Abstract
Significant pharmacokinetic interactions can result between acid-suppressing agents and some protease inhibitors (PIs) in the management of HIV infection. In healthy subjects, famotidine, an H2-receptor antagonist, reduces exposures of atazanavir by 4–28% at doses of 20–40 mg twice daily. This study evaluated the effect of famotidine 20–40 mg twice daily on the pharmacokinetics of atazanavir/ritonavir 300/100 mg once daily with and without tenofovir disoproxil fumarate (TDF) 300 mg in HIV-infected patients (n=40; 87.5% male; mean age 42, range 26–63 years; 55% white). Coadministration of famotidine 40 mg and atazanavir/ritonavir to HIV-infected patients reduced exposures of atazanavir by approximately 20%. This is comparable to reductions seen in HIV-uninfected subjects. Coadministration of famotidine 20 mg had less impact on atazanavir exposures, with no reduction of atazanavir geometric mean plasma concentration at 24 h postdose (Cmin). In the presence of TDF, administration of famotidine 20–40 mg twice daily 2 h after and 10 h before atazanavir/ritonavir reduced exposures of atazanavir by 19–25%. However, all individual atazanavir Cmin values remained at least five-fold above the population mean protein-binding adjusted 90% maximum effect (EC90) against wild-type HIV (14 ng/mL). No viral load rebound was observed at end of study. The results confirmed that coadministration of an H2-receptor antagonist with atazanavir/ritonavir in HIV-infected patients resulted in similar magnitude of reductions in atazanavir exposures as in healthy subjects. This supports the current dose recommendations for coadministration of an H2-receptor antagonist with atazanavir/ritonavir.
Introduction
Atazanavir is a potent once-daily protease inhibitor (PI) for the treatment of HIV type I infection, with proven efficacy in both treatment-experienced and treatment-naïve patients.1–5 Ritonavir-boosted atazanavir given at a dose of 300/100 mg once daily in combination with fixed-dose tenofovir and emtricitabine 300/200 mg once daily offers highly effective antiviral activity with a good safety profile.5,6
Many HIV-infected patients experience clinically significant alterations in gastric pH, and dyspepsia is common.7–9 Symptoms of heartburn and gastroesophageal reflux disease are often treated with prescription or with over-the-counter acid-suppressing agents, including antacids, proton-pump inhibitors, and H2-receptor antagonists.8,9 Gastric acid-suppressing agents may interfere with absorption of drugs when gastric pH is an important determinant of their solubility and/or bioavailability. Atazanavir is a bisulfate salt and its solubility decreases in vitro with increasing pH. The adequate absorption of atazanavir is dependent on an acidic gastric environment,10–13 therefore, a significant pharmacokinetic interaction could result between acid-suppressing agents and atazanavir.12
Previous interaction studies in healthy HIV-uninfected subjects given atazanavir/ritonavir with or without tenofovir disoproxil fumarate (TDF) and the H2-receptor antagonist famotidine have shown that famotidine 40 mg twice daily, when coadministered with atazanavir/ritonavir 300/100 mg once daily without TDF, decreased atazanavir area under the plasma concentration–time curve (AUC) and minimum plasma concentrations (Cmin) by approximately 18% and 28%, respectively.14 Increasing the atazanavir dose to 400 mg plus ritonavir 100 mg once daily when given with famotidine 40 mg twice daily mitigated the effect of famotidine, resulting in similar atazanavir AUC and less decrease in Cmin (14%) when compared with atazanavir/ritonavir 300/100 mg without famotidine.14 In subjects administered atazanavir/ritonavir 300/100 mg with TDF, coadministration of famotidine 20 mg twice daily (with or without a temporal separation from atazanavir) had no significant effect on atazanavir maximum plasma concentrations (Cmax) and AUC, and decreased atazanavir Cmin by 18–20%.15
Since the exposures to atazanavir are usually lower in patients with HIV infection than in healthy individuals16,17 (potentially due to reduced gastric acid secretions in HIV-infected patients), it is suspected that the effect of an H2-receptor antagonist on atazanavir exposure could be more pronounced in HIV-infected patients than has been observed in healthy subjects. This study was therefore designed to evaluate the effect of the H2-receptor antagonist famotidine on the pharmacokinetics of atazanavir/ritonavir in HIV-infected patients. Moreover, since TDF, which as part of an antiretroviral treatment (ART) backbone is often used in conjunction with atazanavir, has also been shown to reduce the exposures of atazanavir, this study included an additional group of patients who received both atazanavir/ritonavir and TDF to fully assess the total impact of an H2-receptor antagonist on the exposures of atazanavir in the presence of TDF.
