Abstract
The use of medicinal plants as natural antimicrobial agents is gaining popularity. Sorrel (Hibiscus sabdariffa) is widely used for the treatment of diseases. The objective of this study was to investigate the antimicrobial activity of sorrel on Escherichia coli O157:H7 isolates from food, veterinary, and clinical samples. Phenolics of the calyces were extracted from 10 g of ground, freeze-dried samples using 100 mL of 80% aqueous methanol. Concentrations of 10%, 5%, and 2.5% methanol extract of sorrel were investigated for its antimicrobial activity. Inhibition zones were indicated by a lack of microbial growth due to inhibitory concentrations of sorrel diffused into semisolid culture medium beneath the sorrel-impregnated disk. The results of this experiment showed that the most potent sorrel concentration was 10%, then 5%, and finally 2.5%. The overall mean zone of inhibition for the sorrel extract was 12.66 mm for 10%, 10.75 mm for 5%, and 8.9 mm for 2.5%. The highest inhibition zones (11.16 mm) were observed in veterinary samples, and the lowest (10.57 mm) in the food samples. There were significant (P<.05) differences among mean zones of inhibition found in the food, veterinary, and clinical sources. Based on the source of samples and concentration of sorrel extract, the lowest mean inhibition was 7.00±0.04 mm from clinical samples, and the highest was 15.37±0.61 mm from a food source. These findings indicated that sorrel was effective at all levels in inhibiting E. coli O157:H7; thus it possesses antimicrobial activity and hold great promise as an antimicrobial agent.
Key Words: antimicrobial activity, Escherichia coli, phenolic, sorrel, zone of inhibition
Introduction
Emerging foodborne pathogens have become a major public health concern. Many naturally occurring compounds found in medicinal plants, herbs, and spices have been shown to possess antimicrobial activities against many foodborne pathogens. The enterohemorrhagic Escherichia coli strain O157:H7 is a major bacterial foodborne pathogen that causes illness from mild watery diarrhea to bloody diarrhea. In children and the elderly the enteritis could lead to complications such as hemolytic uremic syndrome.1 E. coli O157:H7 has been recognized as a significant cause of foodborne and waterborne illness in the industrialized world.2 Cattle are considered to be the major reservoir for E. coli O157:H7, and approximately 80% of herds (beef and dairy) may be affected. This has led to varying numbers of ground beef recalls annually, where contaminated ground beef was the initial source for transmission to humans.3 The U.S. government (2008) ordered the largest beef recall in U.S. history (143.4 million pounds) due to contamination of E. coli and other bacteria.4 More recently, outbreaks have been associated with drinking contaminated water or consumption of contaminated produce.1,5
Synthetic antibiotics provide the main basis for the therapy of bacterial infections. However, overuse of antibiotics has become the major factor for the emergence and dissemination of multi-drug-resistant strains of several groups of microorganisms. Use of herbal products as antimicrobial agents may provide the best alternative to the wide and injudicious use of synthetic antibiotics. The demand for plant-based therapeutics is increasing in both developing and developed countries because of growing recognition that they are natural products, non-narcotic, and easily biodegradable, producing minimum environmental hazards, having no adverse side effects, and being easily available at affordable prices.
