SUMMARY
Alteration of the PTEN/PI3K pathway is associated with late stage and castrate resistant prostate cancer (CRPC). However, how PTEN loss involves in CRPC development is not clear. Here we show that castration-resistant growth is an intrinsic property of Pten-null prostate cancer (CaP) cells, independent of cancer development stage. PTEN loss suppresses androgen-responsive gene expressions by modulating androgen receptor (AR) transcription factor activity. Conditional deletion of Ar in the epithelium promotes the proliferation of Pten-null cancer cells, at least in part, by down-regulating androgen-responsive gene Fkbp5 and preventing PHLPP-mediated AKT inhibition. Our findings identify PI3K and AR pathway crosstalk as a mechanism of CRPC development, with potentially important implications for CaP etiology and therapy.
INTRODUCTION
Prostate cancer (CaP) is the most common male malignancy and a leading cause of mortality in western countries (American Cancer Society, 2010). Androgens are critical both for the development and function of the normal prostate gland and for the maintenance of CaP cells that arise from the secretory epithelium of the prostate. Androgens function through their cognitive receptor, the androgen receptor (AR). Therefore, therapies for advanced CaP usually involve either reducing or blocking the production of androgens or antagonizing the AR and its target genes (Chen et al., 2008). However, all men with metastatic disease develop resistance to these therapies and will progress to castration-resistant prostate cancer (CRPC).
AR is expressed in CRPC and that AR signaling may be activated via autocrine or androgen-independent manner such as through cross-talk with other pro-survival and proliferative pathways (Attard et al., 2009a; Montgomery et al., 2008). However, levels of AR are also heterogeneous and in some instances absent from late stage diseases (Roudier et al., 2003). Such clinical observations raise the possibility that loss of AR expression or activity may serve as an alternative means of escaping androgen withdrawal or AR-targeted therapies, possibly through concomitant activation of compensatory signaling pathways. One of the possible survival and proliferative pathways is the PI3K/AKT/mTOR pathway, which is negatively regulated by the PTEN tumor suppressor (Hill and Wu, 2009; Taylor et al., 2010). PTEN loss occurs frequently during human CaP progression, with up to 70% of late stage samples exhibiting loss of PTEN function or activation of the PI3K pathway (Taylor et al., 2010). PTEN loss or activation of the PI3K/AKT pathway leads to enhanced cell proliferation, survival and migration (Stiles et al., 2004; Vivanco and Sawyers, 2002) as well as castration-resistant growth (Gao et al., 2006; Jiao et al., 2007; Wang et al., 2003).
Progress in defining the mechanisms of CRPC has been limited due to a paucity of xenograft models and scarcity of matched human clinical specimens representing castration-sensitive and castration-resistant disease. Several well-established transgenic CaP models, i.e. TRAMP (Greenberg et al., 1995) and hi-Myc (Ellwood-Yen et al., 2003) have the limitation that the oncogene is driven by an androgen-dependent promoter. Therefore, the effect of androgen ablation on CaP growth is confounded by its effect on transgene expression. Previously, we established the Pten-null CaP model by conditional deletion of Pten in the murine prostatic epithelium and showed that Pten null CaPs progress with defined kinetics that mimic histopathological features of human disease (Wang et al., 2003). We also tested whether the Pten null CaP model could be used for studying CRPC by surgically castrating mutant mice at 16 weeks, when invasive adenocarcinoma had already developed. Despite activation of pro-survival AKT signaling, Pten null cancer cells are sensitive to androgen withdrawal and the cell death index is higher than that of age- and genetic background-matched WT controls and persists 5–10 weeks after castration. However, the cell proliferation index is not changed in comparison to intact Pten null mice. Such androgen-independent growth overrides androgen-dependent cell death and causes castration-resistant growth and invasive adenocarcinoma (Wang et al., 2003). The Pten conditional murine CaP model, therefore, provides a unique opportunity to address the mechanism of resistance to androgen ablation therapy in a genetically defined model where the oncogenic event is androgen - independent. Using this defined genetic model, we tested whether CRPC development is cancer stage-dependent and whether CRPC remains dependent on AR signaling in the epithelium in this study.
RESULTS
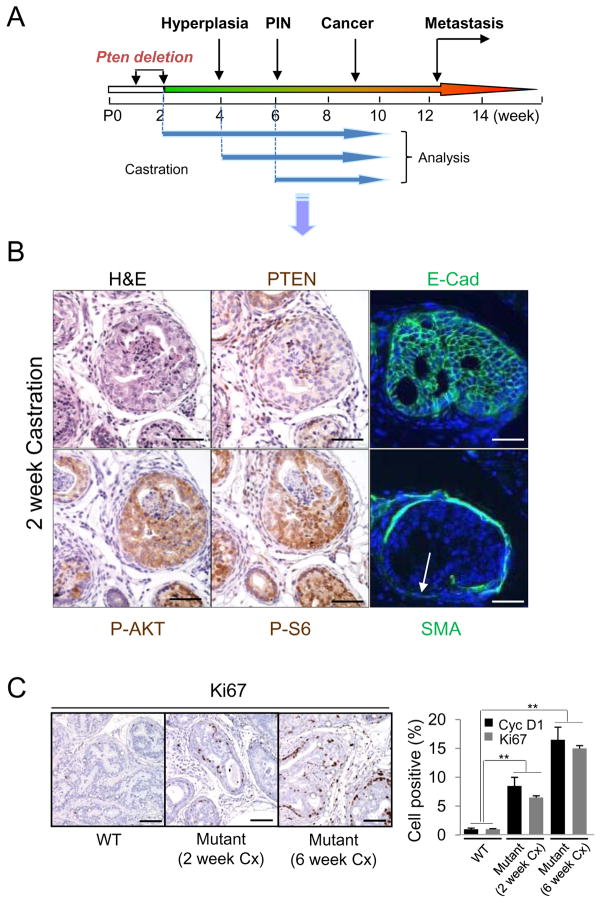
Early castration cannot prevent Pten null CaP initiation and castration-resistant growth
In our previous work (Wang et al., 2003) we castrated Pten conditional knockouts after the development of invasive adenocarcinoma. However the androgen-independent proliferative signal could be either intrinsic to Pten loss or due to other molecular/genetic alterations accumulated during cancer progression. To separate these two possibilities, we tested whether CRPC development depends on cancer stage by castrating the mutant mice at time points corresponding to prostatic epithelial Pten deletion, hyperplasia, and PIN lesion stages, respectively (Wang et al., 2003) (Figure 1A). Age-and genetic background-matched Pten null intact and WT castrated mice were used as controls and all animals were sacrificed at 10 weeks of age. Early castration, particularly when performed at 2 weeks old, significantly reduced the total prostate volume (data not shown). However, E-cadherin-positive PIN lesions and localized invasion, judged by loss of smooth muscle actin (SMA) staining, was clearly evident in the dorsolateral lobes of the mutants (Figure 1B, arrow). Elevated P-AKT (S473) and P-S6 (S240) were also observed, coinciding with Pten loss, in mutant mice castrated at 2 weeks old or 6 week old (Figure 1B and Figure S1). Proliferation index (%Ki67+ cells and Cyclin D1+ cells), was significantly increased in the mutant prostates although those of mice castrated at 2 weeks old were less proliferative than were those of mice castrated at 6 weeks old (Figure 1C), which suggests that other molecular or genetic events may occur between 2 and 6 weeks after the Pten deletion and collaborate with PI3K pathway to promote cell proliferation. Nevertheless, these results suggest that androgen-independent proliferation is intrinsic to Pten loss, which is consistent with previous studies (Jiao et al., 2007) (Gao et al., 2006).
Figure 1. Early castration does not prevent initiation of Pten-null CaP.
(A) Pb-Cre+;PtenL/L mutants were castrated (Cx) at 2, 4 or 6 weeks and aged to 10 weeks. (B) Mutants Cx at 2 weeks were evaluated for carcinoma, PI3K (P-AKT, P-S6) activation coinciding with PTEN loss and invasiveness based on smooth muscle actin (SMA) loss but maintained E-Cadherin expression (arrow). Bar=100 μm (C) Cell proliferation, Ki67+; Cyclin D1+ (Cyc D1) cells, in 2 and 6 week Cx cohorts in comparison to WT Cx controls (**, p<0.01) Bar = 150 μm. Error bars, mean +/− SD. See also Figure S1.
