Summary
Multiple physiological and pathological conditions interfere with the function of Endoplasmic Reticulum (ER). However, much remains unknown regarding the impact of ER stress on inflammatory responses in dendritic cells (DCs) upon the recognition of pathogen molecules. We show that ER stress greatly potentiates the expression of inflammatory cytokines and IFN-β in murine DCs stimulated by polyIC, a synthetic mimic of virus dsRNA. Both toll-like receptor 3 and melanoma differentiation-associated gene-5 are involved in the enhanced IFN-β production, which is associated with increased activation of NF-κB and IRF3 signaling as well as the splicing of X-box binding protein-1 (XBP-1), an important regulator involved in ER stress response. Surprisingly, silencing of XBP-1 reduces polyIC-stimulated IFN-β expression in the presence or absence of ER stress, indicating that XBP-1 may be essential for polyIC signaling and ER stress-amplified IFN-β production. Overexpression of a spliced form of XBP-1(XBP-1s) synergistically augments polyIC-induced inflammatory response. For the first time we show that XBP-1s overexpression-enhanced IFN-β production in DCs markedly suppresses vesicular stomatitis virus infection, revealing a previously unrecognized role of XBP-1 in an antiviral response. Our findings suggest that evolutionarily conserved ER stress response and XBP-1 may function collaboratively with the innate immunity in maintaining cellular homeostasis.
Keywords: endoplasmic reticulum, X-box binding protein-1, polyIC, dendritic cells, inflammatory response, VSV
1. Introduction
The endoplasmic reticulum (ER) plays crucial roles in multiple cellular processes, including intracellular calcium homeostasis, protein secretion and lipid biosynthesis, which are required for cell survival and normal cellular functions [1]. Different stress stimuli, e.g., hypoxia, viral replication, abnormal proteins and starvation, all ultimately lead to accumulation of unfolded or misfolded proteins in the lumen of the ER, and therefore triggering ER stress [2–4]. The ER in higher eukaryotic cells responds to the accumulation of unfolded proteins by activating intracellular signal transduction pathways, collectively called the unfolded protein response (UPR) [5, 6]. The coordinated UPR acts through three main signaling cascades: the inositol-requiring transmembrane kinase/endonuclease 1 (IRE1) pathway generating a splice variant of transcription factor X box binding protein-1 (XBP-1), which activates genes involved in protein degradation and controls the transcription of chaperones[7, 8], the pancreatic ER kinase (PERK) pathway leading to attenuation of cap-dependent translation [9]; and the activating transcription factor 6 (ATF6) pathway [7, 8]. It is known that the ER stress response or UPR is a highly conserved adaptive response and involved in cell survival or death [10]. However, it remains less studied as to how ER stress may influence or alter cellular activities and functions in innate and adaptive immunity. Recent studies showed that ER stress and UPR are linked to synergistic IFN-β production in macrophages [11].
Dendritic cells (DCs), are sentinels distributed around the body that sense pathogens or ‘dangers’ through highly conserved different classes of pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), RIG-I like receptors and NOD-like receptors. PRRs recognize and respond to evolutionarily conserved pathogen-associated molecular patterns (PAMPs) and therefore play a critical role in directing the immune system to initiate appropriate and effective responses against invading pathogens [12, 13]. Activation of these innate PPRs triggers multiple intracellular signaling cascades and expression of a variety of overlapping and unique genes involved in the inflammatory and immune responses [14]. Given the essential roles of DCs in the host immune defense following microbial encounter, it is important to understand the potential impact of the ER stress conditions on DC activities in response to the stimulation of microbial products.
In this study we assess the effect of ER stress on the inflammatory response in DCs upon stimulation with polyriboinosinic: polyribocytidylic acid (polyIC), a synthetic analog of double-stranded RNA. We show that DCs under ER stress conditions respond to polyIC stimulation much more robustly than non-stressed counterparts, as indicated by increased production of IFN-β and other inflammatory cytokines, which depend on both TLR-3 and melanoma differentiation associated protein-5 (MDA-5). Increased production of IFN-β is accompanied by the splicing of XBP-1, a UPR regulator, as well as the activation of NF-κB and IRF3, which are known to be involved in the transcriptional control of IFN-β gene expression. By overexpressing the active form of XBP-1 and knocking down XBP-1, we demonstrate that XBP-1 acts as a positive regulator of polyIC signaling and ER stress-enhanced IFN-β expression in polyIC-stimulated DCs. Furthermore, we provide the first evidence that XBP-1 overexpression significantly enhances DC-mediated antiviral response via increased IFN-β production, emphasizing a critical role that XBP-1 plays in innate immunity against pathogens.
2. Results
Enhanced production of IFN-β in polyIC-stimulated DCs undergoing ER stress
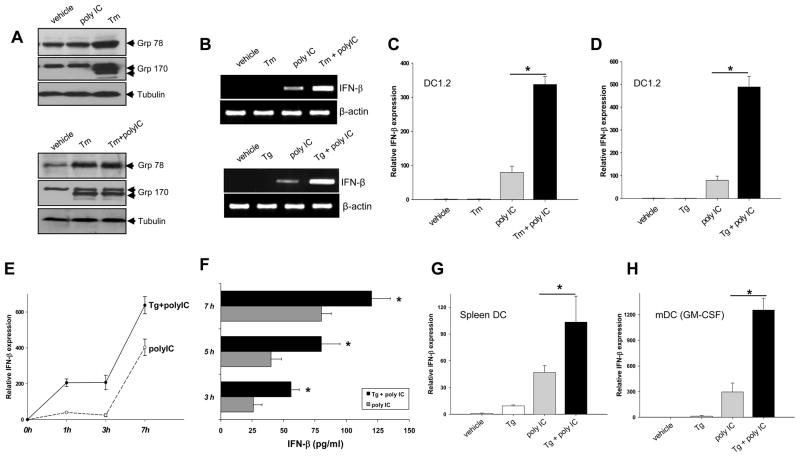
We first assessed the effect of polyIC and ER stressor tunicamycin (Tm, an inhibitor of protein N-glycosylation) on the induction of ER-resident chaperones, grp78 and grp170, an indicator of the ER stress response or UPR. While Tm treatment resulted in a robust induction of grp78 and grp170 in DCs, polyIC did not appear to influence the protein levels of these two chaperone molecules (Fig. 1A, top). In addition, polyIC stimulation did not show an appreciable effect on the Tm-induced expression of chaperone proteins (Fig. 1A, bottom). Similar results were obtained when cells were treated with thapsigargin (Tg), an inhibitor of Ca2+-ATPase, which disrupts Ca2+homeostasis and induces ER stress (data not shown).