Materials and Methods
Study design
This study was an open-label, two-cohort, three-period, multiple-dose, sequential, multicenter study in HIV-infected patients receiving continuous ART containing atazanavir/ritonavir 300/100 mg once daily and two or more nucleoside reverse transcriptase inhibitors (NRTIs; either with or without TDF) for at least 3 months prior to enrollment. The study was conducted at four clinical sites (three in the United States and one in the United Kingdom) from December 2006 to December 2007.
The primary objective of the study was to assess the effect of multiple-dose famotidine twice daily on the multiple-dose pharmacokinetics of atazanavir/ritonavir in HIV-infected patients when famotidine and atazanavir/ritonavir were administered simultaneously (without TDF) or temporally separated (with TDF).
Secondary objectives were to assess the pharmacokinetics of ritonavir coadministered with atazanavir in the presence and absence of famotidine and to assess the safety and tolerability of atazanavir/ritonavir when coadministered with famotidine in the presence of at least two NRTIs.
Subjects
This study was conducted in accordance with Good Clinical Practice and the ethical principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board at each study site. All subjects provided written informed consent prior to participation in the study.
Patients were eligible for the study if they met the following criteria: aged 18–65 years and deemed by the investigator to have an acceptable medical history for inclusion; CD4 count greater than 200 cells/mm3; plasma HIV RNA less than 400 copies per milliliter at least 6 weeks prior to enrollment, within 3 weeks prior to dosing with famotidine, and at 2 days prior to dosing with famotidine; and no history of virologic failure on a PI or documented phenotypic PI resistance or primary PI mutations according to International AIDS Society recommendations (see also www.clinicaltrials.gov study NCT00384904).
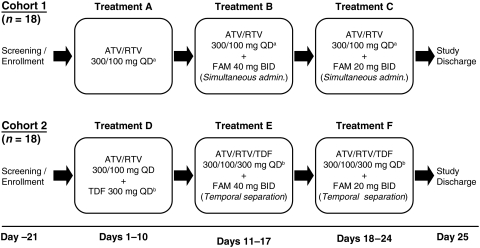
Patients who had been receiving an antiretroviral regimen without TDF were enrolled into cohort 1 and received treatments A, B, and C, while patients who had been receiving an antiretroviral regimen including TDF were enrolled into cohort 2 and received treatments D, E, and F, as shown in Fig. 1. The administration of atazanavir/ritonavir antiretroviral regimens including TDF was temporally separated from the administration of famotidine to avoid further reduction in atazanavir concentrations caused by TDF.
FIG. 1.
Study design. In cohort 1 the morning dose of FAM was administered simultaneously with ATV/RTV and subjects received the following treatments orally: A: Morning dosing of ATV/RTV 300/100 mg QD for 10 days (days 1–10). B: Morning dosing of ATV/RTV 300/100 mg QD+FAM 40 mg twice daily for 7 days (days 11–17). C: Morning dosing of ATV/RTV 300/100 mg QD+FAM 20 mg twice daily for 7 days (days 18–24). In cohort 2 ATV/RTV/TDF was temporarily separated from FAM by administering 2 h before morning FAM and 10 h after afternoon FAM, and subjects received the following treatments orally: D: Morning dosing of ATV/RTV/TDF 300/100/300 mg QD for 10 days (days 1–10); E: Morning dosing of ATV/RTV/TDF 300/100/300 mg QD+FAM 40 mg twice daily for 7 days (days 11–17); and F: Morning dosing of ATV/RTV/TDF 300/100/300 mg QD+FAM 20 mg twice daily for 7 days (days 18–24).+Treatments A, B, and C included ≥2 NRTIs other than TDF.±Treatments D, E, and F included≥1 NRTIs other than TDF. ATV, atazanavir; FAM, famotidine; QD, once daily; RTV, ritonavir.
