 | |
| Non-right | 46 | 44 | |
| Race, n | n.s | ||
| Caucasian | 270 | 269 | |
| African-American | 32 | 27 | |
| Asian | 8 | 6 | |
| Hispanic | 8 | 12 | |
| Other | 10 | 5 | |
| Singleton, n | 208 | 198 | n.s |
| Member of twin pair, n | 120 | 121 | |
| Dizygotic | 59 | 62 | |
| Monozygotic | 61 | 59 | |
| IQ, mean (SD) | 113 (12.7) | 112 (12.3) | n.s |
| SES (SD) | 43 (19.5) | 43 (18.9) | n.s |
| Total number of scans | 667 | 607 | |
| Number of scans, n | n.s | ||
| 1 | 133 | 138 | |
| 2 | 103 | 113 | |
| ≥3 | 92 | 68 | |
| Age at each scan in years, mean (SD) | |||
| First scan | 11.6 (4.7) | 11.7 (5.1) | n.s |
| Second scan | 13.5 (4.4) | 13.2 (4.2) | n.s |
| Third scan | 15.5 (3.9) | 14.5 (4.0) | n.s |
| Age distribution of scans, years | |||
| Mean (SD) | 12.8 (4.9) | 13.2 (5.1) | |
| Range | 3.3–31 | 3.7–30.3 | |
SES, Socioeconomic status; n.s., not significant.
We used mixed models (Pinheiro and Bates, 2000) to estimate the fixed effects of age, sex, and interactions between these terms on each measure. Nested random effects terms modeled within family and within person dependence of observations. Thus (using cubic CT growth with age as an example), CT for ith family's jth individual's kth time-point was modeled as follows: CTijk = Intercept + di + dij + β1(sex) + β2(age) + β3(age2) + β4(age3) + β5(sex × age) + β6(sex × age2) + β7(sex × age3) + eijk.
For each morphometric index of interest, an F test was first used to determine whether a cubic, quadratic, or linear growth model best fit the data. Then, a likelihood ratio test was used to determine whether a model including interactions between age terms and sex predicted significantly more variance in the measure of interest compared with a simpler model including only age terms and a main effect of sex (i.e., if growth curve shape for the measure of interest was significantly different between males and females). Whenever a significant sex difference was not found in the shape of a growth trajectory, a second likelihood ratio test was used to determine whether a model including age and a main effect of sex predicted significantly more variance in the measure of interest compared with a model including age terms alone (i.e., if growth curve height was significantly different between males and females for the measure of interest). All models were run using mean-centered age terms so that the sex term coefficient would refer to the main effect of sex at mean age (13.11 years) rather than age zero. For those morphometric indices that followed nonlinear developmental trajectories, the age-at-peak was determined by solving the first-order derivative of the growth trajectory equation that had been defined for that morphometric index using mixed modeling.
To quantify the relative contribution of CT and SA change to CV change, and of GI and CHA change to SA change, we converted growth trajectories for each measure into annual percentage change trajectories. If the cortex is considered as a sheet, CV is the product of CT and SA. Given this, percentage CV change is the sum of percentage changes in CT and SA. Similarly, given that SA is the product of CHA and GI, the percentage SA change is the sum of percentage changes in CHA and GI. These relationships can be exploited to quantify the relative contribution of changes in CT, SA, CHA, and GI to changes in CV.
Results
By first charting CV change with age in males and females (Fig. 1), we replicated our earlier findings in a smaller sample (Lenroot et al., 2007). We found that CV follows an inverted-U cubic trajectory (F(2,629) = 64.7, p < 0.0001) that peaks in late childhood/early adolescence and then reduces with decreasing velocity before stabilizing in the third decade of life, that absolute CV is greater in males than females (t = 14, p < 0.00005), and that CV trajectories are sexually dimorphic [likelihood ratio (LR) = 18, p = 0.0002], peaking later in males (9.3 years) than females (8 years). The two determinants of CV (CT and SA) also follow an inverted-U cubic trajectory (F(2,629) = 12.8, p < 0.0001 and F(2,629) = 66.8, p < 0.0001, respectively), which peaks earlier for CT than for SA in both sexes. Trajectory shapes and age-at-attaining-peak values are sexually dimorphic for SA (trajectory difference: LR = 32.9, p < 0.0001; peak = 9.7 years in males vs 8.1 years in females), but not CT (LR = 0.45, p = 0.8; peak = 8.6 years in males vs 8.4 years in females). The two determinants of SA (CHA and GI) also follow inverted-U cubic trajectories (CHA: F(2,629) = 26.2, p < 0.0001; GI: F(2,629) = 28.9, p < 0.0001). Trajectory shapes were sexually dimorphic for both CHA (LR = 27.4, p < 0.0001) and GI (LR = 11.1, p = 0.01). Convex hull area increases robustly from age 3 years in both sexes, but reaches its peak much later in males (15.2 years) than females (11.8 years). In contrast, peak GI values occur at the lower limit of our age range in both sexes and decrease steadily thereafter. Both CHA (t = 15.7, p < 0.00005) and GI (t = 4.8, p < 0.00005) are greater in males than females. During the age range studied, estimated group average trajectories for males and females did not intersect for CV, CT, SA, CHA, or GI.