Pten null CaPs are less dependent on AR signaling and Pten loss can suppress androgen-responsive genes expression
That Pten null CaP cells can proliferate under castrate conditions suggests that Pten loss may sensitize AR to castrated androgen levels or even obviate the requirement of androgens. To test this, we compared the AR signaling status, defined by the expression of androgen-responsive genes, in age- and genetic background-matched Pten null and WT prostates at 0, 3, 6 and 14 days post castration (n=3). To determine the AR-responsive genes in normal, non-cancerous prostate, we analyzed a previously published data set (Wang et al., 2007) with two criteria: gene expressions were up- or down-regulated at least 2-fold post castration (castration/intact) and their expressions are reverted upon hormone replacement (hormone replacement/castration). This led to the selection of 148 androgen-responsive genes, including 47 activated and 101 suppressed genes (Table S1). When compared with published human androgen-responsive gene lists (Chauvin and Griswold, 2004; Nelson et al., 2002), we found many overlaps (data not shown).
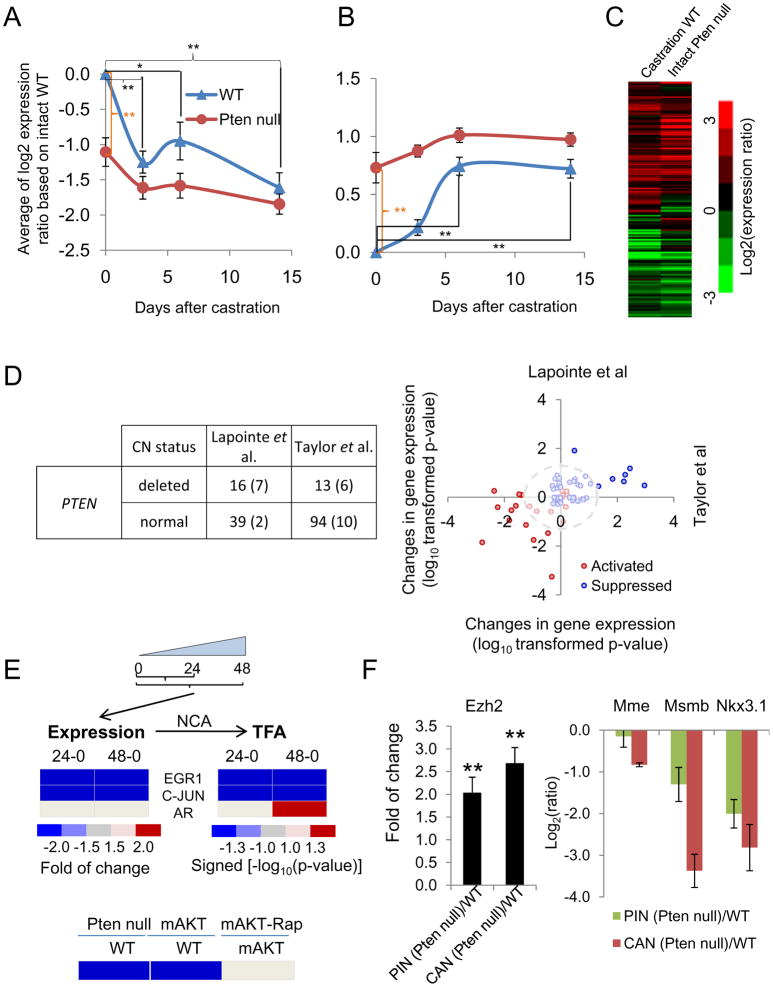
Surprisingly, Pten loss in the epithelium does not sensitize AR signaling, and in the contrary, it suppresses androgen-responsive gene expression or AR transcriptional output in the intact mice (Figure 2A, orange brackets). Although the expressions of the androgen-responsive genes are significantly changed in WT mice after castration the expressions of the majority of these genes are similar in Pten null prostates before and after castration (Figure 2A, B; Figure S2A). Strikingly, the overall AR transcriptional output of 14-day castrated WT prostates is similar to that of intact Pten null prostates (Figure 2C; Figure S2A). Using a rank-rank analysis and a hypergeometric overlap algorithm (Plaisier et al., 2010), we also found the similarity between the global expression profiles of genes altered in Pten null CaPs (Pten null cancer - WT control) with those enriched after castration (castration - intact or day 0 control, Figure S2B and 2C). Together, these results indicate that PTEN loss not only suppresses AR transcriptional output but also drives the overall gene expression profiles towards a castration-like phenotype.
Figure 2. PTEN loss can suppress androgen-responsive gene expression.
(A, B) Expression profile (means +/− s.e.m) of AR activated (A) and suppressed (B) genes in WT and Pten-null murine prostates after castration. (C) Heat map of expression ratios of androgen responsive genes in WT (14-day post castration) and intact Pten-null mutants with respect to intact WT mice. (D) Variation in expression of androgen-responsive genes based on PTEN copy number (CN) in human CaP samples. Left, summary of human samples based on PTEN CN (the numbers inside parentheses indicates the number of metastatic cases); right, a comparative analysis of AR activated (red circles) and suppressed (blue circles) gene expression values in two human CaP datasets. (E) Top, gene expression and NCA derived activities of EGR1, JUN, and AR transcription factors in induced PTEN expression in Pten−/− cells. Bottom, the activity of AR in murine models when PTEN/AKT/mTOR pathway was manipulated genetically or pharmacologically. (F) Expression (means +/− SD) of Ezh2 (left), and AR and EZH2 co-target genes (right) in PIN and cancer (CAN) stages of Pten-null prostate. *, p<0.05; **, p<0.005. See also Figure S2 and Table S1.
To test the relevance of our finding to human CaPs, we analyzed two public datasets (Lapointe et al., 2004; Taylor et al., 2010). Human samples were first stratified according to their PTEN DNA copy number status (PTEN CN; Figure 2D) and then tested if the murine-derived Ar signature genes are differentially expressed according to the PTEN CN status. We found 81/148 murine androgen-responsive genes in both human datasets, including 26 activated and 55 suppressed genes (Figure 2D). The two dimensional comparison illustrates the consistency between the two human datasets for the differential expression of the murine derived androgen-responsive genes in PTEN CN mutant vs. normal cohorts (p value of the correlation coefficient=2.2e−6), suggesting the overall suppression of AR-output in human CaPs with PTEN CN variation, similar to our observation in Pten-null mutants. In contrast, cancers with abnormal copy number (CN) of the MYC oncogene showed less agreement between the two datasets (p value of the correlation coefficient=1.1e−4) (Figure S2D), indicating that suppression of AR transcriptional output may depend on specific oncogenic events.
PTEN controls AR transcription factor activity by regulating EGR1, c-JUN and EZH2 expression
To determine how PTEN loss leads to repression of AR output, we used an inducible system in which PTEN expression is controlled in a doxycycline-dependent manner in Pten null, PtenΔloxp/Δloxp murine cells (Chang et al., 2004). While Ar expression is not influenced by PTEN re-expression, Egr1 and c-Jun transcription factors expressions are down regulated within 24 hrs upon induced PTEN re-expression, which were confirmed in a PTEN-inducible human CaP line, PC3 (Figure 2E and data not shown). Both EGR1 and c-JUN are up regulated in CaPs, especially CRPC, and can physically interact with AR, leading to down regulated AR targeted gene expression and CaP cell growth in androgen-depleted environment (Gitenay and Baron, 2009; Sato et al., 1997; Yang et al., 2006; Yuan et al., 2010). Egr1 deletion impairs prostate tumorigenesis (Abdulkadir et al., 2001). We therefore hypothesized that by negatively regulating EGR1 and c-JUN expression, PTEN upregulates AR function. Using Network Component Analysis (NCA) (Tran et al., 2010), we deduced the transcription factor activities (TFAs) from the expressions of their target genes. Both EGR1 and c-JUN TFAs are reduced upon PTEN re-expression whereas AR TFA is increased 24 hrs after PTEN re-expression and EGR1/c-JUN activity changes (Figure 2E), supporting our hypothesis. The activity of AR was further examined using two murine CaP models, our Pten conditional knockout (Wang et al., 2003) and the transgenic mice over expressing mAKT (Majumder et al., 2004). The AR TFAs are significantly reduced in both models, which can be reverted by rapamycin treatment (Figure 2E), suggesting that the mTOR pathway is involved in PTEN regulation of AR activity.