All regimens containing atazanavir/ritonavir were administered orally with 240 mL of water within 5 min after completion of a light meal (total calories [kcal] approximately 336).
Safety was assessed based on medical review of adverse event (AE) reports, vital signs, electrocardiograms, physical examination findings, and clinical laboratory results. There were safety laboratory profiles obtained at screening, day 2, prior to atazanavir/ritonavir dosing on days 11 and 18, and at study discontinuation (or completion; day 25).
Bioanalytical methods
Blood samples (2 mL) were collected with K3EDTA as anticoagulant on days 10, 17, and 24 for the analysis of complete atazanavir and ritonavir pharmacokinetic profiles. Specimens were processed within 60 min of collection. Plasma samples were analyzed centrally by AtlanBio, St Nazaire, France, by liquid chromatography tandem mass spectrometry (LC-MS/MS). The LC-MS/MS quality control deviations from nominal concentration for atazanavir in the range of 10–10,000 ng/mL were±5.0%, and for ritonavir in the range of 5–5000 ng/mL,±1.0%.
Pharmacokinetic methods
Noncompartmental pharmacokinetic analysis was performed using a validated computer program (Kinetica™ version 4.4.1; Thermo Electron Corporation, Philadelphia, PA) to assess Cmax, time of Cmax (Tmax), Cmin, and AUC curve in one dosing interval (AUC[TAU]).
The Cmax and Tmax were recorded from experimental observations for each treatment period. The Cmin was recorded as the trough plasma concentration at 24 h postdose. The AUC(TAU) was calculated by linear and log-linear trapezoidal summation using a mixed log-linear algorithm.
Statistical analyses
All statistical analyses were carried out using SAS/STAT® Version 8.2 (SAS Institute, Inc., Cary, NC). Point estimates and 90% confidence intervals (CIs) for the geometric mean ratios of atazanavir and ritonavir Cmax, AUC(TAU), and Cmin, with and without famotidine, were constructed for each cohort in order to estimate the effect of different treatments and schedules.
Specifically, analyses were made of the effect of famotidine 40 mg twice daily on the pharmacokinetics of atazanavir/ritonavir when dosed simultaneously (treatment B versus treatment A); famotidine 20 mg twice daily on the pharmacokinetics of atazanavir/ritonavir when dosed simultaneously (treatment C versus treatment A); famotidine 40 mg twice daily on the pharmacokinetics of atazanavir/ritonavir coadministered with TDF when temporally spaced from famotidine (treatment E versus treatment D); and famotidine 20 mg twice daily on the pharmacokinetics of atazanavir/ritonavir coadministered with TDF when temporally spaced from famotidine (treatment F versus treatment D).
Estimates were generated using general linear models on log-transformed data, with treatment as a fixed effect and measurements within each subject as repeated measurements. Point estimates and 90% CIs for differences on the log scale were exponentiated to obtain estimates for ratios of geometric means on the original scale. Treatment periods when atazanavir was administered without famotidine (treatment A and treatment D) were the reference for all comparisons. No adjustments were made for multiplicity.
Although the sample size was not based on statistical power considerations, 18 evaluable subjects in each cohort were to provide at least 85% and 92% confidence that the estimated ratios of the geometric means for atazanavir AUC(TAU) and Cmax, respectively, with or without famotidine, would be within 10% of the true ratios. In addition, 18 evaluable subjects were to provide at least 91% confidence that the corresponding estimate for atazanavir Cmin would be within 20% of the true ratio. To allow for dropouts, an adequate number of subjects would be needed to meet the inclusion/exclusion criteria at screening so that 18 subjects would complete each cohort.
