Abstract
Purpose
Chondroitin sulfate, which is less expensive and more inert than heparinoids, hyaluronan or pentosan polysulfate, has been introduced to restore the barrier function lost due to epithelial dysfunction in interstitial cystitis (IC). The binding of chondroitin sulfate to damaged bladder as a function of the range of pH seen in urine, its efficacy in restoring the bladder's permeability barrier, and the capacity of damaged bladder to bind chondroitin sulfate have not been determined previously.
Methods
Binding of chondroitin sulfate to bladder urothelium was investigated quantitatively using chondroitin sulfate highly labeled with Texas Red and quantitative fluorescence microscopy in a mouse model of acid damage of the urothelium. The efficacy of restoring the barrier function was determined using passage of intravesically instilled 86Rb, a potassium ion mimetic, through the urothelium into the bloodstream in a rat model of bladder damage. The binding capacity of acid-damaged bladder was determined by fluorometry.
Results
Chondroitin sulfate bound tightly and exclusively to the mouse bladder surface that had been damaged by acid but showed only minimal binding to undamaged bladder. There was no systematic variation with pH. The model showed some variability in the degree of damage induced. In rats, chondroitin sulfate instillation restored permeability to 86Rb to control levels. Binding was saturable at 0.67 ± 0.13 mg/cm2 of bladder surface.
Conclusions
Chondroitin sulfate binds preferentially to damaged urothelium and restores the impermeability barrier. This suggests that the GAG layer is a major contributor to the impermeability of bladder urothelium. As determined by the binding capacity, the dose applied to humans in Canada (400 mg per instillation) is sufficient to obtain maximum efficacy.
Keywords: interstitial cystitis, GAG layer, urinary bladder, chondroitin sulfate
Introduction
Interstitial cystitis (IC) is a poorly understood set of symptoms characterized by bladder pain, urgency and often crippling frequency. Long thought to involve only the bladder, recent research suggests that IC may represent the bladder manifestations of a more systemic disorder.1 Although the etiology is unclear, it is clear that the pathophysiology of interstitial cystitis (IC) involves epithelial dysfunction2-5. The bladder maintains a complex set of defenses comprised of tight junction proteins and a dense layer of glycosaminoglycan on the specialized apical cells of the urothelium known as umbrella cells6. These defenses are compromised in IC, and the bladder urothelium becomes leaky, resulting in the appearance of substances added intravesically into circulation7, or reabsorption of fluorescein from the urine8. The potassium sensitivity test, which is the perception of pain from potassium, but not sodium, ions administered intravesically, also suggests loss of the barrier function in IC5. In some patients the pain response is immediately abrogated by intravesical heparin, indicating that it can restore the barrier function5. The dense layer of glycosaminoglycans known as the “GAG layer” has long been proposed to represent a major feature in producing the impermeability barrier5, although its importance has been questioned9. However, patients experience relief of symptoms from intravesically-administered GAG preparations including heparin, pentosan polysulfate10 and chondroitin sulfate11. The proposed mechanism of this “GAG replenishment therapy” is restoration of the GAG layer.
Chondroitin sulfate offers several potential advantages over heparin, pentosan polysulfate, and hyaluronan in simple barrier restoration. It has no effect on the coagulation system12, is inactive against many receptor systems that are affected by heparinoids13 and hyaluronan14, and it is inexpensive. In this paper we quantified binding of chondroitin sulfate to both normal and acid-damaged mouse bladder over the range of pH values expected in urine. Second, in a rat model of acid-damaged bladder, we demonstrated that intravesical treatment with chondroitin sulfate restored bladder impermeability to rubidium ions, a potassium mimetic15, to control levels. Finally, we also quantified the capacity of acid-damaged bladder to adsorb intravesically-applied chondroitin sulfate and showed that this binding is saturable, supporting the intravesical dose of 400 mg that is used currently in Canada.
Materials and Methods
Labeling of chondroitin sulfate
A more reactive aldehyde at the reducing was produced as described16 with 200 mg chondroitin sulfate (Stellar Pharmaceuticals, London, Ontario) and 90 mg (20-fold excess over reducing terminus) of sodium borohydride overnight followed by oxidation with 15 mg of sodium periodate (5-fold excess over reduced termini) at 0° C for 1 hour. Intermediate products were purified by precipitation from 75% ethanol. The oxidized product was labeled with Texas Red Hydrazide (Molecular Probes, Inc.),..17 dried and dissolved in 7.5 mL deionized water to a concentration of 20 mg/mL. F, the fraction of chondroitin sulfate molecules labeled on the reducing terminus, was calculated as follows.