Besides EGR1 and c-JUN, AR target gene expression is also inhibited by the ERG transcription factor, a member of ETS family whose gene is frequently translocated to the AR responding TMPRSS2 gene (Yu et al., 2010). ERG activates EZH2 expression, a member of the polycomb complex associated with human CaP (Sellers and Loda, 2002; Varambally et al., 2002), and EZH2-mediated dedifferentiation program (Yu et al., 2010). Ezh2 expression is upregulated in Pten-null prostate cancer (Figure 2F; Figure S2E) and the expressions of several AR and ERG co-targeted genes, including Nkx3.1, Mme and Msmb (Kunderfranco et al., 2010), are also downregulated in Pten null prostate at PIN and Cancer stages (Figure 2F). Our analyses suggest that PTEN loss suppresses androgen-responsive genes by regulating AR activity through multiple co-regulators, thereby renders Pten null CaP cells less dependent or completely independent of signaling provided by androgens - promoting CRPC growth.
Epithelial AR is not required for the initiation and progression of prostate cancers caused by Pten loss
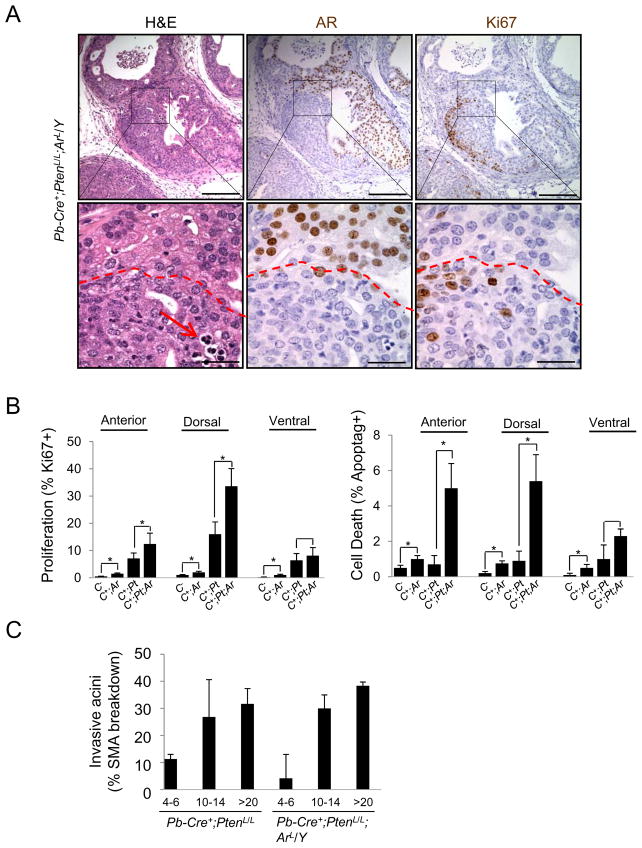
Having demonstrated that Pten null CaP can proliferate independent of androgens, we considered whether CaP can develop in the absence of AR in the epithelium. We crossed Pb-Cre+;PtenL/L (Pten-null) mutants with mice carrying the AR conditional allele (ArL) (De Gendt et al., 2004). Ar deletion led to minimal effect on prostatic epithelium (data not shown), similar to previous publications (Simanainen et al., 2007; Simanainen et al., 2009; Wu et al., 2007). The resulting Pb-Cre+;PtenL/L;ArL/Y (Pten-null;Ar-null) mutants were then analyzed at the age of 4–6, 10–14 and >20 weeks (n=4) and compared to their age- and genetic background-matched Pb-Cre+;PtenL/L mutants. Pten and Ar deletion promoted robust in situ latent adenocarcinoma development in the dorsolateral lobes. Overall cancer progression in the double knockouts is similar to that of Pten single knockout, as indicated by localized loss of SMA staining (Figure 3C and Figure S3A).
Figure 3. Epithelial AR is not required for the initiation of Pten-null CaP.
(A) Deletion of epithelial AR in the anterior lobe of Pten-null CaP (Pb-Cre+;PtenL/L;ArL/Y) mutants and the impact on cell proliferation (Ki67+ cells), apoptosis (red arrow) and cancer formation (bar, top = 200 μm; bottom = 50 μm). (B) Cell proliferation (left) and apoptotic indexes (right) in Cre− (C−), Pb-Cre+;ArL/Y (C+;Ar); Pb-Cre+;PtenL/L and (C+;Pt) and Cre+;PtenL/L;ArL/Y (C+;Pt;Ar) mutants. (C) Frequency of invasiveness based on smooth muscle actin (SMA) break down in Pb-Cre+;PtenL/L and Cre+;PtenL/L;ArL/Y mutants during progression. Error bars, means +/− SD. See also Figure S3.
The anterior and ventral lobes often showed mosaic patterns of conditional deletion (Figure S3A), likely due to lower levels of Cre expression in these lobes (Wu et al., 2001) and less efficient recombination of the ArloxP allele. This allowed us to directly compare the effect of Ar deletion on Pten null CaP cells. In Pten and AR deleted regions, we found enhanced nuclear atypia, cell proliferation (Ki67 staining) and cell death (arrow in the H&E section) in contrast to AR intact regions (comparing areas above and below the red dashed lines in the lower panels of Figure 3A). In Pb-Cre+;PtenL/L;ArL/Y mutants Pten−;AR− adenocarcinomas also appeared less differentiated than Pten−;AR+ regions.
We then examined the content of cells positive for the basal cell markers, p63 and cytokeratin 5 (CK5). We found that Pten−;AR− cancers had a significant expansion of p63+ cells in the proximal region, known to be enriched for stem/progenitor cells (Figure S3B, bottom, 33.5% versus 15.5% of total cells; *, p<0.05), accompanied by increased CK5+;CK8+ transient amplifying and p63+;Ki67+ double positive cells (Figure S3B,). Conversely, the distal regions of Pb-Cre+;PtenL/L;ArL/Y mutants, which compose the bulk of the tumor mass, contained low or no p63+ cells, similar to human CaP (Figure S3C). Proliferation and apoptotic indexes were also increased in Pten-null;AR-null cancers (*, p<0.05), most notably in the proximal regions of the dorsal lateral lobe (Figure 3B). Despite this, AR-independent cell proliferation appeared to override AR-dependent cell death, similar to Pten null cancers under castration conditions (Wang et al 2003), resulting in overall AR-independent tumor growth.
Cell autonomous role of PTEN and PTEN controlled pathway in regulating AR-independent growth
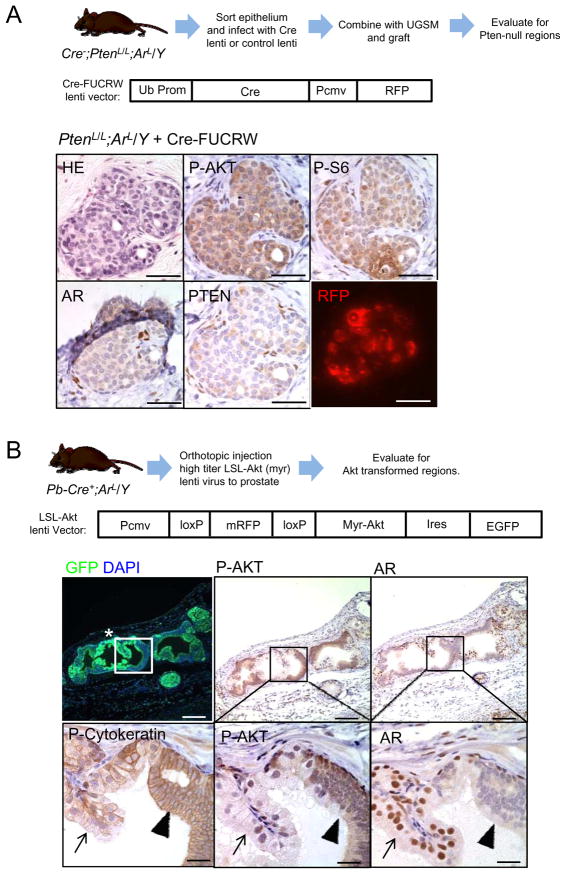
The mosaic patterns of AR deletion in Pb-Cre+;PtenL/L;ArL/Y prostates can potentially complicate our conclusions since AR+ epithelium may supply paracrine factors to “feed” the adjacent AR− cells. Thus, we employed prostate tissue regeneration assay. We FACS sorted prostate epithelium from PtenL/L (n=6) and PtenL/L;ArL/Y (n=4) mice then infected them with either Cre lenti virus (Cre-FUCRW) or control lenti virus (FUCRW). Infected prostate epithelium combined with WT urogenital mesenchyme (UGSM) were grafted under the kidney capsule of NOD;SCID;IL2rγ-null mice (Figure 4A). Recombinants were examined for the presence of pathology coinciding with Cre-FUCRW or FUCRW infection, as visualized by the expression of viral-associated RFP reporter gene (Figure S4A). PtenL/L;ArL/Y cells infected with Cre-FUCRW generated neoplastic pathology with high levels of P-AKT and P-S6 (Figure 4B), similar to the PtenL/L Cre virus infection control (Figure S4A and S4B) while PtenL/L;ArL/Y cells infected with control FUCRW virus displayed normal acinar structure (Figure S4C).