Thus, in light of the evidence of rapid global spread of resistant isolates, the need to find new antimicrobial agents is of paramount importance. Researchers are increasingly turning their attention to herbal products, looking for new leads to develop better drugs against resistant strains.6 Plants are rich in a wide variety of secondary metabolites such as tannins, alkaloids, and flavonoids, which have been found in vitro to have antimicrobial properties.7 There have been several reports on the antimicrobial activity of different herbal extracts.8–11 Survival and growth of both susceptible and antibiotic-resistant Campylobacter strains have been inhibited effectively on agar plates and in contaminated ground beef by application of roselle (Hibiscus sabdariffa L.).12 In a study on blanched spinach and minced cooked beef, using clove and tea tree essential oils at three and four times the minimum inhibitory concentration in in vitro studies was needed to restrict E. coli O157:H7 populations in the food materials.13 Various antimicrobial effects on E. coli have been shown by anzer tea essential oils,14 guarana extract,15 hydrodistilled leaf oil of Cinnamomum chemungianum,16 essential oils from leaves, stems, and flowers of Salvia reuterana (Family Lamiaceae),17 and linalool vapor of bergamot and linalool oils.18 Many plants have been found to cure urinary tract infections, gastrointestinal disorders, respiratory diseases, and cutaneous infections.19 According to the World Health Organization, medicinal plants would be the best source for obtaining a variety of drugs.20
Sorrel, H. sabdariffa L. (Family Malvaceae), a medicinal herb commonly uses to make drink and pickle, is used in folk medicine in the treatment of hypertension, liver diseases, and fever.21–24 Okasha et al.25 reported that a decoction of the seeds is given to augment or induce lactation in poor letdown and maternal mortality. Hibiscus anthocyanins, a group of phenolic natural pigment present in the dried flower of H. sabdariffa and Hibiscus rosa-sinensis, have been found to have cardioprotective,26 hypocholesterolemic,27 antioxidative, and hepatoprotective22 effects in animals. An aqueous extract of H. sabdariffa enhances cardiac Na+,K+-ATPase and Ca2+,Mg2+-ATPase activities.28 Anthocyanin pigments and other phenolic compounds (Hibiscus protocatechuic acid) also isolated from dried flowers of H. sabdariffa demonstrated protective effects against tert-butyl hydroperoxide–induced oxidative damage and hepatotoxicity both in vitro and in vivo.22,29 The aqueous extract was found to be effective against Ascaris galliavium in poultry. Also, the coloring matter of the calyces is said to be lethal to Mycobacterium tuberculosis.30 In India, a decoction of the seeds was given to relieve dysuria and many cases of dyspepsia and debility. H. sabdariffa has been reported to be antiseptic, aphrodisiac, astringent, diuretic, emollient, purgative, sedative, stomachic, and tonic. It is also a folk remedy for abscesses, bilious conditions, cancer, cough, dysuria, and scurvy31 and was also found to be anticarcinogenic.32
These findings contribute to support and qualify the importance of screening natural products. Conclusive information is, however, critical with regard to its role as an antimicrobial. The efficacy of sorrel at 2.5%, 5%, and 10% has not been tested on E. coli O157:H7 isolated from food, veterinary, and clinical sources. Therefore the objective of this study was to evaluate the antimicrobial activities of sorrel (H. sabdariffa) on E. coli O157:H7 isolated from food, veterinary, and clinical sources using the disk diffusion method.
Materials and Methods
Plant material
Dried sorrel calyces were purchased from a local grocery store and freeze-dried. The freeze-dried calyces were grounded to powder and then stored at −20°C until use.
Extraction of phenolics
The phenolics in powdered freeze-dried calyces were extracted by the ultrasound-assisted method.33 Phenolics of the calyces were extracted from 10 g of ground, freeze-dried samples using 100 mL of 80% aqueous methanol. The mixture of freeze-dried powder and 80% aqueous methanol was sonicated for 20 minutes with continual nitrogen gas purging. The mixture was filtered through Whatman (Maidstone, United Kingdom) #2 filter paper using a Buchner funnel and rinsed with 50 mL of 100% methanol. Extraction of the residue was repeated using similar conditions. The two filtrates were combined and transferred into a 1-L evaporating flask with an additional 50 mL of 80% aqueous methanol. The solvent was evaporated using a rotary evaporator at 40°C. The remaining phenolic concentrate was dissolved in 50 mL of 100% methanol and diluted to a final volume of 100 mL using distilled deionized water obtained with a NANOpure™ water system (Barnstead, Dubuque, IA, USA). The mixture was centrifuged at refrigerated temperatures, using a Sorvall (DuPont, Wilmington, DE, USA) RC-5B refrigerated superspeed centrifuge, at 10,000 g for 20 minutes and then stored at −4°C for future use.