Figure 1. ER stress enhances polyIC stimulated IFN-β production in DCs.
A. Induction of ER chaperones by pharmacological activation of ER stress. DC1.2 cells were treated with polyIC, Tm or polyIC plus Tm, and subjected to immunoblotting using antibodies as indicated. B–D. Enhanced IFN-β production by ER stressors and polyIC. Cells were pretreated with Tm for 5 h, and stimulated with polyIC for another 3h. Cells were harvested for PCR (B, top) and qRT-PCR (C) analyses of IFN-β transcription. Similar studies were performed in cells pretreated with Tg for 1 h, followed by PCR (B. bottom) and qRT-PCR (D) analyses (Student t-test, *p<0.001). E and F. Kinetic IFN-β gene expression in DCs treated with Tg and polyIC. Cells were pretreated with Tg, and then stimulated with polyIC for the times indicated. mRNAs were prepared for qRT-PCR analysis (E). Cell culture supernatants were collected for ELISA to measure IFN-β protein levels (F). Student t-test, *p<0.005, polyIC plus Tg versus poly IC alone. G and H. ER stressor-enhanced IFN-β expression in polyIC-stimulated primary DCs. Spleen DCs (G) or bone marrow-derived DCs (H) were treated with Tg and polyIC. IFN-β mRNA levels were measured by qRT-PCR (Student t-test, *p<0.001). The IFN-β expression was normalized to GAPDH expression. The results are presented as means+SD for average fold induction and are representative of three independent experiments.
Given that polyIC primarily results in the production of type I IFNs, we sought to assess the effect of ER stress on polyIC-induced IFN-β expression. Treatment of DCs with either Tm or Tg had a negligible effect on the basal levels of IFN-β, but markedly enhanced IFN-β mRNA levels following polyIC stimulation (Fig. 1B). The synergistic effects of ER stressors and polyIC were confirmed by quantitative real-time PCR (qRT-PCR) (Fig. 1C and D). Kinetic study showed that ER stress-mediated effect appeared as early as 1 h after polyIC stimulation and could still be seen at 7 h (Fig. 1E). The increased IFN-β gene transcription was accompanied by elevated IFN-β protein levels in the culture supernatants (Fig. 1F). The ER stress-potentiated IFN-β expression was similarly seen in freshly isolated spleen DCs (Fig. 1G) and myeloid DCs (mDCs) -derived from bone marrow cells in the presence of GM-CSF( Fig. 1H).
ER stress enhanced IFN-β expression involves both TLR-3 and MDA-5
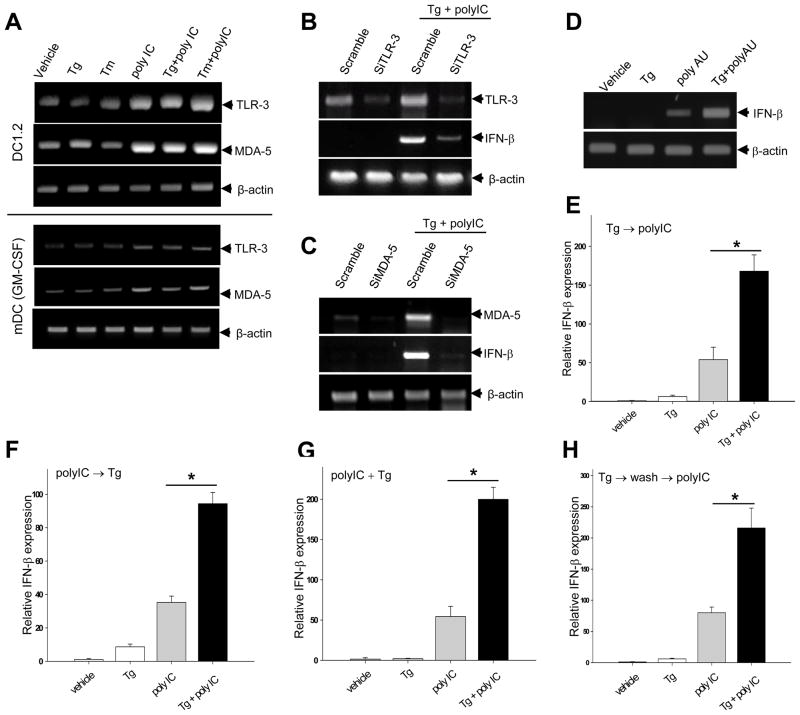
Given that both TLR-3 and MDA-5 have been shown to participate in the polyIC induced inflammatory response [15, 16], we examined the potential role of these two PRRs in the synergistic action of ER stress and polyIC. While polyIC increased TLR-3 and MDA-5 mRNA levels in both DC1.2 cell line and BM-derived mDCs, Tm or Tg-induced ER stress had a negligible effect (Fig. 2A). However, silencing of either TLR-3 (Fig. 2B) or MDA-5 (Fig. 2C) gene using siRNA resulted in diminished IFN-β mRNA expression compared to scramble controls, suggesting that both TLR-3 and MDA-5 are involved in the enhanced IFN-β upregulation. In addition, ER stress increased IFN-β gene activation in a similar fashion when DCs were concurrently treated with polyadenylic-polyuridylic acid (polyAU) (Fig. 2D), which displays specificity for TLR-3 [17].
Figure 2. Both TLR-3 and MDA-5 are involved in ER stress-enhanced IFN-β expression.
A. ER stress does not alter TLR-3 and MDA-5 gene expression. DC1.2 cells (top) or BMDCs (bottom) were treated with ER stressors alone or in combination with polyIC. TLR-3 and MDA-5 mRNA levels were examined. B and C. TLR-3 or MDA-5 silencing results in reduced IFN-β expression. DC1.2 cells were treated with scramble siRNA, TLR3 siRNA (B), or MDA-5 siRNA (C), followed by stimulation with Tg plus polyIC. IFN-β transcription was examined by PCR analysis. D. DC1.2 cells were treated with Tg alone or in combination with polyAU, and were subjected to PCR analysis. E–H. Effect of treatment schedules on the ER stress-enhanced IFN-β expression in polyIC-stimulated DC1.2 cells. E. Cells were pretreated with Tg for 1 h, followed by polyIC stimulation for an additional 2 h. F. Cells were stimulated with polyIC for 2h, followed by incubation in the presence of Tg for an additional 1 h. G. Cells were cultured in the presence of both polyIC and Tg for 3 h. H. Cells were incubated with Tg for 3h and washed extensively, followed by stimulation with or without polyIC for an additional 3h. Cells were subjected to qRT-PCR analyses (Student t-test, *p<0.001). The results are presented as means+SD and are representative of at least two independent experiments.