Figure 4. Epithelial AR is not required for transformation by Pten deletion or myristoylated Akt in regeneration assays.
(A) Evaluating the impact of cre-mediated deletion of Pten and Ar on histopathology and PI3K signaling. The top panel shows the outline of the experiment and the bottom panels show results of tissues stained as indicated. Bar = 100 μm. (B) Evaluating AR deletion and myristoylated-AKT expression in primary, Pb-Cre+;ArL/Y mutants and the impact on prostate histopathology and PI3K signaling. The top panel shows the outline of the experiment and the bottom panels show results of tissues stained as indicated. Bars, top = 200 μm, bottom = 50 μm. See also Figure S4.
One explanation of our observation is that epithelial cells isolated from the PtenL/L;ArL/Y prostate have been “primed” by paracrine factors secreted by AR+ stroma cells (Cunha et al., 2002). To further affirm the cell autonomous role of PTEN in CaP initiation in the absence of epithelial AR, we carried out prostate tissue regenerations using prostate epithelium isolated from E16–17 PtenL/L;Tfm mutant mice obtained from crossing the PtenL/L conditional line with the Tfm mutant mice. Tfm mutant mice express a rapidly degraded AR mutant resulting in complete blockage of prostate development (Cunha and Chung, 1981). Prostate epithelium of E16–17 PtenL/L;Tfm mice therefore should have never been exposed to the potential paracrine factors. We found that PtenΔ/Δ;Tfm epithelial cells formed PIN lesions with activated AKT (Figure S4D; n=3). Therefore, using two in vivo tissue recombinant models, our data indicates that AR-independent carcinogenesis is intrinsic to PTEN inactivation.
To further assess whether it is PTEN or the PTEN controlled signaling pathway that renders CaP development without epithelial AR, we orthotopically injected LSL-AKT virus directly to the anterior lobe of Pb-Cre+;ArL/Y prostates (n=3) (Figure 4B). Using this strategy, only regions expressing Cre and infected by lenti-virus (indicated by GFP+ expression in Figure 4B; Figure S4E) will coordinately delete AR and express Myr-AKT. While AR-positive regions did not show AKT hyperactivation and abnormal phenotypes (Figure 4B, arrows in lower panels), AR-deleted neoplastic regions are pan-cytokeratin positive and showed higher levels of P-AKT (Figure 4B; arrowheads in lower panels). These data support our hypothesis that neoplastic transformation of the prostate by activated cell autonomous PI3K/AKT signaling can occur in the absence of epithelial AR.
AR downregulates AKT activity by stimulating FKBP5 and PHLPP-mediated AKT dephosphorylation
Postnatal epithelial AR deletion sensitizes prostate epithelium to paracrine signaling mediated by AR+ stroma, leading to androgen-induced epithelial hyperproliferation (Cunha, 1994; Cunha et al., 2002; Niu et al., 2008a; Simanainen et al., 2009). To block potential stroma-derived androgen-induced mitogenic signals, we castrated Pb-Cre+;PtenL/L;ArL/Y mutants at 6 weeks (n=4) and analyzed their response. Similar to Pten null mutants, Pb-Cre+;PtenL/L;ArL/Y prostates did respond to castration but developed castration-resistance when analyzed 4 weeks later. Histopathological analysis indicated that castration-resistant cancer outgrowths in the dorsolateral lobes are composed mainly of Pten−;AR− (data not shown) while anterior and ventral lobes contained both AR+ and AR− regions (Figure 5A). Strikingly, in comparison to AR+ regions (arrowheads), AR− regions (arrows) contain much higher levels of membranous P-AKT and intracellular P-S6 as well as elevated cell proliferation and cell death, whether assessed at 4 weeks or even 2 days post-castration (Figure 5A, C and D; Figures S5A).
Figure 5. AR down regulates AKT activity by stimulating FKBP5 and PHLPP-mediated, AKT dephosphorylation.
(A) PI3K activation (P-AKT, P-S6) in AR+ regions (arrows) versus AR− regions (arrow heads) in castrate, Pb-Cre+;PtenL/L;ArL/Y mutants. (Bar, low mag = 150 μm, high mag = 75 μm). (B) Effect of AR deletion on FKBP5 expression in Pb-Cre+PtenL/L;ArL/Y mutants (bar = 50 μm). (C, D) Expression of FKBP5, PHLPP and P-AKT in AR+ regions (arrow heads) compared to AR-null regions (arrows) in castrated Pb-Cre+;PtenL/L;ArL/Y mutants at 2 days (C) and 4 weeks (D) after castration (bar = 75 μm). See also Figure S5.
In searching for androgen-responsive genes that are decreased during castration, we found FKBP5, a member of the cis-trans prolyl isomerase family (Ratajczak et al., 2003). FKBP5 is an established androgen regulated gene in humans (Magee et al., 2006) (Mostaghel et al., 2007) whose expression is immediately suppressed after castration but up-regulated upon hormone replacement in the WT mouse prostate (Figure S5B) and down-regulated in the Pten null intact and castrated prostates (Figure S5C), similar to Mme and Msmb (Figure S5D). We found that FKBP5 expression in Pb-Cre+;PtenL/L;ArL/Y prostate correlates with AR expression (Figure 5B). A recent study showed that FKBP5, by serving as a scaffolding protein for AKT and PHLPP, promotes PHLPP dephosphorylation of AKT at amino acid S473 (Brognard et al., 2007; Gao et al., 2005) and thereby suppressing AKT activity (Pei et al., 2009). In castrated Pb-Cre+;PtenL/L;ArL/Y prostates, we found that regions with Pten and AR deletion have hyperphosphorylated AKT (S473) and reduced PHLPP, especially membrane-associated PHLPP (Figure 5C and D arrows), while Pten−;AR+ regions showed opposite effects (Figure 5C and D, arrow heads). These results suggest that down regulation of FKBP5 either by castration or AR loss could release FKBP5-PHLPP-mediated suppression of AKT activity and promote AKT-dependent but androgen and AR-independent cell proliferation.