Figure 1.
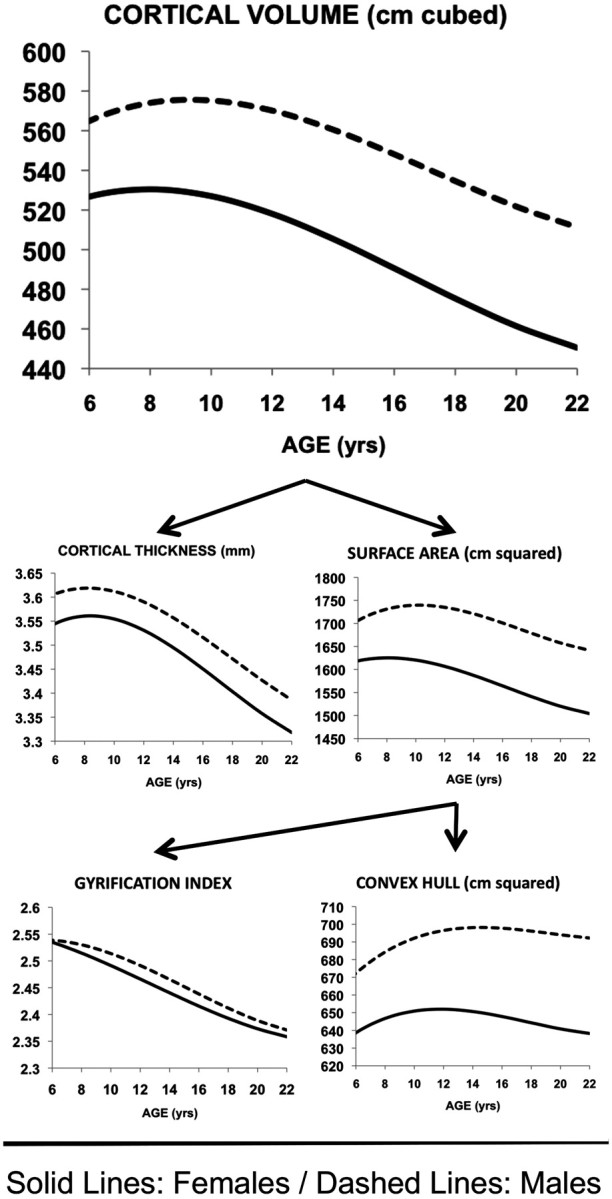
Developmental trajectories for cortical volume and each of its subcomponents in males and females. Measured aspects of cortical anatomy follow cubic developmental trajectories and differ significantly in shape between the sexes. All measures are significantly greater in males than females at the mean age of our sample.
To better illustrate the contributions of CT and SA to CV change, and of CHA and GI to SA change, we plotted the annual percentage change for each of these measures against age (Fig. 2). Figure 2a reveals that, whereas rapid CV expansion during childhood is equally driven by changes in CT and SA in females, approximately two-thirds of CV change in males is accounted for by SA. Also, throughout the age range studied, sex differences in CV change arise because of sex differences in how SA, rather than CT, changes with age. Because the tempo of CT maturation differs little between the sexes, faster and more sustained SA increase in males (followed by slower SA loss) translates into a delayed CV peak relative to females (followed by slower CV loss). Figure 2b shows that the factors driving this sex difference in SA maturation vary with age. Sex differences in the rate of SA expansion during childhood are equally contributed to by sex differences in CHA and GI maturation, but over time, sex differences in GI maturation become a smaller component of sex differences in SA change. Thus, beyond age 12.9 years (when GI change trajectories in males and females converge), sex differences in SA change are almost totally accounted for by CHA. However, in both sexes, CHA loss accounts for a smaller fraction of late adolescent SA loss than reductions in GI.
Figure 2.