We next examined whether the timing of ER stress induction influenced the polyIC-stimulated IFN-β expression. DCs were treated with Tg and polyIC sequentially, e.g., first treated with either Tg (Fig. 2E) or polyIC (Fig. 2F), followed by incubation in the presence of polyIC or Tg, respectively. Alternatively, Tg and polyIC were simultaneously added to the DC culture (Fig. 2G). The synergistic activation of IFN-β gene transcription was similarly shown in all these treatment protocols. Interestingly, ER stressor-enhanced effect still remained when DCs were first primed with Tg, washed extensively and then stimulated with polyIC (Fig. 2H).
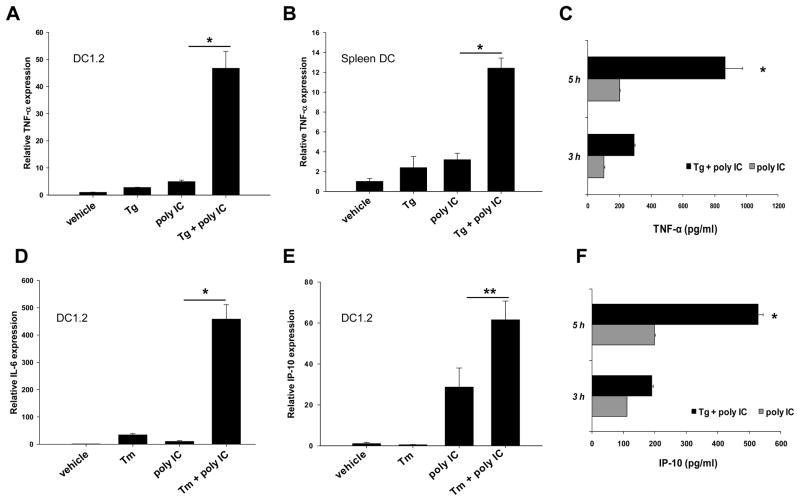
In addition to IFN-β, we assessed the effects of ER stress on inflammatory cytokines induced by polyIC. Tg-triggered ER stress enhanced TNF-α expression in both DC1.2 cell line (Fig. 3A) and spleen DCs (Fig. 3B and C), as indicated by qRT-PCR and ELISA assays. The polyIC-induced expression of IL-6 (Fig. 3D) and IP-10 (Fig. 3E and F) was also enhanced in both mRNA and protein levels upon ER stress. Preincubation with polymyxin B didn’t influence the potentiating effect of Tg (data not shown), suggesting that the observed synergy was not due to a trace of endotoxins within the ER stress inducers.
Figure 3. DCs under ER stress produce more inflammatory cytokines in response to polyIC stimulation.
Tg-pretreated DC1.2 cells (A) or spleen DCs (B) were stimulated with polyIC for 3 h, and harvested for qRT-PCR analysis of TNF-α. Culture supernatants of spleen DCs were collected at the different time points after stimulation and assayed for TNF-α production by ELISA (C). DC1.2 cells were treated with Tm, followed by polyIC stimulation for an additional 3h. Cells were subjected to qRT-PCR analysis of IL-6 (D) or IP-10 (E). Culture supernatants were assayed for the protein levels of IP-10 using ELISA (F). Student t-test, *p<0.001; **p<0.005. The results are shown as means+SD and are from three independent experiments.
Effect of ER stress on the inflammatory response engaged by other TLR ligands
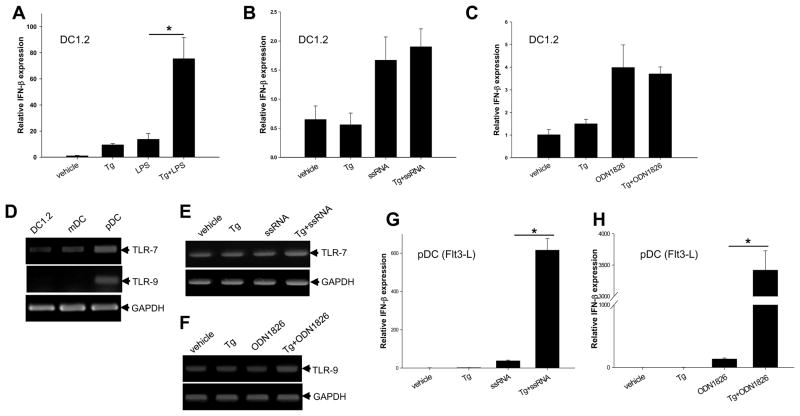
In addition to polyIC, we also assessed the impact of ER stress on inflammatory responses stimulated by other PAMP molecules. Tg treatment of DC1.2 cells strongly enhanced the transcriptional activation of IFN-β gene in response to stimulation with LPS (Fig. 4A), but not with ssRNA (Fig. 4B), a TLR7 agonist, or ODN 1826 (Fig. 4C), a TLR9 agonist. It was also seen that the magnitude of IFN-β activation was much lower in ssRNA and ODN1826-treated DC1.2 cells. We therefore asked the question whether the differential effects of ER stress on the cellular responses to TLR-7 and TLR-9 activation might be attributed to the distribution of these receptors in DCs from different sources. Indeed, plasmacytoid DCs (pDCs) from Flt3 ligand-differentiated BM cells displayed higher levels of TLR-7 and TLR-9 compared to DC1.2 cells or BMDCs (Fig. 4D), which is consistent with previous observation [18]. Similarly, ER stress did not appear to affect the gene transcription of TLR-7 (Fig. 4E) and TLR-9 (Fig. 4F) with or without PAMP stimulation. As expected, Tg-induced ER stress profoundly enhanced IFN-β mRNA levels in pDC treated with ssRNA (Fig. 4G) or ODN1826 (Fig. 4H).
Figure 4. Effects of ER stress on IFN-β expression induced by different TLR ligands.