Results
Patient disposition, demographics, and baseline characteristics
A total of 40 patients were enrolled in the study (18 in cohort 1 and 22 in cohort 2). The demographics and baseline characteristics of patients are shown in Table 1. Within cohort 1, 17 patients completed the study while 1 treated patient was removed due to poor compliance after completing treatment A. This patient was excluded from the pharmacokinetic analysis but included in the safety analysis. Within cohort 2, 19 of the 22 patients completed the study. Four patients were incorrectly dosed with famotidine. Of these 4 patients, 1 completed the study and 3 were discontinued prior to completing the study. The patient who completed the study also incorrectly received atazanavir/ritonavir plus TDF without another NRTI. In this patient, follow-up through 3 weeks after study completion showed that viral loads were undetectable (HIV RNA less than 400 copies per milliliter). The 4 patients incorrectly dosed with famotidine were excluded from the pharmacokinetic analysis but included in the safety analysis.
Table 1.
Patient Demographic Characteristics
| Characteristic | Cohort 1 (n=18) | Cohort 2 (n=22a) |
|---|---|---|
| Mean age yr (range) | 43 (26–63) | 40 (26–54) |
| Gender n (%) | ||
| Male | 14 (78) | 21 (95) |
| Female | 4 (22) | 1 (5) |
| Race n (%) | ||
| Caucasian | 9 (50) | 13 (59) |
| Black | 6 (33) | 4 (18) |
| Asian | 0 | 3 (14) |
| Other | 3 (17) | 2 (9) |
| Body mass index kg/m2 | ||
| Mean (range) | 25.6 (19.3–34.3) | 25.0 (20.4–29.6) |
| CD4 count in cells/μL | ||
| Mean (SD) | 663 (258) | 577 (175) |
| (range) | (261–1000) | (317–907) |
Includes the four subjects who were incorrectly dosed with famotidine.
Cohort 1: atazanavir/ritonavir 300/100 mg once daily with 2 or more NRTIs (without TDF) dosed simultaneously with famotidine twice daily (treatments A, B, and C).
Cohort 2: atazanavir/ritonavir/TDF 300/100/300 mg once daily with 1 or more additional NRTI(s) dosed temporally separated from famotidine BID (treatments D, E, and F).
NRTI(s), nucleoside reverse transcriptase inhibitor(s); TDF, tenofovir disoproxil fumurate; SD, standard deviation.
Pharmacokinetics
Atazanavir exposure in the absence of TDF
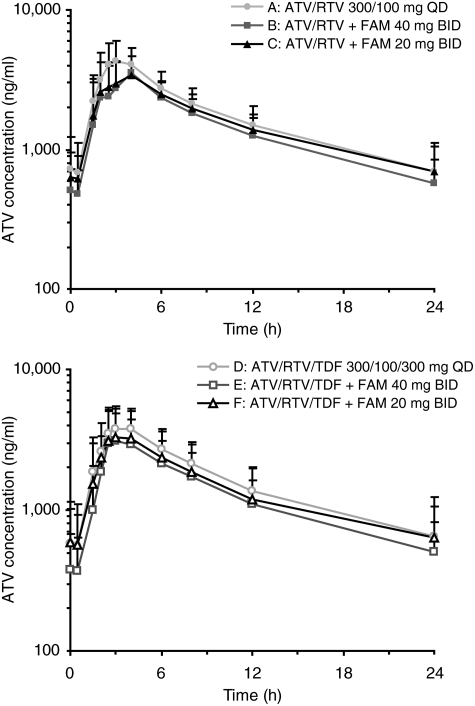
The pharmacokinetic and statistical analyses of atazanavir exposure per treatment group are summarized in Table 2. Plasma concentration-time profiles for atazanavir per treatment group are shown in Fig. 2. Simultaneous or temporally separated administration of famotidine 40 or 20 mg twice daily with atazanavir/ritonavir 300/100 mg once daily decreased atazanavir exposures when compared with atazanavir/ritonavir alone.
Table 2.