A588 is the absorbance at 588 nm, M is the molecular weight of chondroitin sulfate (17,000 g/mol), l is the path length (0.1 cm), ε is the molar extinction coefficient for Texas Red (116,000) and C is the concentration of chondroitin sulfate in g/L.
Mouse model for testing of binding of chondroitin sulfate to damaged bladders
BALB/c mice (Charles River Laboratories) mice were anesthetized with xylazine (1 mg/100 g body weight) followed in 15 minutes by ketamine (12 mg/100 g body weight). After 15 minutes the animals were catheterized with a 24 gauge × ¾ inch intravenous catheter (Terumo Medical). Any urine in the bladder was removed, and the bladder was washed out by 2 cycles of instilling and removing 150 μL phosphate buffered saline (0.010 M phosphate) (PBS). To avoid overdistension, no more than 150μL of solution was ever instilled. The bladder was damaged with 10 mM HCl in water for 10 minutes followed by washes with 150 μL of 150 mM NaHCO3 and PBS. The bladder was instilled with 150 μL of 20mg/mL TR-labeled chondroitin sulfate at pH 8.0, 6.5 or 5.0 which was allowed to remain in the bladder for 1 hour. The bladder was washed with PBS, and the animal immediately euthanized. The bladder was removed and filled with OCT compound in order to inflate it fully. The bladder was then embedded in additional OCT, frozen at -20°C and cut into 5 μm sections, which were mounted on slides. The bladder sections were fixed onto the slide with acetone, washed to remove OCT, and counterstained with 10 μM Hoechst 33258 in PBS to show nuclei. Sections were stained with Alcian Blue as described previously.18
Measurement and Standardization of Fluorescence
The sections were examined under epi-illumination. Fluorescence was standardized against Texas Red beads (Polysciences, Cat. BLI 8938) that provided a fixed-point normalization for each experiment determined from the integrated intensities of at least 100 beads. Normalized images around the entire circumference of the bladder section were captured using a Nikon TE2000-E inverted epi-fluorescent microscope with nanoprecision Z-axis control equipped with a Photometrics Cool Snap ES digital camera under control of Metamorph Software version 7.1.3.0. Fluorescence excitation was performed with an X-Cite 120W lamp and Lamda 10-2 excitation and emission filter wheels. The filters used were Green (for Texas Red) Excitation 555 nm /28 nm width, Emission 617/73; UV (for Hoechst) Excitation 360/30, Emission 457/50. A negative control that was not treated with anything was used to provide a baseline for elimination of autofluorescence.
Analysis of Texas Red-Labeled Chondroitin Sulfate Bound to Urothelium
Using the “lasso” tool (a tool that outlines an area of interest) of PhotoShop, a region of the urothelium was selected on a negative control, and the threshold was set at a level that excluded any background red fluorescence. A region corresponding to urothelium from about 1/8th of a bladder quadrant was selected with the lasso tool, and the average intensity of red pixels (AR), the total number of red pixels (NR) and the total number of pixels (NT) in the selected area were measured using the “measure” functions of PhotoShop. Image analysis programs available from several manufacturers (e.g., Nikon) perform similar analyses. The integrated red intensity (IRI) was calculated for the selected area of interest as follows.
The calculation is independent of the size of the area selected because IRI is normalized to the total area selected. Following image capture, the sections were stained for H&E to show the morphology of the mouse bladder in the controls and after acid damage.
To prevent selection bias, the bladder was mounted to provide cross-sectional cuts. A cross-section for measurement was selected from near the middle of the bladder. High magnification images were obtained from the entire section and were assembled into the circles that comprised the entire bladder section. To further prevent selection bias, a quadrant of the bladder section was randomly selected before the slide was analyzed for imaging. Each region selected for measurement comprised about 1/8th of a quadrant.