Correlations among PTEN, AR, FKBP5 and PHLPP in human CaPs
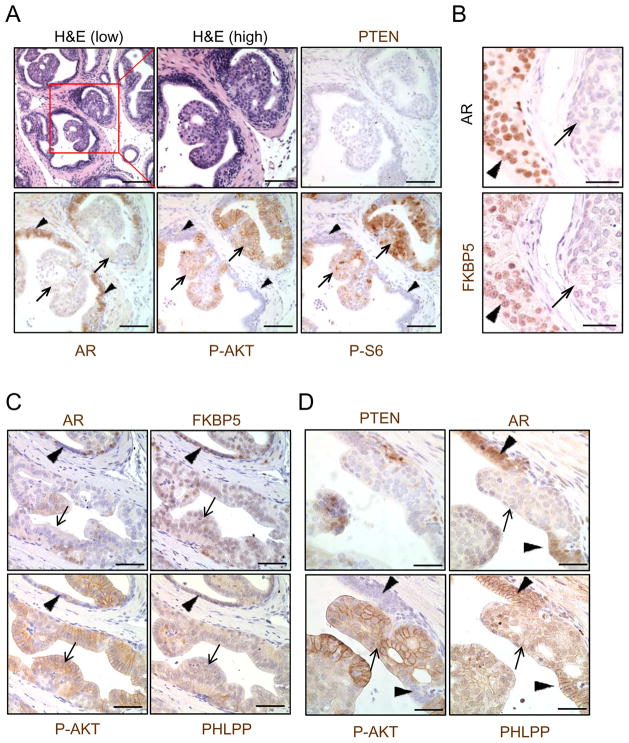
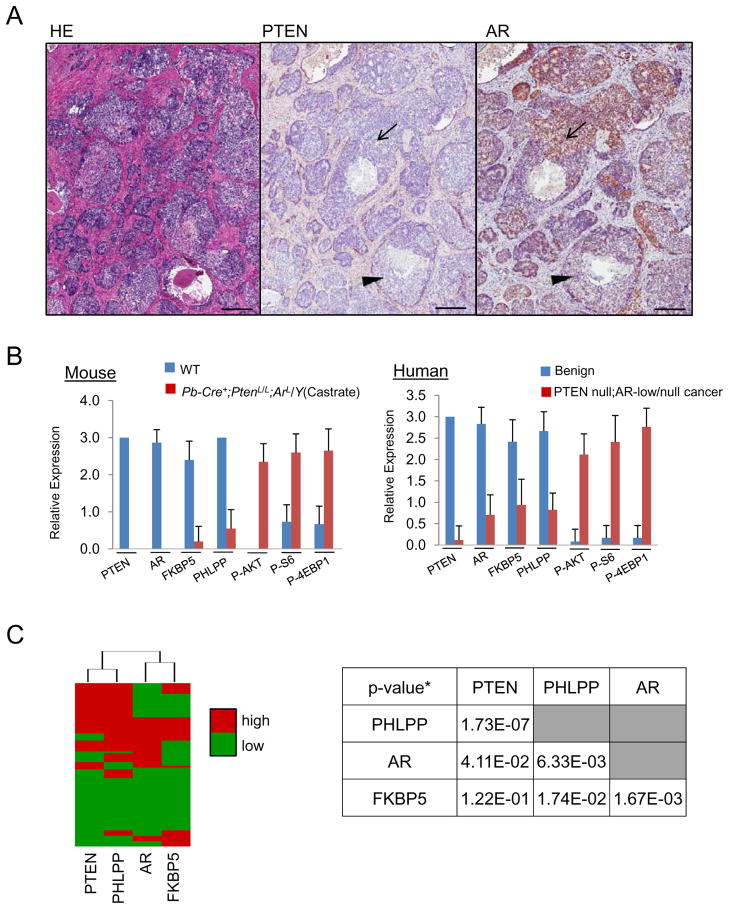
To assess the relevance of our finding in human CaPs, we took consecutive sections from a whole mounted fresh surgically resected human CaP sample and performed IHC analyses for PTEN, AR, FKBP5, PHLPP, P-AKT, P-S6 and Ki67 levels. We observed considerable heterogeneity of AR expression in PTEN null cancerous regions (Figure 6A; arrow, PTEN−;AR+; arrow head, PTEN−;AR− while adjacent non-cancer regions showed uniformed PTEN+ and AR+ staining (Figure S6A). Importantly, high AR expression regions are associated with higher FKBP5 and PHLPP but lower P-AKT and P-S6, similar to our observations in the murine model (Figure S6B). We then took 40 high power images from benign and PTEN-negative;AR-low/negative cancer regions and scored the expression of PTEN, AR, FKBP5, PHLPP, P-AKT, P-S6 and P-4EBP1 in a range of 0–3, with 3 being the highest expression level. Similar to the Pb-Cre+;PtenL/L;ArL/Y mouse model (Figure 6B, left panel), we observed moderate to high levels of PI3K pathway effectors in PTEN-negative;AR-low/negative human cancer regions (Figure 6B, right panel). Moreover, PTEN-negative;AR-low/negative regions maintained elevated cell proliferation in comparison to benign regions (19.2% vs. 1.4%, p<0.01) (Figure S6C). We then surveyed CaP tissue microarrays generated by the UCLA Prostate SPORE program. Again, consecutive sections were used for IHC analyses and only cores show >30% epithelium were scored. Among 91 cores we analyzed, we found extensive AR heterogeneous expression levels, ranging from nearly 100% AR+ to complete AR− (Figure S6D). Using unsupervised cluster analysis, we affirmed that PTEN is significantly correlated to PHLPP expression (p = 1.73e−7, χ2 test), while AR and FKBP5 are closely clustered together (p = 1.67e−3, χ2 test) (Figure 6C, Figure S6E). These data suggest that the AR-FKBP5-PHLPP feedback loop may also function in human PTEN-negative;AR-low/negative CaP.
Figure 6. Heterogeneous AR expression in human CaPs correlating with PI3K/AKT signaling and FKBP5 and PHLPP levels.
(A) PTEN and AR expression in human CaP (bar = 500 μm). (B) PI3K pathway components (P-AKT, P-S6, P-4EBP1) in Pten−;AR− regions of Pb-Cre+;PtenL/L;ArL/Y mice and in PTEN-negative;AR-low/negative regions of human CaPs. Error bars, means +/− SD. (C) Unsupervised clustering analysis of PTEN, PHLPP, AR, and FKBP5 in human TMA samples (N=91) (left). Chi squared test P-values* (N = 91) were used to quantitate the strength of association between each pair (right, table). Protein level was categorized to high (IHC> 1) and low (< 1) levels). See also Figure S6.
Combined AR/androgen ablation and mTOR inhibition results in enhanced therapeutic efficacy in Pten-null CaP
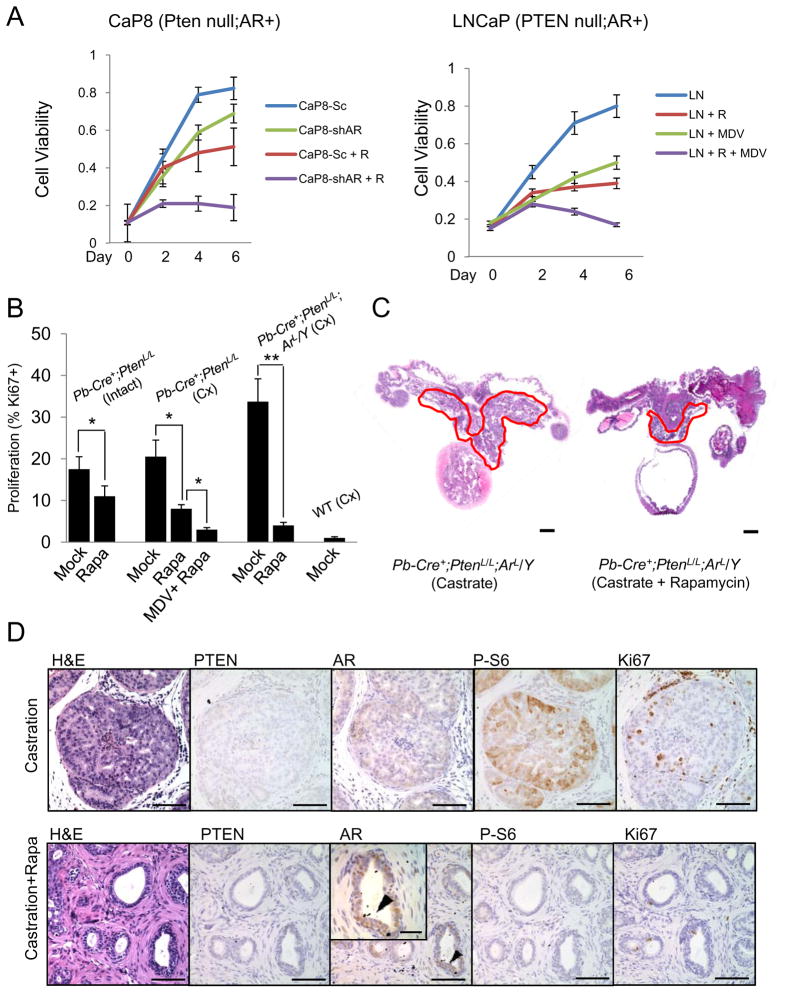
Since ablation of the AR/androgen signaling axis further activates AKT, we hypothesized that cancers with low levels of AR and PTEN loss would have greater dependency on the PI3K/AKT/mTOR signaling axis. To test this in vitro, we used CaP8 cells, a Pten-null;AR+ cell line derived from the Pb-Cre+;PtenL/L CaP model (Jiao et al., 2007), infected with either a scramble or a sh-AR RNAi lenti-virus containing a RFP tag for FACS sorting. Analysis of cell viability at 0, 2, 4 and 6 days indicated a cooperative reduction in cell viability using AR knockdown combined with the mTOR inhibitor rapamycin (1 nM) (Figure 7A, left graph) (n=6). We also treated the PTEN-null;AR+ LNCaP human CaP cell line with rapamycin (1 nM), the 2nd generation anti-AR inhibitor MDV3100 (10 μM) (Scher et al., 2010; Tran et al., 2009), or both. Again, we observed a cooperative inhibition effect when both AR and PI3K/mTOR pathways were co-targeted (Figure 7A, right graph) (n=6).