Pharmacokinetic Analyses for Atazanavir Exposurea
| Treatment | Cmax (ng/mL) | AUC(TAU) (ng.h/mL) | Cmin (ng/mL) |
|---|---|---|---|
| Cohort 1 | |||
| A: ATV/RTV | 4,572 (32) | 39,831 (33) | 588 (61) |
| B: ATV/RTV+famotidine 40 mg | 3,592 (33) | 31,787 (34) | 492 (49) |
| C: ATV/RTV+famotidine 20 mg | 3,687 (33) | 35,322 (30) | 610 (51) |
| Treatment B/A: Rb (90% CI) | 0.77 (0.67–0.88) | 0.77 (0.68–0.86) | 0.80 (0.69–0.92) |
| Treatment C/A: Rb (90% CI) | 0.80 (0.68–0.93) | 0.87 (0.75–1.01) | 0.99 (0.84–1.18) |
| Cohort 2 | |||
| D: ATV/RTV/TDF | 3987 (35) | 36,015 (41) | 542 (62) |
| E: ATV/RTV/TDF+famotidine 40 mg | 3057 (54) | 27,178 (45) | 409 (64) |
| F: ATV/RTV/TDF+famotidine 20 mg | 3130 (56) | 28,586 (61) | 441 (91) |
| Treatment E/D: Rb (90% CI) | 0.77 (0.64–0.92) | 0.76 (0.64–0.89) | 0.75 (0.53–1.07) |
| Treatment F/D: Rb (90% CI) | 0.79 (0.64–0.96) | 0.79 (0.66–0.96) | 0.81 (0.63–1.05) |
The values are reported as geometric means (coefficient of variation %) unless otherwise indicated
R is the ratio of adjusted geometric means (90% CI) for the treatment periods when atazanavir was administered with famotidine (treatments B, C, E, and F) to those when atazanavir was administered without famotidine (treatments A and D).
ATV, atazanavir; RTV, ritonavir; CI, confidence interval; TDF, tenofovir disoproxil fumarate.
FIG. 2.
Mean (standard deviation, SD) plasma concentration–time profiles for atazanavir by treatment.
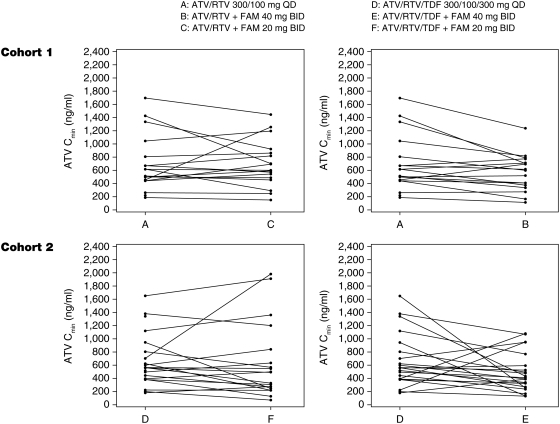
Using the geometric mean ratios (Table 2), atazanavir Cmax, AUC(TAU), and Cmin were 23%, 23%, and 20% lower, respectively, after patients received famotidine 40 mg coadministered with atazanavir/ritonavir (treatment B) compared with atazanavir/ritonavir alone (treatment A). Coadministration of famotidine 20 mg twice daily (treatment C) had less impact on atazanavir exposures. Using the geometric mean ratios, atazanavir Cmax, AUC(TAU), and Cmin were 20%, 13%, and 1% lower, respectively, in treatment C than in treatment A. Figure 3 shows the comparison of atazanavir Cmin for each individual patient in cohort 1. Despite the individual variation, the trend of reduction in atazanavir exposures with famotidine administration was consistent in most patients.
FIG. 3.
Individual atazanavir pharmacokinetic exposures (Cmin) by treatment.