Rat model for testing restoration of the permeability barrier by chondroitin sulfate
The animals (300 g Sprague-Dawley rats) with a cannula surgically implanted in the dome of the bladder with tubing that ran under the skin and emerged behind the neck were obtained from Charles River Laboratories (Cambridge, MA). To prevent problems from acute inflammation resulting from the surgery, the rats were allowed to acclimate at our institution for at least 3 days prior to the experiment. The rats were anesthetized with acepromazine (2 mg/100 g body weight) followed in 10 minutes with ketamine (5 mg/100 g body weight) according to an IACUC-approved protocol. After a 15 minute wait to ensure anesthesia, the cannulae were attached to a 1 ml syringe with a ½ inch blunt 23 gauge needle. The contents of the bladder were aspirated, the bladder was washed twice with 0.4 ml of PBS, and damage was induced in 2 rats by injecting 400 μL of 10 mM HCl in 0.154 M NaCl through the cannula into the bladder. The negative control received 400 μL of PBS. The solutions remained in the bladders for 10 min. In case of incomplete recovery of the instilled solution, the animal was lifted to a vertical position to facilitate complete draining. To one animal was added 400 μL of 20 mg/mL chondroitin sulfate in 0.154 M NaCl provided as a sterile solution for intravesical use (trade name Uracyst from Stellar Pharmaceuticals, London, Ontario). The negative control and the positive controls both received 400 μL of PBS alone. The solutions were allowed to remain in the bladder for 20 minutes, at which time the solutions were removed and 3 × 107 dpm of 86Rb was instilled. After 30 minutes the contents of the bladder were removed and blood (800 μL) was obtained by cardiac puncture. The animals were then euthanized. For the 86Rb studies, 800 μL of whole blood was counted after bleaching with 1.5 ml of 30% H2O2. Excess H2O2 was destroyed with 450 μL of saturated ascorbic acid, and the solution was mixed with 5 mL of Ultimate Gold scintillation fluid (Perkin-Elmer). The counting efficiency for this isotope is 95%. Studies were always performed with 3 animals per experiment: a negative control, a positive control and a chondroitin sulfate-treated animal with a minimum of 3 replicates.
Determination of binding capacity of acid-damaged rat bladder for chondroitin sulfate
In order to calculate the capacity of the acid-damaged bladder to adsorb chondroitin sulfate, the amount of labeled chondroitin sulfate remaining in the bladder was determined as a function of the instilled dose by fluorometry with excitation at 589 nm and emission at 615 nm. The dose was normalized to the surface area by using the experimentally determined bladder capacity and the relation between surface area (A) and volume (V) of a sphere, A = 4.84V2/3. V was determined by filling the bladder of anesthetized rats through the catheter at a low flow rate until leakage occurred. The capacities of three rat bladders were between 0.7 and 0.8 mL. The concentration of chondroitin sulfate was varied to provide the indicated doses in a constant volume of 0.5 mL. Equivalent doses for humans were calculated for doses of 80, 200, 400 and 800 mg assuming a 350 mL bladder capacity for an IC patient.
Statistical analysis
The data were analyzed using Prism 4 (GraphPad Software, Inc.). The binding data were analyzed using a one-way ANOVA and Bonferroni's Multiple Comparison of individual pairwise comparisons. More exact pairwise comparisons were also performed with a two-tailed Student's t-test assuming unequal variances.