Antimicrobial activities of the sorrel disk diffusion method
Food samples (ground beef, spinach, and vegetable packs) were purchased from randomly selected area grocery stores (Huntsville, AL, USA). Clinical samples (human fecal matter) were obtained from Huntsville Hospital (Huntsville). Veterinary samples (rabbit carcasses) were acquired from the Alabama A&M University rabbit farm (Normal, AL, USA). Bacterial isolates were grown in Mueller–Hinton broth for 24 hours, swabbed evenly onto the surface of Mueller–Hinton agar using sterile cotton swabs, and allowed to dry. Filter paper disks (Difco, Detroit, MI, USA) impregnated in triplicates with control or 2.5%, 5%, and 10% sorrel were placed on the surface of each plate for each isolate and then incubated at 37°C for 16 hours. Inhibition zones were then measured to the nearest millimeter. Inhibition zones were indicated by a lack of microbial growth due to inhibitory concentrations of sorrel diffused into semisolid culture media (agar) beneath the sorrel-impregnated disc.
Statistical analysis
The data were analyzed by SAS version 9.1 software (SAS Institute, Cary, NC, USA) using a completely randomized design, and means separation was done using Tukey's Studentized range test at a significance level of P<.05.
Results
Antimicrobial activities of various concentrations (2.5%, 5%, and 10%) of sorrel extract against E. coli O157:H7 isolates from food, veterinary, and clinical samples are presented in Table 1. Based on source of samples and concentration of sorrel extract, the lowest mean zone of inhibition was 7.00±0.04 mm from a clinical source, whereas the highest was 15.37±0.61 mm from a food source.
Table 1.
Antimicrobial Activity of Sorrel on E. coli O157:H7 from Food samples
| |
|
Zones of inhibition (mm) |
||
|---|---|---|---|---|
| E. coli O157:H7 isolates | Source | 2.5% sorrel | 5% sorrel | 10% sorrel |
| MF-AAMU-1 | Ground beef | 7.59±0.2e | 9.33±0.1e | 11.51±0.4e |
| MF-AAMU-2 | Ground beef | 8.33±0.1d | 10.46±0.1d | 12.52±0.0d |
| MF-AAMU-3 | Ground beef | 7.40±0.1e | 11.59±0.0c | 12.41±0.1d |
| MF-AAMU-4 | Ground beef | 8.42±0.1d | 10.07±0.4d | 11.54±0.3e |
| MF-AAMU-5 | Ground beef | 7.26±0.1e | 8.83±0.1d | 10.56±0.1d |
| MF-AAMU-7 | Ground beef | 8.64±0.0d | 10.37±0.0d | 11.78±0.0e |
| MF-AAMU-119 | Vegetable pack | 8.62±0.1d | 10.63±0.0d | 12.13±0.1d |
| MF-AAMU-10 | Ground beef | 7.76±0.0e | 9.7±0.07e | 12.67±0.0d |
| MF-AAMU-11 | Ground beef | 10.02±0.0b | 11.25±0.1c | 15.37±0.6a |
| MF-AAMU-12 | Ground beef | 10.02±0.0b | 12.2±0.0b | 14.59±0.1b |
| MF-AAMU-13 | Ground beef | 9.84±0.1c | 11.9±0.01c | 12.83±0.2d |