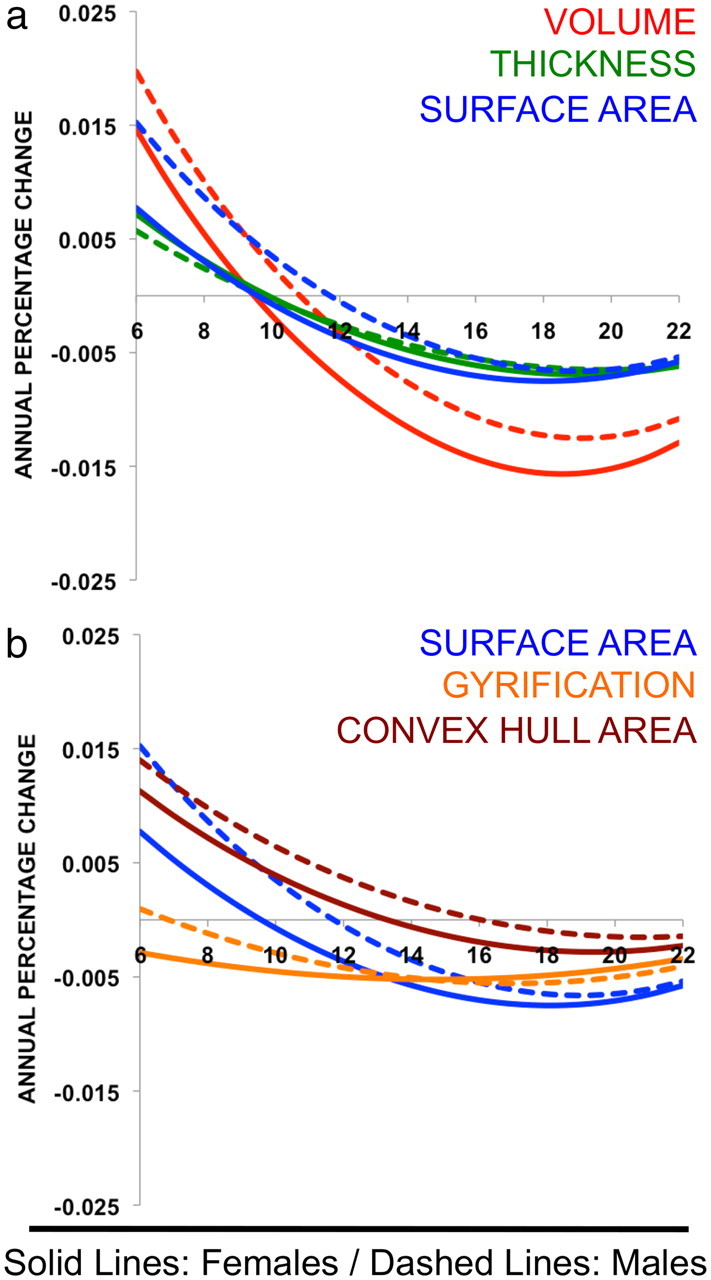
a, Percentage change in CV, CT, and SA plotted against age for males and females. Relative contributions of CT and SA to CV increases in childhood are sexually dimorphic. Sex differences in volume change with age are largely driven by sex difference in SA (rather than CT) maturation. b, Percentage change in SA, CHA, and GI plotted against age for males and females. Sex differences in the tempo of SA change are equally driven by sex differences in CHA and GI change during childhood, but sex differences in CHA change become the main source of sex differences in SA change in adolescence. Both sexes show a developmental shift from CHA to GI as the principal component of SA change, but this shift is delayed in males.
Discussion
Our study shows for the first time how maturational changes and sexual dimorphisms of CV arise through the complex interplay of several distinct facets of cortical anatomy. Not all CV change is created equal, because CV change is so differently composed of changes in CT, CHA, and GI, depending on the sex and developmental phase under consideration. Knowing about these dissociations permits a new narrowing-down of the search for those genetic and environmental factors that underlie normative and pathological variations in cortical development. For example, studies of sexually dimorphic brain anatomy in healthy humans have only recently moved on from cross-sectional comparison of volumetric measures, to focus more on sex differences in the tempo of volumetric brain maturation (Lenroot et al., 2007). We now show that sexual dimorphism in the dynamics of CV maturation is itself very much an emergent property of developmental sex differences in CHA (which varies with radial brain size), rather than the degree of cortical folding (indexed by GI) or CT. Specifically, sex differences in CV change with age and are largely driven by the delayed peak and slower subsequent decline of CHA in males compared with females. Moreover, since we did not find significant sex differences in the tempo of overall mean CT change, our study suggests that other, noncortical determinants of radial brain size must underlie sexually dimorphic CHA and CV development. Thus, crucial next steps in unraveling sex differences in the tempo of CV change will be to longitudinally investigate how radial dimensions of the tissue mass underlying the cortical mantle change with age and then to establish how sex differences in this radial maturation arise out of developmental changes in the volume of cerebral white matter, subcortical gray matter, and CSF.