Results
Nature of Damage Induced by 10 mM HCl
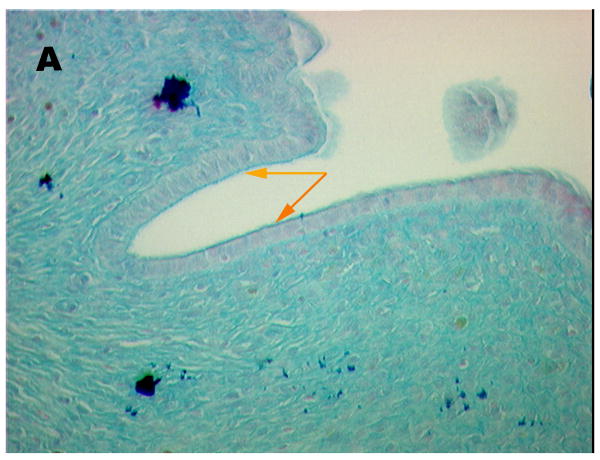
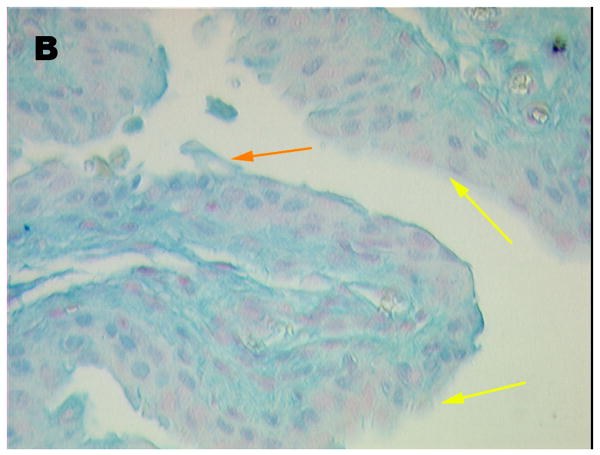
Fig 1, shows an Alcian Blue stained section of control (A) and acid-damaged (B) bladder, respectively. The control shows an intact umbrella cell layer capped by a distinct GAG layer. Some areas with umbrella cells were evident on the luminal surface of the acid-treated bladder, whereas in others, the umbrella cells were absent and only the basal cells and intermediate cells remained. However, in all areas examined, some urothelium remained. Note the area in the treated bladder marked with an orange arrow where the luminal cell layer with its attached GAG layer is peeling away. Some areas probably were protected from acid because care was taken to avoid fully inflating the bladder and producing an artifact in which the urothelium could split due to overdistension, thereby allowing the label to leak under the urothelium.
Figure 1.
Alcian Blue stained sections of (A) control (20 × magnification) and (B) rat bladder damaged with 10 mM HCl for 10 min. (40 × magnification). Orange arrows are used to denote areas of minimal damage. Yellow arrows point to areas where umbrella cells are denuded.
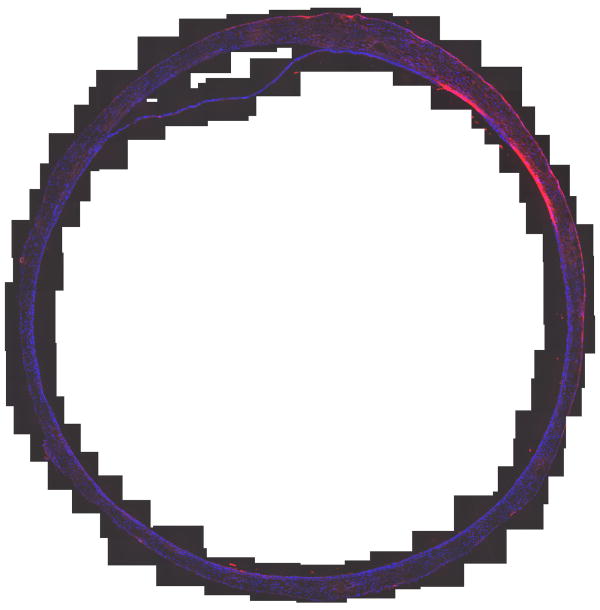
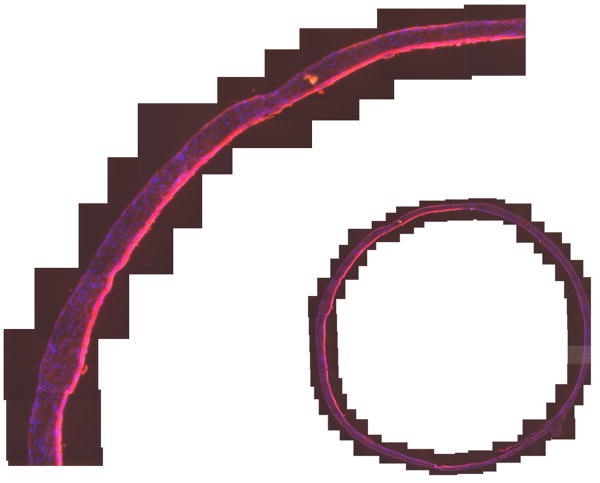
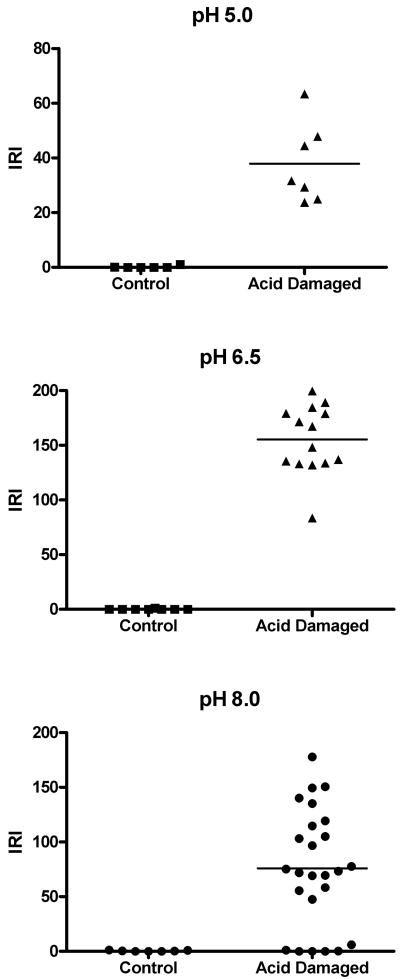
Quantification of Binding to Mouse Bladder
The absorbance of the labeled chondroitin sulfate was 0.285 at 40 mg/mL and a 0.01 cm pathlength, corresponding to F = 0.10. The results of quantifying binding as a function of pH are shown in Table 1 and Figures 2 and 3. Figure 2 shows each measurement of a section of mouse bladder. Table 1 summarizes the statistical analysis and Figure 3 shows the images of an entire cross section of a control and one of the chondroitin