| MF-AAMU-14 | Ground beef | 7.52±0.1e | 10.2±0.1d | 12.42±0.1d |
| MF-AAMU-15 | Ground beef | 8.31±0.0 d | 9.83±0.0e | 11.78±0.0e |
| MF-AAMU-120 | Vegetable pack | 7.45±0.1e | 9.65±0.0e | 11.55±0.1e |
| MF-AAMU-17 | Ground beef | 9.75±0.1c | 11.3±0.1c | 13.5±0.0c |
| MF-AAMU-121 | Vegetable pack | 10.29±0.0b | 11.42±0.0c | 12.44±0.0d |
| MF-AAMU-22 | Ground beef | 9.3±0.0c | 10.43±0.0d | 12.36±0.1d |
| MF-AAMU-23 | Ground beef | 7.47±0.2e | 11.2±0.0c | 13.93±0.0 c |
| MF-AAMU-24 | Ground beef | 7.13±0.0e | 9.02±0.0e | 11.49±0.1e |
| MF-AAMU-26 | Ground beef | 10.35±0.1b | 11.23±0.1c | 12.49±0.1d |
| MF-AAMU-31 | Ground beef | 9.59±0.2c | 11.13±0.0c | 13.05±0.2c |
| MF-AAMU-34 | Ground beef | 8.68±0.2d | 13.42±0.1c | 14.4±0.1b |
| MF-AAMU-35 | Ground beef | 9.63±0.1c | 10.78±0.0d | 11.35±0.2e |
| MF-AAMU-40 | Ground beef | 7.98±0.0e | 9.50±0.2e | 12.21±0.1d |
| MF-AAMU-46 | Ground beef | 8.56±0.1d | 10.22±0.1d | 12.63±0.1d |
| MF-AAMU-47 | Ground beef | 7.98±0.0e | 9.50±0.2e | 12.21±0.1d |
| MF-AAMU-52 | Ground beef | 9.43±0.2c | 12.4±0.1b | 14.65±0.0b |
| MF-AAMU-63 | Ground beef | 9.76±0.0c | 10.46±0.0d | 11.36±0.0e |
| MF-AAMU-66 | Ground beef | 8.78±0.0d | 9.52±0.1e | 11.04±0.0e |
| MF-AAMU-67 | Ground beef | 9.80±0.0c | 11.72±0.1c | 12.45±0.0d |
| MF-AAMU-72 | Ground beef | 7.97±0.0e | 9.91±0.0e | 11.02±0.0e |
| MF-AAMU-73 | Ground beef | 7.78±0.0e | 9.12±0.0e | 11.89±0.0e |
| MF-AAMU-79 | Ground beef | 8.26±0.0d | 9.23±0.0e | 12.14±0.0d |
| MF-AAMU-84 | Spinach | 9.74±0.1c | 11.44±0.1c | 13.64±0.0c |
Food samples included ground beef, spinach, and vegetable packs containing lettuce, carrot, and cabbage obtained from local grocery stores. Data are mean±SEM values.
abcde: Different letters denote differences in zones of inhibition for 2.5%, 5%, and 10% Sorrel.
Results showed that within each isolate from food source, the mean zone of inhibition produced at the 2.5% level of sorrel concentration ranged from a low of 7.13±0.0 mm to a high of 10.02±0.04 mm. At 5% sorrel concentration, mean inhibition zones varied from 8.83±0.1 mm to 13.42±0.11 mm. In the case of 10% sorrel concentration, variation was between a low of 10.56±0.16 mm and a high of 15.37±0.61 mm (Table 1).
The highest mean inhibition against the veterinary samples at the 2.5% sorrel concentration was 11.33±0.06 mm, whereas the lowest was 7.59±0.23 mm (Table 2). At the 5% sorrel concentration mean inhibition varied from 9.71±0.11 mm to 12.57±0.11 mm. The mean inhibitions produced at the 10% sorrel concentration were between 11.42±0.12 mm and 14.62±0.3 mm.
Table 2.