Atazanavir exposure in the presence of TDF
Using the geometric mean ratios (Table 2), atazanavir Cmax, AUC(TAU), and Cmin were 23%, 24%, and 25% lower respectively, after receiving famotidine 40 mg temporally separated from atazanavir/ritonavir/TDF (treatment E) compared with atazanavir/ritonavir/TDF alone (treatment D). Administration of famotidine 20 mg twice daily with atazanavir/ritonavir/TDF with temporal separation (treatment F) had slightly less impact on atazanavir exposures. Using the geometric mean ratios atazanavir Cmax, AUC(TAU), and Cmin were 21%, 21%, and 19% lower, respectively (for treatment F), than those for treatment D. Figure 3 shows the comparison of atazanavir Cmin for each individual patient in cohort 2.
Ritonavir exposure
The ritonavir exposures were comparable across all treatments, such that simultaneous or temporally separated administration of famotidine 40 or 20 mg twice daily with atazanavir/ritonavir with or without TDF once daily had minimal effects on ritonavir exposures (data not shown).
Safety
There were no deaths, serious AEs, or discontinuations due to AEs in the study. Elevations in total bilirubin occurred in 7 (17.5%) patients (4 in cohort 1 and 3 in cohort 2). One patient experienced jaundice following administration of famotidine 20 mg temporally separated from atazanavir/ritonavir/TDF (treatment F). Headache was the most frequently observed treatment-related AE reported in 12 subjects (30%), followed by diarrhea in 6 subjects (15%). The AEs of headache were mild in intensity and those of diarrhea were mild or moderate in intensity. The remainder of reported AEs occurred only one or two times per treatment. There were no significant electrocardiographic or vital sign changes in any patients.
All patients who completed the study remained virologically suppressed at a level of less than 400 copies per milliliter at study completion.
Discussion
This study assessed the effect of coadministration of a commonly used H2-receptor antagonist, famotidine, on atazanavir/ritonavir exposure in HIV-infected patients. While many antiretroviral regimen pharmacokinetic studies have been performed in healthy HIV-uninfected subjects, it is known that atazanavir exposures in HIV patients can be 30–50% lower than those in healthy HIV-uninfected subjects.16 Through direct study of atazanavir/ritonavir drug interactions in HIV-infected patients, it is possible to investigate drug interactions in clinically relevant populations and test the relevance of pharmacokinetic results obtained from studies conducted in HIV-uninfected cohorts.
In this study of HIV-infected patients, coadministration of famotidine 40 mg twice daily and atazanavir/ritonavir 300/100 mg once daily reduced the exposure of atazanavir by 20–23%. The magnitude of this reduction is comparable to that noted in healthy HIV-uninfected subjects, in whom 14–28% decreases in atazanavir exposure were observed.14 In addition, this study in HIV-infected patients found that a lower dose of famotidine (20 mg twice daily) had less impact on atazanavir exposures, particularly Cmin (approximately 1% decrease).
In the presence of TDF, the magnitude of the famotidine (40 mg twice daily)-induced reduction in atazanavir exposure seen in the present study in HIV-infected subjects (23–25%) is generally comparable to the 21–28% reductions noted in healthy subjects.15 The impact of famotidine 20 mg twice daily was somewhat greater on Cmax and AUC in HIV-infected patients than in healthy subjects (i.e., decreases of 20% versus no effect), whereas a similar effect on Cmin was observed (19% versus 18% in healthy subjects).15 The mechanism and clinical relevance for the observed difference is uncertain. However, the magnitude of the reduction in atazanavir exposures by coadministration of TDF (approximately 10%, treatment D versus A) was less than the previously observed 25% reduction in healthy subjects.18 This difference may be explained by the different study design in the two studies: this study was conducted in parallel groups whereas the previous study was conducted in a crossover fashion. Nonetheless, the effects of TDF on atazanavir exposure do not appear to impact the antiretroviral efficacy of the combination regimen as demonstrated in the CASTLE study.5
Despite the reduction in atazanavir Cmin with famotidine and/or TDF administration, an important finding of the present study is that all individual Cmin values for atazanavir on days 10, 17, and 24 (data not shown) remained at least fivefold above the population mean protein-binding adjusted concentration required to obtain 90% of the maximum effect (EC90) against wild-type HIV (EC90=14 ng/mL after correcting for human serum binding effects).19 The lowest atazanavir Cmin among all treated patients in the current study was 70.5 ng/mL. These findings are in keeping with the observation in this study that concomitant use of famotidine and/or TDF with atazanavir was associated with continued virologic suppression. These results support the recommendation that in treatment-naive patients, further dose adjustment for atazanavir is not necessary. However, for treatment-experienced patients, a higher ratio of atazanavir Cmin to EC90 may be needed to achieve optimal antiviral efficacy. It has been shown previously in healthy subjects that an increase in atazanavir dose to 400 mg can mitigate the reduction in atazanavir exposures caused by TDF and famotidine.14,18 Increasing the atazanavir dose to 400 mg would alleviate the potential double impact on atazanavir exposures by both of these coadministered drugs and ensure that atazanavir Cmin is maintained within the therapeutic range in treatment-experienced patients.