sulfate-treated bladders at pH 6.5. The control was chosen to illustrate the worst case. No other control showed any appreciable binding except this one, which showed a small area that bound chondroitin sulfate. We speculate that catheter damage could be responsible. Chondroitin sulfate bound in a broad area on the luminal surface encompassing more than the exposed cell surface. Comparison with Fig. 1 suggests it not only bound to the surface of intermediate layer cells exposed by loss of the umbrella cells but also penetrated between cells. Some variability in binding was observed in the treated bladders, as shown in Figure 3B, probably because of variability in the degree of damage induced by acid treatment as shown in Fig. 1. Very clearly, even with less damaged areas included, the damaged bladders bound more Texas Red-labeled chondroitin sulfate than did the controls. No systematic differences were seen in binding at different pH values.
Table 1. Mean ±SD of Integrated Red Intensity (IRI) of mouse bladder sections by pH at which binding occurred with highly labeled chondroitin sulfate.
| pH of ChS | Control (1 mouse) | Acid-Damaged | p |
|---|---|---|---|
| 5.0 | 0.21 ± 0.46 N=6 | 37.94 ± 14.56 N=7 (1 mouse) | <0.0005 |
| 6.5 | 0.00 ± 0.00 N=8 | 153.54 ± 31.57 N=14 (2 mice) | <0.0001 |
| 8.0 | 0.41 ± 0.53 N=7 | 75.67 ± 53.85 N=25 (3 mice) | <0.0001 |
Figure 2.
(A) Mouse Bladder, Control, No acid damage, treated with TR-Chondroitin sulfate at pH 6.5 (B) Mouse Bladder, Acid damaged, treated with TR-Chondroitin sulfate at pH 6.5
Figure 3.
Dot plots of normalized integrated red intensities (IRI) of mouse bladder labeled with Texas Red-labeled chondroitin sulfate as a function of pH and treatment. Each symbol represents one measurement of an area of interest. Horizontal bars are mean values.