Antimicrobial Activity of Sorrel on E. coli O157:H7 on Veterinary Samples
| |
|
Zones of inhibition (mm) |
||
|---|---|---|---|---|
| E. coli O157:H7 isolates | Source | 2.5% sorrel | 5% sorrel | 10% sorrel |
| MF-AAMU-89 | Veterinary | 10.86±0.0b | 11.17±0.1c | 14.62±0.3b |
| MF-AAMU-101 | Veterinary | 9.27±0.1c | 10.51±0.0d | 12.89±0.1d |
| MF-AAMU-90 | Veterinary | 8.38±0.1d | 11.17±0.1c | 14.17±0.0b |
| MF-AAMU-92 | Veterinary | 9.31±0.1c | 12.57±0.1b | 13.11±0.1c |
| MF-AAMU-93 | Veterinary | 10.56±0.0b | 11.06±0.1c | 12.72±0.3d |
| MF-AAMU-94 | Veterinary | 10.72±0.1b | 12.29±0.0b | 13.52±0.0c |
| MF-AAMU-99 | Veterinary | 10.44±0.2b | 11.41±0.2c | 12.67±0.1d |
| MF-AAMU-100 | Veterinary | 10.67±0.2b | 11.47±0.1c | 13.61±0.1c |
| MF-AAMU-102 | Veterinary | 7.59±0.2e | 9.86±0.05e | 11.42±0.1e |
| MF-AAMU-103 | Veterinary | 8.76±0.1d | 10.31±0.0d | 12.4±0.22d |
| MF-AAMU-104 | Veterinary | 9.57±0.1c | 10.47±0.1d | 12.36±0.0d |
| MF-AAMU-105 | Veterinary | 8.55±0.2d | 10.51±0.1d | 13.61±0.1c |
| MF-AAMU-107 | Veterinary | 9.23±0.0c | 12.46±0.2b | 12.51±0.0d |
| MF-AAMU-108 | Veterinary | 9.16±0.0c | 11.23±0.1c | 12.61±0.1d |
| MF-AAMU-109 | Veterinary | 8.67±0.1d | 9.71±0.1e | 12.62±0.1d |
| MF-AAMU-110 | Veterinary | 8.7±0.0d | 10.67±0.1d | 12.54±0.0d |
| MF-AAMU-111 | Veterinary | 8.51±0.2d | 11.46±0.1c | 12.41±0.0d |
| MF-AAMU-112 | Veterinary | 9.32±0.2c | 11.66±0.1c | 12.42±0.1d |
| MF-AAMU-113 | Veterinary | 9.99±0.0c | 12.30±0.1b | 12.47±0.2d |
| MF-AAMU-114 | Veterinary | 8.75±0.0d | 10.69±0.1d | 13.65±0.1c |
| MF-AAMU-117 | Veterinary | 11.33±0.0c | 12.37±0.0b | 13.33±0.3c |
Veterinary samples were from rabbit carcasses. Data are mean±SEM values.
abcde: Different letters denote differences in zones of inhibition for 2.5%, 5%, and 10% sorrel.
Antimicrobial activities evidenced by the effect of sorrel on clinical samples produced an overall mean zone of inhibition ranging from 7.00±0.04 mm to 14.98±0.00 mm (Table 3). At the lowest sorrel concentration (2.5%), the mean zone of inhibition ranged from a low of 7.00±0.04 mm to a high of 10.15±0.15 mm. At the highest level of sorrel concentration (10%), mean inhibition zones produced were between 7.97±0.07 mm and 14.98±0.00 mm. At the 5% sorrel concentration, mean inhibition zones varied from 7.6±0.06 mm to 13.37±0.11 mm (Table 3).
Table 3.