Figure 7. Cooperative effects of AR and mTOR inhibition in vitro and in vivo.
(A) In vitro response of Pten-null;Ar+ mouse (CaP8) and human (LNCaP) CaP cells to AR knockdown (sh-AR) or pharmacological inhibition of AR (MDV3100, 10 μM) with and without rapamycin (R: 1 nM) treatment. (Sc = control sh oligo). (B, D) In vivo response to treatments with castration, MDV3100, rapamycin or their combinations as measured by cell proliferation (Ki67+ cells) and (C, D) tumor burden in Pb-Cre+;PtenL/L and Pb-Cre+;PtenL/L:ArL/Y mutants (C, bar = 2 mm; D, bar = 200 μm; D, inset bar = 75 μm). Error bars = means +/− SD. See also Figure S7.
To test our hypothesis in vivo, we treated intact and castrated Pb-Cre+;PtenL/L and Pb-Cre+;PtenL/L;ArL/Y mice at cancer stage with rapamycin and evaluated the effects by Ki67 index (Figure 7B) and histopathology (Figure 7C and Figure S7; n=4). Rapamycin treatment (4mg/kg daily for 4 weeks) led to a reduction of Ki67 index in Pb-Cre+;PtenL/L intact mutants from 17% to 11%; castration and castration plus MDV3100 (10mg/kg/d) further reduced the rates of cell proliferation to 8% and 3%, respectively (Figure 7B). Similarly, when Pb-Cre+;PtenL/L;ArL/Y mutants were castrated and treated with or without rapamycin for 4 weeks, we found a significant reduction of prostate volume in comparison to the placebo cohort (Figure 7C). At the histological level, double mutants with combination of rapamycin treatment showed significant atrophy in most glandular structures, similar to that of WT castrated and Pten null castrated mice treated with combination of rapamycin and MDV3100 (Figure S7). A reduction in P-S6 (Ser240) staining and reduced cell proliferation (Figure 7B and D; 33.7% versus 4%; **, p<0.01) was also observed. Interestingly, many residual epithelia in rapamycin treated castrated mutants appeared to be AR+ (high power insert). Collectively, these data suggest that CaPs with AR loss have greater reliance upon the PI3K/AKT/mTOR signaling pathways and that combined AR/androgen blockage in conjunction with PI3K/AKT/mTOR inhibition is more effective for CaPs initiated by PTEN loss or PI3K/AKT activation.
DISCUSSION
PTEN loss or PI3K pathway activation represents one of the most frequent genetic alterations found in human CaPs (Taylor et al., 2010). Dysregulation of the PTEN/PI3K pathway has also been associated with resistance to conventional anti-androgen therapies (Ham et al., 2009). Despite these clinical observations, the consequence of PTEN loss and how its loss influence androgen/AR signaling axis and CRPC development are unclear. By genetically deleting Pten and Pten/Ar in the prostatic epithelium and analyzing human CaP samples, our studies support the hypothesis that PTEN loss or PI3K pathway activation may function in a cell autonomous manner to promote androgen/AR-independent CaP progression and CRPC development. Although Pten null epithelial cells remain sensitive to androgen withdrawal or AR ablation, the resulting activated PI3K/AKT pathway is sufficient to compensate androgen/AR signaling blockage, mobilize basal and transient amplifying stem/progenitor cells and promote cell proliferation. Therefore, in CaPs initiated by PTEN loss or PI3K activation, the overall outcome of cancer development, especially CRPC development, depends on the balance of androgen-dependent cell survival/differentiation and androgen-independent cell proliferation. PTEN loss enhances the expressions of EGR1, c-JUN and EZH2, which in turn suppresses AR transcription factor activity and output. Inhibiting FKBP5/PHLPP-mediated negative feedback to AKT activation, as a result of castration or AR inhibition, may further enhance the strength of the PI3K/AKT pathway and tilt the balance toward androgen-independent growth (Figure 8). Importantly, results derived from our genetically engineered mouse models, including PTEN controlled AR transcription output and AR-FKBP5-PHLPP regulatory loop, can also be observed in human CaP samples. Given commonly observed heterogeneous PTEN deletion/mutation and AR expression patterns within individual human CaPs, shown in our study and published by others (Attard et al., 2009b; Taylor et al., 2010), we would expect to observe a greater range of phenotypic correlating between PTEN loss, AR expression and CRPC development in human patients.
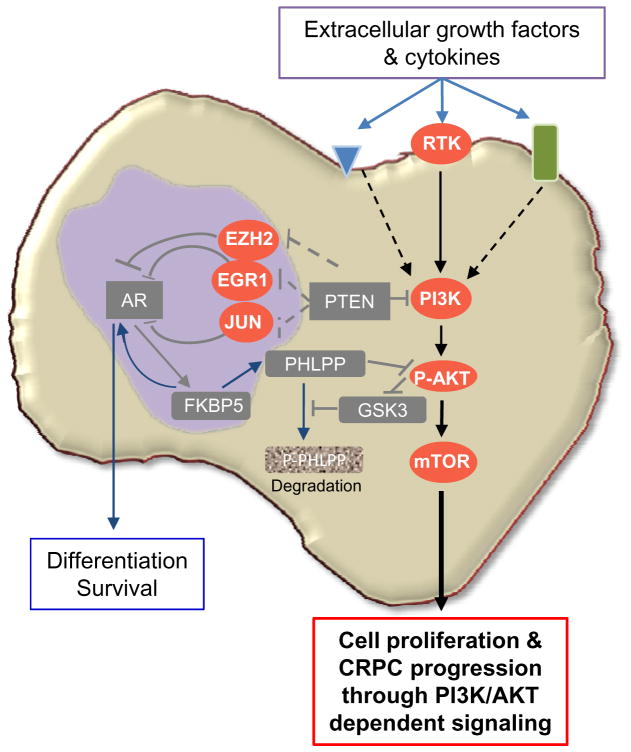
Figure 8. PTEN loss promotes CRPC development by two collaborative mechanisms.
By regulating EGR1, c-JUN and EZH2 expression and activities, PTEN loss suppresses AR transcription factor activity and output, leading to reduced prostate epithelial differentiation and survival. Collaboratively, PTEN loss activates the PI3K/AKT signaling pathway and reduces the AR-regulated FKBP5-PHLPP negative feedback loop to AKT activation, further enhances AKT activation, leading to androgen/AR-independent prostate epithelial proliferation.
Using genetically engineered animal models, our findings may provide potential insight to the clinical settings. Although hormone therapy immediately after radical prostatectomy improves survival and reduces the recurrence (Messing et al., 1999), it is not clear whether early androgen deprivation therapy (ADT) will prevent cancer progression and CRPC development. That early castration of the Pten null CaP model does not significantly impede carcinoma development and castration-resistant growth suggests that patients with PTEN loss or PI3K pathway activation may not benefit from aggressive early hormone treatment. While neoadjuvant ADT may partially reduce tumor load and PSA levels, it may select for cells with activated compensatory cell survival and proliferating signaling pathways, such as those PTEN null or PI3K/AKT-activated, and ultimately facilitate resistance to anti-androgen therapy.