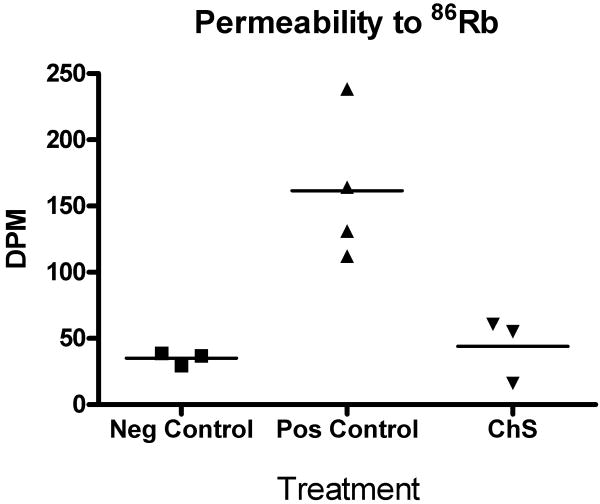
The effect of intravesical chondroitin sulfate on the permeability of acid-damaged rat bladders
Figure 4 shows the effect of intravesical chondroitin sulfate on the permeability of rat bladders damaged with 10 mM HCl. Absorption of 86Rb into systemic circulation after instillation into rat bladders (mean ± SEM) was 35.15 ± 2.10 dpm/mL for the negative controls, 161.5 ± 27.8 dpm/mL for the positive controls, and 44.02 ± 14.03 dpm/mL for the chondroitin sulfate-treated animals. The statistical comparisons listed in the figure legend strongly support the hypothesis that acid damage causes increased permeability, but that ChS treatment immediately restores the permeability barrier to control levels.
Figure 4.
Dot plot of the concentration of 86Rb measured in rat serum as a function of treatment. Horizontal bars represent mean values. A one-way ANOVA showed the differences between the means within the data set were significant at p=0.0056. Bonferroni's Multiple Comparison Test showed the differences between means of negative control and acid-damaged bladders (126.4, t = 4.3) and ChS-treated and acid damaged bladders (117.5, t = 4.0) were both statistically significant (p<0.05, two tailed); the difference between chondroitin sulfate-treated and acid-damaged bladders (8.9, t = 0.28) was not statistically significant. In pair-wise comparisons the two-tailed p-values of t-tests using Welch's correction for unequal variances were p = 0.0203, 0.0196 and 0.5991, respectively.
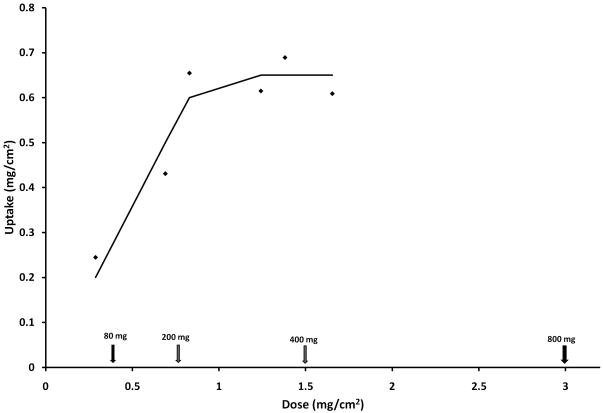
Titration of binding capacity for chondroitin sulfate of acid-damaged bladder
Figure 5 demonstrates that over a range of doses of chondroitin sulfate available per unit area that the acid-damaged rat bladder exhibits a saturable dose-response curve. Fitting to a sigmoidal dose-response curve yielded a saturation capacity of 0.67 ± 0.13 mg/cm2 (R2 = 0.855). Lower doses, such as 80 mg used previously 19, would not appear to have achieved saturation, even though they did show efficacy.
Figure 5.
Binding capacity of rat bladder for Texas Red-labeled chondroitin sulfate following surface damage with 10 mM HCl. The arrows represent the equivalent doses in human bladder (left to right) of 80, 200, 400 and 800 mg, respectively, and were calculated assuming a 350 ml bladder for IC patients. The 400 mg dose currently is in use in Canada.
Discussion
The role of the GAG layer in protecting the urothelium has been controversial. On the basis of Ussing chamber experiments, Lewis concluded it contributed little or nothing to the barrier function of mammalian urothelium9, whereas Parsons has claimed it represents a major contributor to barrier function and that loss of the GAG layer is responsible for the loss of impermeability in IC5. Recently, findings demonstrating edema resulting from chondroitinase removal of bladder surface GAG without other damage supports the role of the GAG layer in contributing to impermeability.20 Clearly, the urothelium in IC is dysfunctional and the permeability barrier is lost3-5,7. The urothelium shows altered structural properties with loss of the GAG layer, as clearly demonstrated by immunohistochemical analysis for both GAG chains and the proteoglycans to which they are bound.4 Other molecules, including uroplakin, have been proposed to represent major defense molecules, but the only approximately two-fold increase in permeability of bladders of uroplakin knockout mice over wild type mice21 brings uroplakin as the major barrier into question. In this study we clearly demonstrate that chondroitin sulfate binds preferentially to damaged urothelium, as reported previously17, but also that the binding occurs over the range of pH likely to be found in the human bladder. We also showed that adding exogenous chondroitin sulfate intravesically to the acid-damaged bladder restored the impermeability to 86Rb ions to control levels. Rubidium belongs to the same family of elements as potassium and is used as a model for potassium movement across membranes15 because of the impractically short half-lives of potassium isotopes.