Antimicrobial Activity of Sorrel on E. coli O157:H7 from Clinical Samples
| |
|
Zones of inhibition (mm) |
||
|---|---|---|---|---|
| E. coli O157:H7 isolates | Source | 2.5% sorrel | 5% sorrel | 10% sorrel |
| MF-AAMU-25 | Clinical | 9.49±0.1c | 10.83±0.0d | 13.26±0.1c |
| MF-AAMU-27 | Clinical | 7.66±0.2e | 11.61±0.1c | 14.53±0.1b |
| MF-AAMU-28 | Clinical | 7.75±0.0e | 9.52±0.2e | 13.59±0.1c |
| MF-AAMU-29 | Clinical | 8.81±0.0d | 9.56±0.0e | 14.84±0.1b |
| MF-AAMU-30 | Clinical | 9.43±0.2c | 12.4±0.1b | 14.65±0.0b |
| MF-AAMU-32 | Clinical | 7.40±0.2e | 12.82±0.4b | 13.44±0.2c |
| MF-AAMU-33 | Clinical | 9.26±0.1c | 10.56±0.1d | 12.90±0.2d |
| MF-AAMU-36 | Clinical | 9.21±0.0c | 10.63±0.2d | 12.37±0.5d |
| MF-AAMU-37 | Clinical | 9.87±0.2c | 10.26±0.1d | 11.47±0.1e |
| MF-AAMU-38 | Clinical | 9.41±0.1c | 11.01±0.1c | 13.99±0.0c |
| MF-AAMU-44 | Clinical | 7.44±0.2e | 9.25±0.7e | 12.62±0.3d |
| MF-AAMU-45 | Clinical | 8.68±0.1d | 13.37±0.1c | 14.69±0.0b |
| MF-AAMU-42 | Clinical | 10.15±0.1b | 12.99±0.1b | 14.98±0.0b |
| MF-AAMU-54 | Clinical | 8.78±0.0d | 9.52±0.1e | 11.04±0.0e |
| MF-AAMU-56 | Clinical | 7.00±0.0e | 7.6±0.0e | 7.97±0.0e |
| MF-AAMU-59 | Clinical | 8.81±0.2d | 11.0±0.3c | 14.77±0.0b |
| MF-AAMU-60 | Clinical | 7.02±0.1e | 11.2±0.3c | 13.22±0.3 c |
| MF-AAMU-62 | Clinical | 8.91±0.0d | 10.32±0.0d | 12.34±0.3d |
| MF-AAMU-65 | Clinical | 7.81±0.0e | 8.90±0.0e | 11.77±0.0e |
Clinical samples were human fecal specimens obtained from a hospital. Data are mean±SEM values.
abcde: Different letters denote differences in zones of inhibition for 2.5%, 5%, and 10% sorrel.
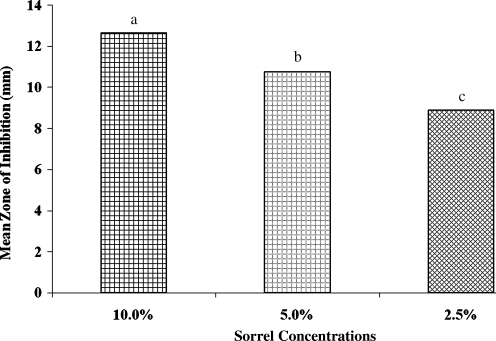
Results of this experiment showed that the extract of H. sabdariffa exhibited antibacterial activity against the E. coli bacterial strains. The results indicated that the most potent sorrel concentration was 10%, followed by 5%, whereas the least potent was 2.5% (Fig. 1). There were significant (P<.05) differences observed in mean zone inhibition among the 10%, 5%, and 2.5% concentrations of sorrel extract (Fig. 1). The overall mean zone of inhibition for the 10% concentration of sorrel was 12.66 mm, whereas for the 2.5% concentration it was 8.90 mm; at the 5% level of concentration the mean zone of inhibition was 10.75 mm (Fig. 1).
FIG. 1.
Antimicrobial activities of 2.5%, 5%, and 10% sorrel concentration on zones of inhibition. Data are mean±SEM values. abcDifferent letters denote significantly (P<.05) differences in zones of inhibition for 2.5%, 5%, and 10% sorrel.
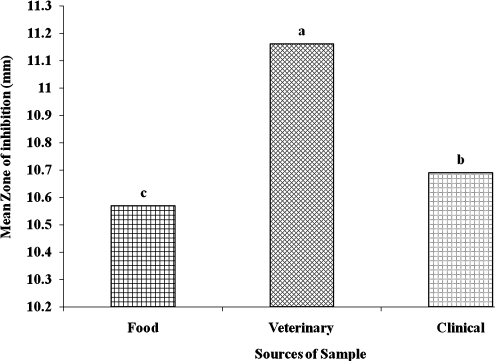
There were significant (P<.05) differences between mean zones of inhibition displayed in the food, veterinary, and clinical sources (Fig. 2). The highest inhibition zones were observed in veterinary isolates, whereas the least were observed in the food isolates. The overall mean zone of inhibition observed in the veterinary isolates was 11.16 mm, whereas in the food isolates it was 10.57 mm; the overall mean zone of inhibition among the clinical isolates was 10.69 mm.