A limitation of the current study is the low percentage of women (12.5%) enrolled, which is not untypical for PK studies conducted in HIV patients.
In summary, the findings of the current study are consistent with previous data from healthy HIV-uninfected subjects, and support the current dosing recommendations when atazanavir and H2-receptor antagonist coadministration is indicated in antiretroviral-naive patients that an H2-receptor antagonist dose equivalent to famotidine 20–40 mg twice daily can be used with atazanavir/ritonavir 300/100 mg once daily. For treatment-experienced patients who are taking the atazanavir/ritonavir regimen, an H2-receptor antagonist may be given at a dose not to exceed the equivalent of famotidine 20 mg twice daily. For treatment-experienced patients who are also taking TDF, the atazanavir dose should be adjusted to 400 mg with ritonavir 100 mg. For all patients taking an H2-receptor antagonist, atazanavir/ritonavir should be given simultaneously with or at least 10 h after the dose of the H2-receptor antagonist.2,20 These approaches to antiretroviral dosing and temporal spacing of coadministered H2-receptor antagonists ensure that atazanavir exposures are adequate across all groups of HIV-infected patients.
Acknowledgments
This study was funded by Bristol-Myers Squibb. Editorial support was provided by W McFadzean and J Turner of PAREXEL.
Part of this research was presented as a poster at the 10th International Workshop on Clinical Pharmacology of HIV Therapy, April 15–17, 2009, Amsterdam, The Netherlands.
Author Disclosure Statement
X.W., J.Z., E.C., L.Z., Y.W., M.C., and R.B. are employees of Bristol-Myers Squibb. L.M. was an employee of Bristol-Myers Squibb at the time the study was conducted.
M.B. has received travel and research grants from, and has acted as a consultant for, Bristol-Myers Squibb, Abbott, Tibotec, Johnson & Johnson, Pfizer, GSK, MSD, and Gilead.
A.K. has received research support from Bristol-Myers Squibb, Abbott, Tibotec, Gilead and Pfizer, and consultant fees from Boehringer Ingelheim and Bristol-Myers Squibb, Tibotec, and Merck.
K.P. has received research support from Bristol-Myers Squibb, Abbott, Tibotec, Gilead, and Pfizer, and consultant fees from Tibotec.
P.T. has received consultant fees from Bristol-Myers Squibb, Tibotec, Merck, Pfizer, and GlaxoSmithKline.