The acid-damage model was used rather than the protamine model because of questions whether protamine and similar peptides open ion-conducting channels in the membrane that can be competed away with GAG as claimed by Lewis9 or whether the cationic peptide neutralizes the charge of the GAG layer as claimed by Parsons22. Previous studies with 14C urea did not demonstrate any radioactivity in circulation and “uptake” was defined as loss from the bladder22 rather than appearance in circulation. Most likely the dose of radioactivity was insufficient to demonstrate appearance in the circulation. We therefore used the least ambiguous definition possible, appearance in circulation, of an ion that cannot be metabolized and which mimics the alleged pathologic agent, potassium ions.5 The results presented above clearly demonstrate that intravesically administered chondroitin sulfate immediately restores the permeability barrier of acid-damaged urothelium at least to the degree that the amount of marker (86Rb) that penetrates into the blood stream is reduced to levels seen in controls with intact bladders.
The cause of epithelial dysfunction in IC is not known but may involve either the “antiproliferative factor peptide”3, other cytotoxins23 or cytokine-induced changes.24 The acid damage model provides a model that at least superficially resembles IC in that the umbrella cells also are missing from areas of IC bladder.4 To the extent that this model duplicates the properties of the dysfunctional urothelium, these findings suggest intravesical chondroitin sulfate also should be effective in mitigating the symptoms of IC as well as the wider range of “Painful Bladder Syndrome” because such patients also respond to intravesical GAG replenishment25. In an open-label study performed in Canada, of 53 IC/PBS patients naïve to intravesical GAG treatment, 48 patients had a positive potassium sensitivity test, and 32 patients had moderate or marked improvement after intravesical chondroitin sulfate treatment.11 Chondroitin sulfate has several advantages over other GAG products such as heparin, hyaluronate, or pentosan polysulfate in being much more biologically inert12-14 and less expensive. To the extent that binding saturation experiments in rat bladders can be extrapolated to human IC, the dosage of 400 mg chondroitin sulfate currently used in Canada likely should sufficient to saturate the bladder surface.
Conclusions
The GAG layer represents a major contributor to bladder impermeability. Bladders lacking the apical layer of cells become more permeable, but impermeability can be restored with exogenous chondroitin sulfate. Extrapolating from animal studies, a dose of 400 mg should saturate the surface of an IC patient with a 350 ml capacity bladder. The mode of action is most consistent with a simple coating to restore the missing GAG layer.
Acknowledgments
The authors gratefully acknowledge the assistance of C. Dirk Engles, Jean Coffman and Kim Kyker.
This work was supported in part by R01 DK069808 (REH), by a grant from Stellar Pharmaceuticals, Ltd. (REH), a research contract from Watson Pharmaceuticals (REH) and by the Department of Urology.