A–C. ER stress does not enhance IFN-β expression in ssRNA and CpG-ODN-stimulated DC1.2 cells. DC1.2 cells were pretreated with Tg and stimulated with LPS (A), ssRNA (B) or CpG-ODN1826 (C) for an additional 3h. Cells were harvested for qRT-PCR analysis of IFN-β mRNA levels (Student t-test, *p<0.001). D. Plasmacytoid DCs express higher levels of TLR-7 and TLR-9. DC1.2 cells, BMDCs and pDC were assessed for the mRNA levels of TLR-7 and TLR-9. E and F. ER stress does not influence the gene expression of TLR-7 and TLR-9. pDCs were treated with Tg, ssRNA or Tg plus ssRNA (E). Alternatively, pDCs were treated with Tg, ODN1826 or Tg plus ODN1826 (F). mRNA levels of TLR-7 and TLR-9 were examined. G and H. pDCs are highly responsive to the stimulation with ER stressor in combination with TLR-7 or TLR-9 agonist. pDCs were preincubated with Tg for 1 h and then stimulated with 0.5μg/ml ssRNA or 5μg/ml ODN1826 for 3 h. Cells were subjected to qRT-PCR analysis of IFN-β levels (Student t-test, *p<0.001). The results are presented as means+ SD and are representative of three independent experiments
ER stress enhances the activation of signaling pathways controlling IFN-β
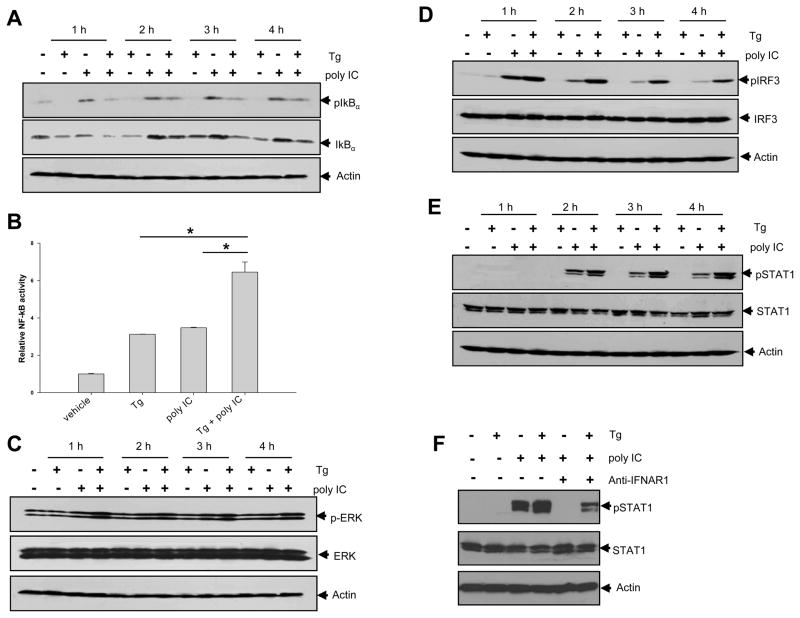
Rapid induction of IFN-β following virus infection involves a cooperative action of transcription factors, including NF-κB, (ATF2)/c-Jun (controlled by MAP kinases) and Interferon Regulatory Factors (IRFs) [19, 20]. We showed that Tg treatment greatly enhanced polyIC-induced IκBα degradation at 1h time point, which was independent of the phosphorylation of IκBα (Fig. 5A). Luciferase reporter assays using DC1.2 cells confirmed that Tg treatment was able to induce and enhance the activation of NF-κB (Fig. 5B). Tg treatment also modestly increased ERK1 phosphorylation (Fig. 5C). Interestingly, IRF3 phosphorylation was profoundly enhanced following Tg treatment (Fig. 5D), suggesting that ER stress modulated the MAPK and IRF3 signaling pathways. However, Tg treatment did not alter the polyIC-induced phosphorylation of P38 and JNK (data not shown). Interestingly, the phosphorylation of Signal Transducer and Activator of Transcription 1 (STAT1) was also substantially increased in DCs treated with Tg plus polyIC (Fig. 5E). The levels of phopho-STAT1 was greatly reduced when blocking antibodies for the interferon-α/β receptor1 (IFNAR1) were used (Fig. 5F), indicating that the produced IFN-β may be able to bind to IFNAR and engage the STAT1 signaling in an autocrine or paracrine fashion [21].
Figure 5. Effects of ER stress on polyIC-induced signaling involved in IFN-β expression.
A. DC1.2 cells were treated with Tg, polyIC or Tg plus polyIC, and were subjected to immunoblotting of phospho-IκBα and IκBα. B. Cells were transfected with pGL4-NF-κB-luciferase plasmid, followed by treatment with Tg and polyIC. Cells were lysed and assayed for NF-κB luciferase activity (Student t-test, *p<0.005). In addition, treated cells were subjected to immunoblotting of phospho-ERK (C), phospho-IRF3 (D), and phospho-STAT1 (E). The results are representative of three independent experiments. F. Cells were preincubated with 10μg/ml anti-IFNAR1 antibodies (+) or control anti-mouse IgG (−) for 1 h, and stimulated with polyIC. Immunoblotting was performed to assess the levels of phospho-STAT1. The results are representative of two independent experiments.
XBP-1 contributes to ER stress amplified IFN -β production upon polyIC stimulation
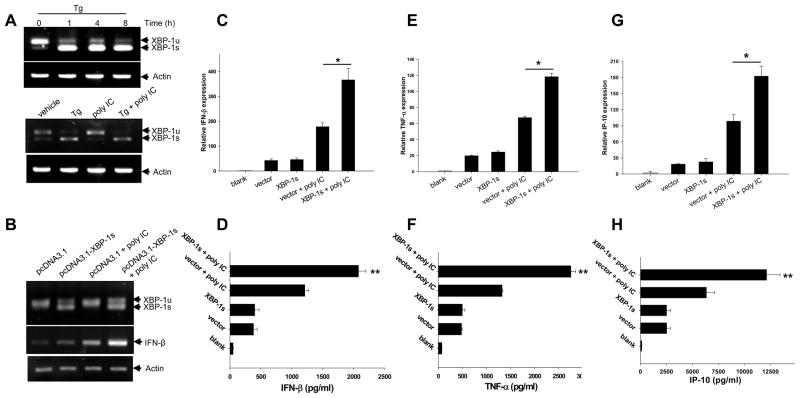
Given the important roles of XBP-1 in ER stress-induced cellular signaling pathways and UPR, we examined the potential involvement of XBP-1 in ER stress promoted inflammatory response. A modest but constitutive XBP-1 splicing was observed in un-stimulated DC1.2 cells (Fig. 6A, top) and freshly isolated spleen DCs, but not in BM-derived mDCs (data not shown). Tg stimulation resulted in a time -dependent increase of the spliced form of XBP -1(XBP-1s) (Fig. 6A, top). Treatment of DCs with polyIC did not appear to affect the Tg-triggered XBP-1 splicing (Fig. 6A, bottom). Enforced expression of XBP-1s was associated with the elevation of IFN-β mRNA levels upon poly IC stimulation, as indicated by PCR assays (Fig. 6B). Subsequent qRT-PCR analyses showed that overexpression of XBP-1s greatly enhanced the transcriptional activation of IFN-β (Fig. 6C), TNF-α (Fig. 6E) and IP-10 (Fig. 6G). XBP-1s overexpression-promoted inflammatory response was subsequently validated by ELISA ( Fig. 6D, F and H).