Our results also have significant implications for future studies of atypical brain development. For example, it is well established that males are at greater risk than females for developing autism, and that emergence of the disorder in early childhood is strongly associated with CV overgrowth (Raznahan and Bolton, 2008). Our fractionation of CV maturation now shows how these two core aspects of the autism phenotype could potentially be simultaneously accounted for by the novel (but testable) hypothesis that autism risk factors operate by derailing SA (rather than CT) maturation. Figure 2a illustrates why aberrant SA maturation would be expected to have its maximal impact on CV development during early childhood and in males rather than females. Such insights represent badly needed leads in the biological study of highly heritable neurodevelopment conditions like autism, given that the genetic influences on CT and SA are known to be largely non-overlapping (Panizzon et al., 2009) and differentially altered by allelic variation within established risk genes for developmental disorders (Joyner et al., 2009). Our results also provide the strongest evidence to date that identifying dissociated alterations of different CV components in disease states may help to date developmental insults. For example, because we find that peak GI is achieved during or before toddlerhood [which converges with in utero studies of other primates (Kochunov et al., 2010)], variation in cortical gyrification emerges as a potentially useful marker of this crucial early developmental window.
The findings we present here should be considered in light of certain caveats. First, exclusion criteria were applied during participant recruitment (for details, see Giedd et al., 1996) to limit potential confounding factors such as a personal or family history of mental illness or developmental disability. The resultant benefits of strengthening the internal validity of our large longitudinal sample have to be balanced against the potential drawbacks of inadvertent selection bias for other attributes that also may be associated with differences in brain development, such as IQ. Second, although our study is the first to longitudinally characterize developmental trajectories for several distinct CV determinants in parallel, we were unable to examine how specific genetic and environmental differences might act and interact over developmental time to give rise to each of the anatomical trajectories described. Third, to address our question about how changes in CT, CHA, and GI contribute to CV change, estimates of absolute anatomical change for each of these diverse measures had to be converted to a common and comparable metric of percentage change. Absolute change may however be a more suitable phenotype than percentage change for the purpose of addressing different questions about brain development. Fourth, many of the CV subcomponents examined in our study could, in principle, be further fractionated (e.g., thickness changes in different cortical layers contributing to CT, changes in sulcal depth vs sulcal length as contributors to GI), and this will be an important focus for future work as methods for sMRI acquisition and processing continue to advance.
Despite these limitations, our study shows for the first time how biologically distinct determinants of CV contribute to CV change in a manner that varies dramatically as a function of sex and developmental stage. Knowledge of these dissociations allows future questions about the causes and consequences of cortical maturation to be asked in more efficient ways.
Footnotes
This study was supported by the National Institutes of Health, National Institute of Health Intramural Research, and a UK Medical Research Council Clinical Research Training Fellowship (G0701370 to A.R.). We thank the participants who took part in this study.
The authors have no competing financial interests.
References
- Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65:1259–1268. doi: 10.1001/archpsyc.65.11.1259. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Konopka G. Neuroscience in the era of functional genomics and systems biology. Nature. 2009;461:908–915. doi: 10.1038/nature08537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2189. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Joyner AH, J CR, Bloss CS, Bakken TE, Rimol LM, Melle I, Agartz I, Djurovic S, Topol EJ, Schork NJ, Andreassen OA, Dale AM. A common MECP2 haplotype associates with reduced cortical surface area in humans in two independent populations. Proc Natl Acad Sci U S A. 2009;106:15483–15488. doi: 10.1073/pnas.0901866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Castro C, Davis D, Dudley D, Brewer J, Zhang Y, Kroenke CD, Purdy D, Fox PT, Simerly C, Schatten G. Mapping primary gyrogenesis during fetal development in primate brains: high-resolution in utero structural MRI of fetal brain development in pregnant baboons. Front Neurosci. 2010;4:20. doi: 10.3389/fnins.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imag Rev. 2005;1:105–113. [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee JD, Kim EY, Park B, Oh MK, Kim JJ. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage. 2009;47:98–106. doi: 10.1016/j.neuroimage.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Bolton PF. Autism spectrum disorders. Medicine (Baltimore) 2008;36:489–492. [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton PF, Paus T, Murphy DG. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 2010a;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, Murphy DD. Cortical anatomy in human X monosomy. Neuroimage. 2010b;49:2915–2923. doi: 10.1016/j.neuroimage.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Kochunov P, Zilles K, Shelledy W, Lancaster J, Thompson P, Duggirala R, Blangero J, Fox PT, Glahn DC. On the genetic architecture of cortical folding and brain volume in primates. Neuroimage. 2010;53:1103–1108. doi: 10.1016/j.neuroimage.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA. Structural and functional analyses of human cerebral cortex using a surface-based atlas. J Neurosci. 1997;17:7079–7102. doi: 10.1523/JNEUROSCI.17-18-07079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