References
- 1.Hanno P, Nordling J, van OA. What is new in bladder pain syndrome/interstitial cystitis? Curr Opin Urol. 2008;18:353. doi: 10.1097/MOU.0b013e3282fcea88. [DOI] [PubMed] [Google Scholar]
- 2.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal Expression of Molecular Markers for Bladder Impermeability and Differentiation in Urothelium of Interstitial Cystitis Patients. J Urol. 2004;171:1554. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 3.Chai TC, Keay S. New theories in interstitial cystitis. Nat Clin Pract Urol. 2004;1:85. doi: 10.1038/ncpuro0057. [DOI] [PubMed] [Google Scholar]
- 4.Hauser PJ, Dozmorov MG, Bane BL, Slobodov G, Culkin DJ, Hurst RE. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol. 2008;179:764. doi: 10.1016/j.juro.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 6.Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology. 2007;69:17. doi: 10.1016/j.urology.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 7.Erickson DR, Herb N, Ordille S, Harmon N, Bhavanandan VP. A new direct test of bladder permeability. J Urol. 2000;164:419. [PubMed] [Google Scholar]
- 8.Buffington CA, Woodworth BE. Excretion of fluorescein in the urine of women with interstitial cystitis. J Urol. 1997;158:786. doi: 10.1097/00005392-199709000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Tzan CJ, Berg J, Lewis SA. Effect of protamine sulfate on the permeability properties of the mammalian urinary bladder. J Membr Biol. 1993;133:227. doi: 10.1007/BF00232022. [DOI] [PubMed] [Google Scholar]
- 10.Parsons CL. Epithelial coating techniques in the treatment of interstitial cystitis. Urology. 1997;49:100. doi: 10.1016/s0090-4295(97)00180-5. [DOI] [PubMed] [Google Scholar]
- 11.Nickel JC, Ergerdie B, Downey J, Singh R, Skekhan A, Carr L, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int. 2009;103:56. doi: 10.1111/j.1464-410X.2008.08028.x. [DOI] [PubMed] [Google Scholar]
- 12.Brecher AS, Adamu MT. Coagulation protein function: enhancement of the anticoagulant effect of acetaldehyde by sulfated glycosaminoglycans. Dig Dis Sci. 2001;46:2033. doi: 10.1023/a:1010668005729. [DOI] [PubMed] [Google Scholar]
- 13.Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 14.Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- 15.McKay NG, Kirby RW, Lawson K. Rubidium efflux as a tool for the pharmacological characterisation of compounds with BK channel opening properties. Methods Mol Biol. 2008;491:267. doi: 10.1007/978-1-59745-526-8_21. [DOI] [PubMed] [Google Scholar]
- 16.Raja RH, LeBoeuf RD, Stone GW, Weigel PH. Preparation of alkylamine and 125I-radiolabeled derivatives of hyaluronic acid uniquely modified at the reducing end. Anal Biochem. 1984;139:168. doi: 10.1016/0003-2697(84)90402-0. [DOI] [PubMed] [Google Scholar]
- 17.Kyker KD, Coffman J, Hurst RE. Exogenous glycosaminoglycans coat damaged bladder surfaces in experimentally damaged mouse bladder. BMC Urol. 2005;5:4. doi: 10.1186/1471-2490-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurst RE, Rhodes SW, Adamson PB, Parsons CL, Roy JB. Functional and structural characteristics of the glycosaminoglycans of the bladder luminal surface. J Urol. 1987;138:433. doi: 10.1016/s0022-5347(17)43180-6. [DOI] [PubMed] [Google Scholar]
- 19.Steinhoff G, Ittah B, Rowan S. The efficacy of chondroitin sulfate 0.2% in treating interstitial cystitis. Can J Urol. 2002;9:1454. [PubMed] [Google Scholar]
- 20.Huygens A, Crnolatac I, Maes J, Van CB, Van PH, Roskams T, et al. Influence of the glycosaminoglycan layer on the permeation of hypericin in rat bladders in vivo. BJU Int. 2007;100:1176. doi: 10.1111/j.1464-410X.2007.07167.x. [DOI] [PubMed] [Google Scholar]
- 21.Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:F1200. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 22.Parsons CL, Boychuk D, Jones S, Hurst RE, Callahan H. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol. 1990;143:139. doi: 10.1016/s0022-5347(17)39897-x. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran M, Stein P, Parsons CL. Toxic factors in human urine that injure urothelium. Int J Urol. 2006;13:409. doi: 10.1111/j.1442-2042.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Chen M, Lowentritt BH, Van Zijl PS, Koch KR, Keay S, et al. EGF and HB-EGF modulate inward potassium current in human bladder urothelial cells from normal and interstitial cystitis patients. Am J Physiol Cell Physiol. 2007;292:C106. doi: 10.1152/ajpcell.00209.2006. [DOI] [PubMed] [Google Scholar]
- 25.Parsons CL. Interstitial cystitis and lower urinary tract symptoms in males and females-the combined role of potassium and epithelial dysfunction. Rev Urol. 2002;4(Suppl 1):S49. [PMC free article] [PubMed] [Google Scholar]