Figure 6. XBP-1s overexpression synergizes with polyIC to induce inflammatory cytokines.
A. XBP-1 splicing in Tg-treated DCs. DC 1.2 cells were treated with Tg for the times indicated and analyzed for XBP-1 splicing using PCR (top). DCs were treated with Tg, poly IC or Tg plus polyIC, followed by PCR analysis of XBP-1 splicing (bottom). B. Cells were transfected with pcDNA3.1 or pcDNA3.1-XBP-1s, followed by poly IC stimulation for 3h. Cells were assayed for XBP-1 splicing and IFN-β expression using PCR. C–H. Cells were transfected with XBP-1s plasmid or empty vector, and stimulated with polyIC for 3h. Cells were examined for mRNA levels for IFN-β (C), TNF-α (E) and IP-10 (G) using qRT-PCR. Culture supernatants were collected 24h after treatment and assayed for protein levels of IFN-β (D), TNF-α (F) and IP-10 (H). The results are presented as means+SD and are representative of three independent experiments (Student t-test, *p<0.005, **p<0.005).
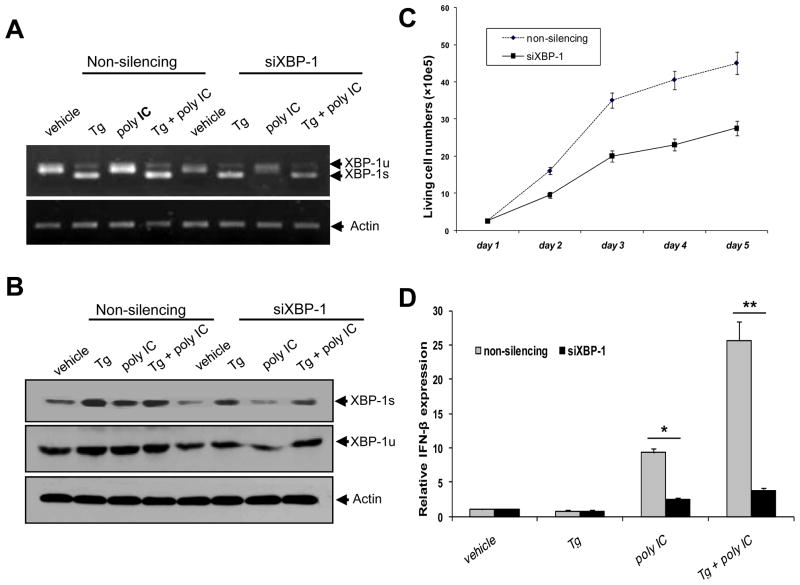
To examine whether loss of XBP-1 impaired ER stress-mediated effect on polyIC-stimulated IFN-β expression, XBP-1 in DCs was silenced by siRNA for XBP-1. The efficiency of XBP-1 knockdown was confirmed using RT-PCR (Fig. 7A) and immunoblotting (Fig. 7B) analyses. Down-regulation of XBP-1 significantly inhibited the growth of DC1.2 cells (Fig. 7C), suggesting that XBP-1 may act as a pro-survival factor in DCs as reported previously [22]. It was evident that XBP-1 knockdown abrogated ER stress-enhanced IFN-β expression in polyIC stimulated DCs (Fig. 7D), suggesting that XBP-1, at least in part, coupled ER stress to IFN-β production in the context of polyIC stimulation. Intriguingly, XBP-1 silencing also greatly reduced polyIC-induced IFN-β expression, implicating the dual roles of XBP-1 in the ER stress response and pathogen recognition.
Figure 7. Silencing XBP-1 abolishes ER stress-enhanced IFN-β gene activation by polyIC.
DC1.2 cells were transfected with control siRNA or XBP-1 siRNA. Knock-down efficiency was confirmed by PCR (A) and immunoblotting (B). C. XBP-1 silence inhibits DC1.2 cell growth. Control or XBP-1 siRNA transduced DCs were followed by counting the viable cells at different time points. D. Following XBP-1 silencing, cells were treated with polyIC, or Tg plus polyIC, followed by qRT-PCR analysis of IFN-β (Student t-test, *p<0.005; ** p<0.001). The results are representative of three independent experiments.
XBP-1s overexpression enhances DC-mediated antiviral response
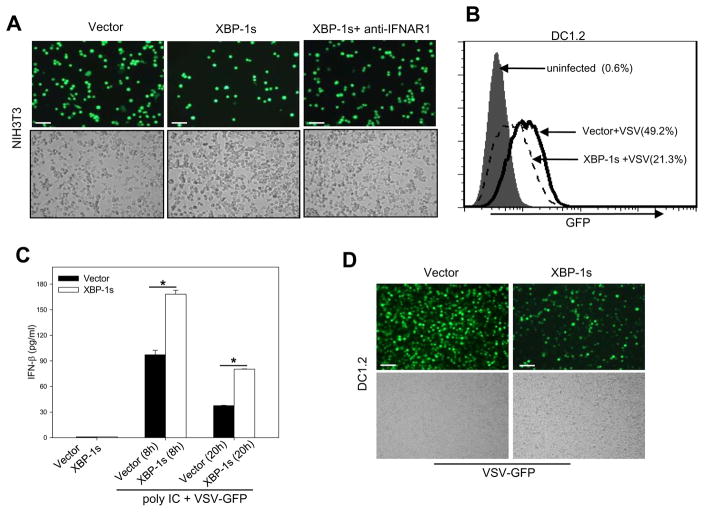
Given that XBP-1 is important for polyIC-induced IFN-β production, which is known to be involved in virus suppression, we next performed a biological assay to confirm its role in an antiviral response. DC1.2 cells were transfected with XBP-1s construct or control vector and then treated with polyIC to induce IFN-β biosynthesis. The conditioned media were added to NIH-3T3 cells prior to the infection with recombinant VSV harboring a GFP reporter gene (VSV-GFP). The percentage of GFP-positive cells postinfection was decreased profoundly in the presence of the media from XBP-1s overexpressing DCs compared with control vector transduced cells (Fig. 8A). In addition, biofunctional grade IFNAR1 antibodies were used for neutralization of IFNAR-1 bioactivity. IFNAR1 blockade abrogated XBP-1 facilitated antiviral response( Fig. 8A), suggesting the involvement of type-I IFNs in the antiviral activity.