References
- 1.Swainston HT. Scott LJ. Atazanavir: A review of its use in the management of HIV infection. Drugs. 2005;65:2309–2336. doi: 10.2165/00003495-200565160-00010. [DOI] [PubMed] [Google Scholar]
- 2.Reyataz (atazanavir). [US prescribing information] Princeton, NJ: Bristol-Myers Squibb; 2010. [Google Scholar]
- 3.Perez-Elias MJ. Atazanavir: Simplicity and convenience in different scenarios. Expert Opin Pharmacother. 2007;8:689–700. doi: 10.1517/14656566.8.5.689. [DOI] [PubMed] [Google Scholar]
- 4.Johnson M. Grinsztejn B. Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 5.Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services; Dec 1, 2009. [Dec 10;2010 ]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. [Google Scholar]
- 7.Welage LS. Carver PL. Revankar S. Pierson C. Kauffman CA. Alterations in gastric acidity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:1431–1438. doi: 10.1093/clinids/21.6.1431. [DOI] [PubMed] [Google Scholar]
- 8.Luber A. Garg V. Gharakhanian S. Team VHP. Survey of medication used by HIV-infected patients that affect gastrointestinal acidity and potential for negative drug interactions with HAART [Abstract 206]. Presented at: 7th International Congress on Drug Therapy in HIV Infection; Glasgow, Scotland. Nov 14–18;2004 . [Google Scholar]
- 9.Luber AD. Use of acid-reducing agents in protease inhibitor-based HAART and the potential for negative treatment outcomes. AIDS Read. 2005;15:692–700. [PubMed] [Google Scholar]
- 10.Beique L. Giguere P. la Porte C. Angel J. Interactions between protease inhibitors and acid-reducing agents: A systematic review. HIV Med. 2007;8:335–345. doi: 10.1111/j.1468-1293.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 11.Falcon RW. Kakuda TN. Drug interactions between HIV protease inhibitors and acid-reducing agents. Clin Pharmacokinet. 2008;47:75–89. doi: 10.2165/00003088-200847020-00001. [DOI] [PubMed] [Google Scholar]
- 12.Fulco PP. Vora UB. Bearman GM. Acid suppressive therapy and the effects on protease inhibitors. Ann Pharmacother. 2006;40:1974–1983. doi: 10.1345/aph.1H022. [DOI] [PubMed] [Google Scholar]
- 13.Eley T. Agarwala S. Wang R, et al. Analysis of intra-gastric pH and atazanavir bioavailability in healthy subjects. Presented at: 7th Workshop on Clinical Pharmacology in HIV Therapy [Abstract 40]; Lisbon, Portugal. Apr 20–22;2006 . [Google Scholar]
- 14.Agarwala S. Eley T. Villegas C, et al. Pharmacokinetic effect of famotidine on atazanavir with and without ritonavir in healthy subjects [Abstract 11]. Presented at: 6th International Workshop on Clinical Pharmacology of HIV Therapy; Quebec City, Canada. Apr 28–30;2005 . [Google Scholar]
- 15.Agarwala S. Perssson A. Eley T, et al. Effect of famotidine 20 and 40 mg dosing regimens on the bioavailability of atazanavir with ritonavir in combination with tenofovir in healthy subjects [Abstract 568]. Presented at: 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. Feb 25–28;2007 . [Google Scholar]
- 16.Dickinson L. Khoo S. Back D. Differences in the pharmacokinetics of protease inhibitors between healthy volunteers and HIV-infected persons. Curr Opin HIV AIDS. 2008;3:296–305. doi: 10.1097/COH.0b013e3282f82bf1. [DOI] [PubMed] [Google Scholar]
- 17.Taburet AM. Piketty C. Chazallon C, et al. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:2091–2096. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwala S. Eley T. Villegas C. Wang Y. Hughes E. Grasela D. Pharmacokinetic interaction between tenofovir and atazanavir coadministered with ritonavir in healthy subjects [Poster 2.9]. Presented at: 6th International Workshop on Clinical Pharmacology of HIV Therapy; Quebec City, Canada. Apr 28–30;2005 . [Google Scholar]
- 19.Zhu L. Liao S. Child M, et al. CASTLE study: pharmacokinetics and inhibitory quotient of atazanavir/ritonavir 300/100 mg once daily compared to lopinavir/ritonavir 400/100 mg twice daily, each in combination with fixed dose tenofovir-emtricitabine once daily, are supportive of 48 week efficacy and safety in HIV-1 infected, treatment-naïve subjects [Poster 023]. Presented at: 9th International Workshop on Clinical Pharmacology of HIV Therapy; New Orleans, LA. Apr 7–9;2008 . [Google Scholar]
- 20.Reyataz. Summary of product characteristics. Uxbridge, UK: Bristol-Myers Squibb; 2010. [Google Scholar]