An important finding from our study is that PTEN loss suppresses AR transcription factor activity and androgen-responsive gene expression in both murine models and human prostate samples. Interestingly, AR itself is not down-regulated in Pten null CaPs, even after castration (data not shown), similar to human cancers after short-term castration or after 9 months of neoadjuvant ADT (Mostaghel et al., 2007). Previous studies suggest that the PI3K pathway can either activate or suppress AR activity (Kaarbo et al., 2010; Lei et al., 2006; Lin et al., 2004; Lin et al., 2003). Our analyses demonstrate that PTEN, by regulating the expressions and activities of EGR1 and c-JUN transcription factors as well as the level of EZH2, controls AR transcription output, and thereby, render PTEN null CaP cells less or completely independent of signaling provided by androgens and epithelial AR, hence promote castration-resistant growth. However, how PTEN/PI3K pathway controls EGR1, c-JUN and EZH2 expression and activities requires further study.
The mosaic pattern of AR deletion observed in our in vivo model also mimic the heterogeneous AR expression observed in our human CaP studies and those reported by others (Attard et al., 2009b). After castration of Pb-Cre+;PtenL/L;ArL/Y mutants, we observed significant outgrowth of Pten−;AR− cancerous regions, accompanied by elevated P-PAKT and cell proliferation. Adjacent to these outgrowth are AR+ regions. Therefore, it is possible that within heterogeneous malignant CaPs, those with low or negative AR expression fail to respond to conventional anti-androgen treatment and continue to proliferate and survive as a consequence of PTEN loss and PI3K/AKT activation. While such cells would not constitute PSA producing cancer cells, they would contribute towards overall tumor load.
An intriguing finding of our study is the elevated P-AKT (S473) levels despite genetic deletion of Ar and surgical castration. The recently defined relationship between FKBP5 and the AKT phosphatase, PHLPP (Gao et al., 2005) (Brognard et al., 2007) (Pei et al., 2010), provides a potential mechanism as how PI3K/AKT signaling can be activated upon inhibition of the AR/androgen signaling axis. Specifically, while in normal cells PHLPP levels are high, which keeps P-AKT levels low, down-regulation of FKBP5 in Pten-negative cells that have undergone AR/androgen ablation would lead to a reduction of PHLPP’s association with P-AKT and consequently enhance AKT activity. Although it is difficult to assess P-AKT levels in all human CaP samples due to various sample harvesting and preparation procedures used, we did observed statistically significant correlation between the levels of PTEN and PHLPP as well as AR and FKBP5. Importantly, when a fresh resected CaP sample was analyzed, we found that regions with low or no AR expression have lower levels of FKBP5 and PHLPP and enhanced P-AKT and P-S6 staining. Besides the AR-FKBP5-PHLPP feedback loop, we also observed changes in other scaffold proteins and mediators of the PI3K pathway (Table S1). It will be interesting for future studies to ascertain whether other AR/androgen regulated genes, such as FKBP11 and IGFBP3/6, also have the capacity to modulate PI3K/AKT signaling in a manner similar to FKBP5.
Increasing evidence suggests that different cellular compartments may contribute differently towards cancer initiation. For example, the prostate epithelial AR may confer some suppressor function while the stromal AR has been postulated to be mitogenic (Cunha et al., 2002; Simanainen et al., 2009), thus raising the possibility that stromal AR plays a dominant role in Pten null cancer initiation or during the development of CRPC. In fact, using the TRAMP CaP model, a previous report has shown that reduction of stromal AR content may lead to reduced tumor progression (Niu et al., 2008b). However, extrapolating these data to human disease is challenging because neuroendocrine carcinoma observed in the TRAMP model, a phenotype which is rare in human disease (Abbas et al., 1995; Tetu et al., 1987). It is also possible that while AR loss or degradation in differentiated luminal epithelium results in cell death, AR loss in basal or transient amplifying initiating cells is well tolerated. In fact, recent reports have shown that the basal “LSC” stem/progenitor cells either from the Pten null CaP model (Mulholland et al., 2009) or from primary human tissue that has been transformed (Goldstein et al., 2010) are sufficient for tumor reconstitution. Thus, it would be interesting and potentially clinically relevant to ascertain if AR-null stem progenitor cells would be capable of cancer initiation. Interestingly, cells with high N-cadherin expression, a marker associated epithelial-mesenchymal transition and cancer metastasis at late stage disease are also AR-null or low and forced expression of N-cadherin results in reduced AR expression and elevated P-AKT in human CaPs (Tanaka et al., 2010).
Previous studies have shown that mTOR inhibition alone is relatively ineffective at reducing overall tumor load in Pb-Cre+;PtenL/L mutants (Kinkade et al., 2008) (Zhang et al., 2009). To test the hypothesis that combined AR and mTOR targeting may be more effective where single agent use is not, we evaluated the impact of total AR/androgen ablation in conjunction with mTOR inhibition. When comparing Pb-Cre+;PtenL/L;ArL/Y mutants under castration alone to those with rapamycin, we observed marked inhibition of cell proliferation and reduction of tumor load. Thus, these pre-clinical data suggests that complete abolishment of the AR/androgen signaling axis combined with mTOR inhibition is superior to single agent use, most likely by inhibiting the crosstalk between the two pathways. Thus, more effective blockade of the androgen/AR axis with new generation inhibitors such as abiraterone and MDV3100 in combination with mTOR or PI3K/mTOR dual inhibitors may prove to be further advantageous in treating CRPC cases initiated by alterations of PTEN/PI3K pathway.
EXPERIMENTAL PROCEDURES
Generation of Pb-Cre+;PtenL/L;ArL/Y and PtenL/L;Tfm mutant mice
Mice with conditional deletion of Pten and AR in the murine prostate were developed by crossing male, Pb-Cre+;PtenL/L mice with Cre−;ArL/ArL female mice on a mixed background. The AR conditional line (ARloxP-exon2-loxP) was obtained from and previously described by the laboratory of Dr. Guido Verhoeven (De Gendt et al., 2004) while Pb-Cre+;PtenL/L mutants were described by our laboratory (Wang et al., 2003). PtenL/L;Tfm mutant mice were obtained by crossing PtenL/L mutants with Tfm mice (JAX) (Cunha and Chung, 1981). All animal experiments were approved by the UCLA Animal Research committee and conducted according to relevant regulatory standards.
Cell Isolation and Flow Cytometry
The isolation of murine prostate epithelium was carried out by mechanical dissociation (5 min mincing) and enzymatic digestion (collagenase type I digestion at 37 C for 2 hours) of all prostate lobes. Single cell suspension were generated and stained in DMEM 10% FBS for 20 min at 4 oC with anti-CD49f (Biolegend), and with a Lin cocktail comprised of anti-CD45, anti-CD31, and anti-Ter119 (ebiosciences). Cell sorting was performed on the BD FACS Vantage or BD FACS Aria II (BD Biosciences). Lin−CD49fhi/mid cells were collected and subsequently used for FACS analysis and/or tissue regeneration assays (Mulholland et al., 2009)
Prostate regenerations
Donor (Lin−CD49fhi/mid) epithelia isolated from Cre−, Cre−;PtenL/L or Cre−;PtenL/L;ArL/Y or PtenL/L;Tfm mutant mice were infected with high titer (>1×109) Cre-lenti virus (Cre-FUCRW) by low speed centrifugation over 90 min. Infected epithelium (1–2 × 105) were recombined with UGSM (2 × 105) in 15 μl collagen pellets and incubated for 6–10 hours in DMEM (high glucose, 10% FBS; Insulin, 0.005 mg/ml, Bovine pituitary extract; 10.7 μg/ml; EGF/FGF, 3.0 ng/ml). Tissue recombinants were then surgically implanted below the kidney capsule of NOD;SCID;IL2rγ-null mice for 6–10 weeks (Mulholland et al., 2009; Lawson et al., 2010, Goldstein et al., 2010).
Plasmids
The murine Ar-specific short hairpin RNAs (shRNA) (Jiao et al., 2007) with a 6 promoter were cloned into the BamHI-EcoRI site the lentivector FUCRW. The open reading frame for the human AR gene with flag tag was cloned at the XbaI site under the human ubiquitin promoter.
Orthotopic lenti-virus injection
The LSL-Akt (loxP-RFP-loxP-myr-Akt-Ires-EGP) lenti vector and virus generated as previously described (Marumoto et al., 2009). To carry out orthotopic injections, 2–3 μl of high titer lenti virus (> 1×108) was injected to the base of the anterior prostate lobe (proximal to urethra) of Pb-Cre+;ArL/Y mice aged 2–3 weeks using a cemented, 10 μl microsyringe (Hamilton). Mice were then aged for 6–8 weeks and subsequently evaluated at the histological level.