Figure 8. XBP-1s overexpression in DCs promotes an antiviral response dependent on type I IFN.
A. XBP-1s-enhanced type I IFN production protects cells from viral infection. NIH3T3 cells were preincubated with conditioned media derived from polyIC-stimulated DC1.2 cells transfected with XBP-1s or empty vector. The NIH3T3 cells were then infected with VSV-GFP viruses and expression of GFP were examined using a fluorescence microscopy. Anti-IFNAR1 antibodies were also used to assess the involvement of type I IFNs. B. Reduced viral infectivity in polyIC-stimulated XBP-1s-overexpressing DCs. DC1.2 cells transduced with XBP-1s or empty vector were stimulated with polyIC, followed by infection with VSV-GFP. The percentage of GFP-expressing cells was measured by flow cytometry analysis. C. Antiviral activity correlates with increased IFN-β levels in media derived from XBP-1s-overexpressing DCs. Culture supernatants were collected at the times indicated after virus infection and IFN-β levels were measured by ELISA (Student t-test, *p<0.001). D. Resistance of XBP-1s-overexpressing DCs to direct VSV infection. DC1.2 cells transduced with XBP-1s or empty vector were infected with VSV-GFP at an MOI of 10 in serum-free medium. 20 h later, cells were examined using a fluorescence microscope. The results are representative of two independent experiments. For microscopy results, cells were photographed using a Nikon fluorescence microscope with a 10× objective lenses and a SPOT digital camera (Bars, 200nm).
A greatly reduced viral infectivity was also observed in polyIC-stimulated XBP-1s-overexpressing DCs compared to vector-transduced cells upon direct infection with VSV-GFP viruses (Fig. 8B), which also confirmed the increased antiviral bioactivity of conditioned media from XBP-1 transduced DCs as shown in Figure 7A. The GFP-positive DCs decreased from 49.2% to 21.3%, as measured by flow cytometry analysis (Fig. 8B). As expected, culture media derived from VSV-GFP infected XBP-1s-expressing DCs displayed higher levels of IFN-β than control cells (Fig. 8C). Lastly, DC1.2 cells transfected with XBP-1s construct or control vector were infected with recombinant VSV-GFP in the absence of polyIC stimulation. The overall percentage of GFP-positive cells postinfection were decreased significantly in XBP-1s overexpressing DC1.2 cells compared with control vector transduced cells (Fig. 8D).
3. Discussion
DCs play a crucial role in sensing pathogens through PPRs and initiation of innate as well as adaptive immune responses. In the present study, we have investigated the impact of ER stress on the inflammatory response engaged by polyIC, a mimic of virus dsRNA. Interestingly, activation of an ER-stress response markedly potentiates DC-mediated innate immune response to microbial products, implicating an inter-connection and coordination of these two evolutionarily conserved cellular responses. Furthermore, our studies establish an important role of XBP-1 in dsRNA signaling and innate antiviral immunity.
The current study shows that signals produced by ER stressed DCs are able to modulate microbial molecule-induced inflammatory responses. Treatment of DCs (e.g., a DC line as well as primary DCs) with Tm and Tg greatly enhanced polyIC-induced expression of IFN-β and inflammatory cytokines, which agrees with the recent report from Colbert’s group [11]. It appears that, in addition to sensing and responding to disturbances (e.g., misfolded proteins) in the ER, the UPR may directly or indirectly engage PPR-initiated signaling cascades. ER stress-induced UPR response represents an adaptive pro-survival cellular mechanism. Thus, the collaborative and synergistic actions of the ER stress response and PPR-augmented innate immune response may facilitate the clearance of pathogens and maintenance of homeostasis.
IFN-β is transcriptionally regulated by three distinct families of transcription factors, NF-κB, (ATF2)/c-Jun and IRF3 [19, 20]. Although Tg-induced ER stress promoted polyIC-induced NF-κB activity, it did not appear to work through canonical signaling pathways (i.e., phosphorylation-induced degradation of IκBα). Our data are in line with a previous study showing that ER stress activated the NF-κB pathway through the PERK-eIF2α-mediated translational repression [23]. ER stress-associated NF-κB activation has also been reported to result from the oxidative stress of excessive protein folding and/or from an ER-stress-mediated leakage of calcium into the cytosol [24]. Interestingly, we show for the first time that ER stress substantially enhances polyIC-induced IRF3 activation, even though Tg alone had no apparent effect on IRF3 phosphorylation. While NF-κB and MAPKs are activated in response to numerous stimuli, including inflammation, IRFs are mainly activated during virus infection, and known to determine the specificity of type I IFN induction [19]. Indeed, IRF3 is critically involved in the initial induction of IFN-β upon cell infection by viruses [25], and is activated through phosphorylation by the noncanonical IκB kinases [26, 27].
The innate immune system has developed two pathways for the recognition of dsRNA, which include TLR-3 and RIG-I–like helicases (RLHs), e.g., retinoic acid–inducible gene I (RIG-I) and MDA-5 [14]. PolyIC can be recognized predominantly by endosomally localized TLR-3 when directly added to the culture medium [28] or mainly by cytosolic MDA-5 when transfected using cationic liposome [16, 29]. However, gene silencing studies demonstrates that ER stress-promoted IFN-β expression involves both TLR-3 and MDA -5, although ER stress does not alter the expression levels of these two PRRs in DCs. In addition, ER stress-enhanced IFN-β expression was similarly observed in pDCs stimulated with ssRNA or CpG-ODN, implicating a general phenomenon of synergistic activities of ER stress response and the innate immunity initiated by the recognition of PAMPs. Given the differential expression of TLRs in DC populations, it is conceivable that DC subsets under stress conditions display distinct cellular responses when engaged by different microbial molecules and the net effect of these various factors will determine the consequent innate immunity.
In our studies, pharmacologic induction of ER stress results in a spliced and active form of XBP-1(XBP-1s) in DCs, probably due to the endoribonuclease activity of IRE1 that causes the excising of an intron from the mRNA of transcription factor XBP-1 as previously described [7, 30]. Interestingly, overexpression of XBP-1s greatly enhanced polyIC-augmented innate immune response. That enforced expression of XBP-1s in DCs recapitulated the effects of Tg- or Tm-induced ER stress on polyIC-triggered inflammatory response suggests that XBP-1 may play a role in bridging ER stress and the potentiated polyIC signaling in DCs and macrophages [11, 31].
Using a gene silencing approach, we have confirmed the involvement of XBP-1 in Tg and polyIC-induced synergistic activation of IFN-β. Unexpectedly, XBP-1 knockdown also abrogated polyIC-induced signaling and IFN-β expression in the absence of ER stress, suggesting that XBP-1 and an intact UPR response not only are involved in ER stress-amplified inflammatory response, but also is important for the polyIC-induced innate immunity. In support of our hypothesis, we have provided the first evidence that XBP-1s overexpression markedly enhances DC-mediated antiviral activities through increased production of IFN-β and possibly other inflammatory factors. Our result supports a recent finding by Glimcher’s group that the XBP-1 branch of UPR acts as a positive regulator of TLR2/4 responses in macrophages [32]. While TLR2 and TLR4 agonists (i.e., Pam3CSK4 or LPS) were able to trigger the activation of IRE1α and XBP-1 in the absence of an ER-stress response, polyIC treatment did not cause apparent cytosolic splicing of XBP-1 in our studies, suggesting dsRNA-sensing signaling may differ from LPS-sensing TLR2/4 signaling pathways in regulating XBP-1 activation and ER stress responses. It is also possible that the levels of spliced XBP-1 were too low to be detected in polyIC-treated DCs. Nonetheless, our findings clearly indicate that XBP-1 participates in the regulation of ER stress response or UPR as well as dsRNA signaling and the host response against pathogens.
In the present study, we also show for the first time that ER stress synergizes with the activation of TLR-7 and TLR-9 in pDCs. It is conceivable that the complex interaction of pathogen (e.g., VSV) with DCs may involve coordination of multiple innate PRRs and consequent downstream signaling cascades [33–35]. Therefore, our results suggest that synergistic actions of ER stress/XBP-1 and PAMP-triggered innate immunity are not exclusively restricted to TLR-3 or MDA-5. Indeed, overexpression of XBP-1s profoundly reduces the infectivity of VSV in DC1.2 cells even in the absence of polyIC treatment. Although receptors involved in the sensing of VSV remain to be defined, we have provided convincing evidence supporting the notion that XBP-1s-promoted production of IFN-β by DCs in response to PAMP stimulation is able to effectively limit virus infection. The molecular mechanisms underlying the actions of XBP-1 and ER stress in the context of PAMP stimulation is under investigation.
In summary, our studies demonstrate that ER stress and its key regulator XBP-1 promote the polyIC-induced inflammatory response in DCs, supporting the idea that these two evolutionarily conserved cellular responses are interconnected and may act together to maximize innate immunity against foreign pathogens. Given the complex and cooperative nature of the ER-stress response as well as the important role of XBP-1 in the immune system, future studies will need to define the molecular links between ER stress and/or XBP-1 activation and PAMP-initiated innate response as well as the physiological significance of this connection. Improved understanding of the inter-connection of these cellular machineries or networks may lead to development of novel strategies that may be used to our benefits in the setting of pathogen infections or inflammatory diseases.
4. Materials and Methods
Animals and Cells
DC1.2 cells, a murine dendritic cell line [36] were kindly provided by Dr. Kenneth Rock (University of Massachusetts Medical Center). Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics at 37°C in a humidified incubator containing 5% CO2. Hamster kidney cells (BHK-21; American Type Culture Collection) and NIH-3T3 cells were maintained in DMEM containing 10% FBS. Bone marrow (BM)-derived mDCs were prepared in the presence of GM-CSF as described previously [37]. In brief, BM cells at 106 cells/ml were plated placed in 12-well plates in 2 mL medium with 10 ng/mL GM-CSF. At days 2 and 4, supernatant containing nonadherent cells were removed, the wells were washed gently, and fresh medium containing GM-CSF was added. At day 6, nonadherent cells were collected, centrifuged, resuspended in fresh medium with GM-CSF, and cultured for an additional 24 h in Petri dishes. BM-derived pDCs were generated by culturing BM cells in culture medium supplemented with 100 ng/mL Flt3 ligand (R & D Systems, Minneapolis, MN, USA) for 10 days in 12-well plates. On day 5, half of medium was replaced with fresh medium containing Flt3 ligand [18]. To prepare splenic DCs, spleens were minced and digested in RPMI media containing collagenase D (2 mg/mL) and DNase I (100 ng/mL) for 90 minutes at 37°C [38]. After filtration through a 70-μm sieve, CD11c+ DCs were isolated by positive selection with CD11c antibody-coated microbeads (Miltenyi Biotec, Auburn, CA).
Cell stimulation
To induce ER stress, DCs were pretreated with tunicamycin (Tm, 10 μg/mL) from Sigma for 5 h or thapsigargin (Tg, 1 μM) from Invitrogen (Carlsbad, CA) for 1 h. After washing, cells were incubated with the indicated stimuli as follows: polyIC (25 μg/ml) from Invivogen (San Diego, CA); LPS (100 ng/mL) from Sigma; CpG ODN 1826 (5 μg/mL) from Invivogen; ssRNA (0.5 μg/mL) from Invivogen. For gene silencing, control scramble siRNA, TLR-3, MDA-5 or XBP-1 siRNA from Santa Cruz Biotechnology (Santa Cruz, CA) was transfected into DCs accordingly to the manufacturer’s instructions. 48 h later, cells were treated as described above. For XBP-1 overexpression, cells were transfected with pcDNA3.1-XBP-1s plasmid, kindly provided by Dr. Laurie Glimcher (Harvard School of Public Health), or vector with Fugene HD. Cells were stimulated with polyIC 36 h later and subjected to analyses.