Collection of Patient Samples
Clinical samples were obtained with informed consent under approval from the University of California at Los Angeles (UCLA) Institutional Review Board. All samples were de-identified to protect patients’ confidentiality.
Human whole mount and Tissue Microarrays (TMA)
Pathologic specimens (whole mount specimens) were acquired from the UCLA Tissue Procurement Core Laboratory in a de-identified manner. IHC analysis and scoring was carried out on cores containing >30% epithelial content (Thomas et al., 2004).
Drug Treatment
Pb-Cre+;PtenL/L and Pb-Cre+;PtenL/L;ArL/Y were castrated and immediately treated with either 4 mg/kg/d (I.P.) rapamycin (Selleck Chem.), 10 mg/kg/d MDV3100 or vehicle control (P.O.). Mice were treated for 4 weeks and then evaluated at the histological level.
Accession Numbers
Gene expression microarray data have been deposited in the GEO data base with the accession number GSE29010.
Supplementary Material
SIGNIFICANCE.
Resistance to androgen deprivation therapy is the major hurdle for managing patients with advanced CaP. Therefore, understanding the molecular mechanisms underlying CRPC will be helpful for the design of therapeutic strategies to overcome the resistance. We found that CaPs initiated by PTEN loss result in suppression of AR transcription output and can progress to CRPC independent of epithelial AR, providing a mechanism of escaping the requirement of androgen/AR axis for castration-resistant growth. AR loss or inhibition, on the other hand, can further activate AKT activity via down regulation of the FKBP5 scaffold protein and PHLPP phosphatase. Our study suggests that co-targeting both AR and PTEN/PI3K pathways may enhance therapeutic efficacy for CaPs initiated by PI3K pathway alterations.
HIGHLIGHTS.
Castration-resistant proliferation of Pten null prostate cancer cells.
PTEN loss suppresses AR transcription factor activity promoting AR-independence.
AR inhibits AKT through FKBP5 scaffold protein and PHLPP phosphatase.
Co-targeting of AR and AKT/mTOR signaling leads to better therapeutic outcome.
Acknowledgments
We appreciate Drs. Guido Verhoeven and Karel De Gendt for generous supplying the AR conditional knockout line; UCLA Prostate SPORE for whole mount human CaP and TMA sections; Dr. Charles Sawyers for MDV3100 and communicating unpublished results. We thank the helpful comments and suggestions from Drs. Owen Witte, Robert Reiter, Peter Nelson and colleagues in our laboratories; DJM was supported by NIH F32 CA112988-01 and CIRM TG2-01169 and LMT is supported by NIH T32 CA009056; This work has been supported in part by award from the Prostate Cancer Foundation (to HW and IPG), Jean Perkins Foundation and Department of Defense (to IPG) and grant from NIH (R01 CA107166 and RO1 CA121110 to HW).
References
- Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995;46:617–630. doi: 10.1016/S0090-4295(99)80290-8. [DOI] [PubMed] [Google Scholar]
- Abdulkadir SA, Qu Z, Garabedian E, Song SK, Peters TJ, Svaren J, Carbone JM, Naughton CK, Catalona WJ, Ackerman JJ, et al. Impaired prostate tumorigenesis in Egr1-deficient mice. Nat Med. 2001;7:101–107. doi: 10.1038/83231. [DOI] [PubMed] [Google Scholar]
- Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009a;16:458–462. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009b;69:4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Freeman DJ, Wu H. PTEN regulates Mdm2 expression through the P1 promoter. J Biol Chem. 2004;279:29841–29848. doi: 10.1074/jbc.M401488200. [DOI] [PubMed] [Google Scholar]
- Chauvin TR, Griswold MD. Androgen-regulated genes in the murine epididymis. Biol Reprod. 2004;71:560–569. doi: 10.1095/biolreprod.103.026302. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–1044. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LW. Stromal-epithelial interactions--I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–485. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in Nkx3.1; Pten mice. Cancer Res. 2006;66:7929–7933. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Gitenay D, Baron VT. Is EGR1 a potential target for prostate cancer therapy? Future Oncol. 2009;5:993–1003. doi: 10.2217/fon.09.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham WS, Cho NH, Kim WT, Ju HJ, Lee JS, Choi YD. Pathological effects of prostate cancer correlate with neuroendocrine differentiation and PTEN expression after bicalutamide monotherapy. J Urol. 2009;182:1378–1384. doi: 10.1016/j.juro.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Hill R, Wu H. PTEN, stem cells, and cancer stem cells. J Biol Chem. 2009;284:11755–11759. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, Wu H. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- Kaarbo M, Mikkelsen OL, Malerod L, Qu S, Lobert VH, Akgul G, Halvorsen T, Maelandsmo GM, Saatcioglu F. PI3K-AKT-mTOR pathway is dominant over androgen receptor signaling in prostate cancer cells. Cell Oncol. 2010;32:11–27. doi: 10.3233/CLO-2009-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, Malek A, Chiorino G, Catapano CV, Carbone GM. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS One. 2010;5:e10547. doi: 10.1371/journal.pone.0010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J, Gleave ME, Witte ON, Liu X, Wu H. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9:367–378. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18:2409–2423. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- Lin HK, Hu YC, Yang L, Altuwaijri S, Chen YT, Kang HY, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–598. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, Hood L, Lin B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008a;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Altuwaijri S, Yeh S, Lai KP, Yu S, Chuang KH, Huang SP, Lardy H, Chang C. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci U S A. 2008b;105:12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Lou Z, Wang L. Emerging role of FKBP51 in AKT kinase/protein kinase B signaling. Cell Cycle. 2010;9:6–7. doi: 10.4161/cc.9.1.10290. [DOI] [PubMed] [Google Scholar]
- Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38:e169. doi: 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak T, Ward BK, Minchin RF. Immunophilin chaperones in steroid receptor signalling. Curr Top Med Chem. 2003;3:1348–1357. doi: 10.2174/1568026033451934. [DOI] [PubMed] [Google Scholar]
- Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- Sato N, Sadar MD, Bruchovsky N, Saatcioglu F, Rennie PS, Sato S, Lange PH, Gleave ME. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J Biol Chem. 1997;272:17485–17494. doi: 10.1074/jbc.272.28.17485. [DOI] [PubMed] [Google Scholar]
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers WR, Loda M. The EZH2 polycomb transcriptional repressor--a marker or mover of metastatic prostate cancer? Cancer Cell. 2002;2:349–350. doi: 10.1016/s1535-6108(02)00187-3. [DOI] [PubMed] [Google Scholar]
- Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148:2264–2272. doi: 10.1210/en.2006-1223. [DOI] [PubMed] [Google Scholar]
- Simanainen U, McNamara K, Gao YR, Handelsman DJ. Androgen sensitivity of prostate epithelium is enhanced by postnatal androgen receptor inactivation. Am J Physiol Endocrinol Metab. 2009;296:E1335–1343. doi: 10.1152/ajpendo.00017.2009. [DOI] [PubMed] [Google Scholar]
- Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010 doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NG. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LM, Hyduke DR, Liao JC. Trimming of mammalian transcriptional networks using network component analysis. BMC Bioinformatics. 2010;11:511. doi: 10.1186/1471-2105-11-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wang XD, Wang BE, Soriano R, Zha J, Zhang Z, Modrusan Z, Cunha GR, Gao WQ. Expression profiling of the mouse prostate after castration and hormone replacement: implication of H-cadherin in prostate tumorigenesis. Differentiation. 2007;75:219–234. doi: 10.1111/j.1432-0436.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- Yang SZ, Eltoum IA, Abdulkadir SA. Enhanced EGR1 activity promotes the growth of prostate cancer cells in an androgen-depleted environment. J Cell Biochem. 2006;97:1292–1299. doi: 10.1002/jcb.20736. [DOI] [PubMed] [Google Scholar]
- Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Young CY, Tian Y, Liu Z, Zhang M, Lou H. Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol Cell Biochem. 2010;339:253–262. doi: 10.1007/s11010-010-0388-7. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhu J, Efferson CL, Ware C, Tammam J, Angagaw M, Laskey J, Bettano KA, Kasibhatla S, Reilly JF, et al. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a Pten-deficient mouse model of prostate cancer. Cancer Res. 2009;69:7466–7472. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.