PCR and quantitative real-time PCR
Total RNA was extracted from cells using TRIzol reagent and treated with DNase from Fermentas (Glen Burnie, MD). Reverse transcription (RT) was performed using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas), followed by PCR assays using the PCR primers as follows: mIFN-β, sense primer: 5′-CGTTCCTGCTGTGCTTCTC-3′, antisense primer: 5′-ATCTCTGCTCGGACCACCA-3′; mTNF-α, sense primer: 5′-AGCACAGAAAGCATGATCCG-3′, antisense primer: 5′-GTTGTCTTTGAGATCCATGCCGTTGG-3′; mXBP-1, sense primer: 5′-ACACGCTTGGGA ATGGACAC-3′, antisense primer: 5′-CCATGGGAAGATGTTCTGGG-3′; mTLR3, sense primer: 5′-AAGAGGGCGGAAAGGTG-3′, antisense primer: 5′-GAAGCGAGCATTTACTA-3′; mTLR7, sense primer: 5′-CTCTCTGTCTCAGAGGACTCC-3′, antisense primer: 5′-TCTTCCAGATGGTT CAGC-3′; mTLR9, antisense primer: 5′-GCTTTGGCCTTTCACTCTTG-3′, antisense primer: 5′-AACTGCGCTCTGTGCCTTAT-3′; mMDA5, sense primer: 5′-GAATCAGACACAAGT TTGGCAG-3′, antisense primer: 5′-CATTACCTTATTGACAAGAAC-3′; β-actin, sense primer: 5′-GTCCCTCACCCTCCCAA AAG-3′, antisense primer: 5′-GCTGCCTCAACA CCTCAACC-3′. For qRT-PCR, all primers and reagents were purchased from Applied Biosystems, and reaction was run on the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene expression was quantified relative to the expression of the housekeeping gene β-actin, and normalized to that measured in control by standard 2(−Δ ΔCT)calculation.
Dual-luciferase reporter (DLR) assay
Approximately 3 × 105 DC1.2 cells were seeded into a 24-well culture plate. After 24 h, cells were cotransfected with 500 ng pGL4-NF-κB-luciferase plasmid and 20 ng pRL-TK-luciferase plasmid by Fugene HD. Each transfection experiment was performed in triplicates. 24h later, Tg or vehicle was added into the cells and incubated for 1h, followed by the addition of poly IC or vehicle. Cells were incubated for additional 3h and then assayed for both firefly and renilla luciferase activities using the dual-luciferase reporter assay system on a GloMax™ luminometer (Promega, Madison WI).
VSV-GFP virus package
Vesicular stomatitis virus (VSV)-GFP, a gift of Dr. Brian Lichty (McMaster University, Hamilton, Canada), was propagated using BHK-21 cells. Briefly, cells were infected with VSV-GFP virus at an MOI of 0.5 for 2 h at 37 °C. Residual input viruses were removed and 10 ml of growth media were added. Viral supernatants were harvested 36 h later, filtered using a 0.45-μm filter and concentrated by centrifugation at 28,000 rpm.
Preparation of DC conditioned media and VSV-GFP virus infection assays
Preparation of DC conditioned media and virus infection studies were performed as previously described [39]. Briefly, DC1.2 cells were transfected with pcDNA3.1-XBP-1s plasmid or vector with Fugene HD in 10 cm dishes. 48 h later, cells were resuspended at a density of 5 × 106/ml and stimulated with 25 μg/ml of polyIC for another 8h. The conditioned media were stored at −80°C for later usage. For VSV infection assays, 1 ml of the conditioned media were added to NIH-3T3 cells seeded in a 6-well plate and incubated for another 3 h. Cells were then infected with VSV-GFP at a multiple of infection of 1.0. Viruses were incubated with cells for 1 h to allow adsorption. Residual input viruses were removed and replaced with fresh growth media. 16 h later, cells were photographed using a Nikon fluorescence microscope with a 10× objective lenses and a SPOT digital camera. The percentage of GFP-expression cells was measured by flow cytometry analysis. For antibody blocking, 10μg/ml MAR1-5A3 monoclonal antibodies that react with mouse IFN-α/β receptor subunit 1 (IFNAR-1) from eBioscience (San Diego, CA) or control anti-mouse IgG were added to cells and incubated for 1 h before viral infection.
Acknowledgments
We gratefully thank Dr. Laurie Glimcher (Harvard School of Public Health), Dr. Kenneth Rock (University of Massachusetts Medical Center), and Dr. Brian Lichty (McMaster University) for kindly providing the XBP-1 plasmids and VSV-GFP.
This work was supported by National Cancer Institute Grants CA129111, CA154708, American Cancer Society Grant RSG-08-187-01-LIB, Harrison Endowed Scholarship and NCI Cancer Center Support Grant to Massey Cancer Center.
Abbreviations
- DC
dendritic cells
- ER
endoplasmic reticulum
- IFNAR
Interferon-α/β receptor
- PAMP
pathogen-associated molecular pattern
- polyIC
polyriboinosinic:polyribocytidylic acid
- PRR
pattern recognition receptor
- TLR
toll-like receptor
- Tg
Thapsigargin
- Tm
tunicamycin
- UPR
unfolded protein response
- VSV
vesicular stomatitis virus
- XBP-1
X-box binding protein-1
Footnotes
Conflict of interest:
The authors declared no financial or commercial conflict of interest
References
- 1.Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 10.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur J Immunol. 2008;38:1194–1203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 13.Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Uematsu S, Takeuchi O. Pathogen Recognition and Innate Immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 16.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrot I, Deauvieau F, Massacrier C, Hughes N, Garrone P, Durand I, Demaria O, Viaud N, Gauthier L, Blery M, Bonnefoy-Berard N, Morel Y, Tschopp J, Alexopoulou L, Trinchieri G, Paturel C, Caux C. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J Immunol. 185:2080–2088. doi: 10.4049/jimmunol.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development. The Journal of Experimental Medicine. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda K, Takaoka A, Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 21.Malmgaard L, Salazar-Mather TP, Lewis CA, Biron CA. Promotion of alpha/beta interferon induction during in vivo viral infection through alpha/beta interferon receptor/STAT1 system-dependent and -independent pathways. J Virol. 2002;76:4520–4525. doi: 10.1128/JVI.76.9.4520-4525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 28.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 29.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 30.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Blanc I, Luyet PP, Pons V, Ferguson C, Emans N, Petiot A, Mayran N, Demaurex N, Faure J, Sadoul R, Parton RG, Gruenberg J. Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 37.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. The Journal of Experimental Medicine. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, Kim HL, Subjeck JR, Wang XY. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–5828. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renn CN, Sanchez DJ, Ochoa MT, Legaspi AJ, Oh CK, Liu PT, Krutzik SR, Sieling PA, Cheng G, Modlin RL. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J Immunol. 2006;177:298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]