FIG. 2.
Mean zones of inhibition produced among food, veterinary, and clinical sources. Data are mean±SEM values. abcDifferent letters denote significantly (P<.05) differences in zones of inhibition in food, veterinary, and clinical samples.
Discussion
The use of plant extracts and phytochemicals with known antimicrobial properties can be of great significance in therapeutic use. The results of the present study support the use of H. sabdariffa for human and animal disease therapy and reinforce the importance of the ethnobotanical approach as a potential source of bioactive substances. This may be attributed to two reasons: first, the nature and potentiality of biologically active components (alkaloids, flavonoids, phenolics, and biterpenoids); and second, the stronger extraction capacity of methanol could have produced higher numbers and amounts of active constituents responsible for antibacterial activity.9,34
H. sabdariffa produced zones of inhibition against E. coli O157:H7 isolates from food, veterinary, and clinical sources (Tables 1–3, respectively). Our study showed that the most effective sorrel concentration was 10%, whereas the least effective was the 2.5% concentration. There were significant (P<.05) differences in the effects of 10%, 5%, and 2.5% sorrel concentration against E. coli O157:H7 strains (Fig. 1). The antimicrobial activity may be the result of phenolic compounds, including flavonoids. This present study was comparable with other studies done using plant extracts on foodborne microorganisms. Krasaekoopt and Kongkarnchanatip35 used phenolic compounds, including flavonoids, and reported that the highest zones of inhibition were displayed by Staphylococcus aureus, a Gram-positive organism, compared with Gram-negative bacteria (E. coli) with the plant extracts studied. This study was also supported by the previous studies of Nair and Chanda20 and Rabe and van Staden.36 Siriponputikorn et al.37 reported similar results on the antimicrobial effect of Thai seasoning on common foodborne pathogens. In addition, Krasaekoopt and Kongkarnchanatip35 found the antimicrobial activity of Senna and Sesbania flowers were higher than that of Talisman extract on the bacterial strains; the percentages of flavonoid found in Sesbania flower, Senna flower, and Talisman were 8.4%, 8.6%, and 3.4%, respectively.
The antimicrobial activity due to flavonoids may be because of their structure, as they have the ability to form a combined complex with bacterial cell walls.38 Also, with the number of hydroxyl groups present on the phenolic ring there is increased hydroxylation, and with increased hydroxylation there will be increased antimicrobial activity. Cowan38 reported that the site(s) and number of hydroxyl groups on the phenol group are thought to be related to their relative toxicity to microorganisms, with evidence that increased hydroxylation results in increased toxicity. Flavonoids are also hydroxylated phenolic substances but occur as a C6–C3 unit linked to an aromatic ring. Because they are known to be synthesized by plants in response to microbial infection, it should not be surprising that they have been found in vitro to be effective antimicrobial substances against a wide array of microorganisms.
E. coli strains are Gram-negative microorganisms, and the effect of H. sabdariffa may vary depending on Gram-negative or -positive microorganisms. In our study H. sabdariffa at different concentration was bactericidal against E. coli O157:H7. Nair and Chanda20 also found similar effects, and they reported that standard ATCC strains of Gram-positive bacteria were more sensitive than Gram-negative ones toward the plant extracts. The mechanism of action may be by inhibition of various cellular processes, followed by an increase in plasma membrane permeability and finally ion leakage from the bacterial cells.39 These processes include the inhibition of electron transport, protein translocation, phosphorylation steps, and other enzyme-dependent reactions. Nair and Chanda20 showed inhibition at concentrations as low as 9.75 μg/mL. Antibacterial activities (minimum inhibitory concentrations of 0.30±0.2–1.30±0.2 mg/mL) were exhibited against S. aureus, Bacillus stearothermophilus, Micrococcus luteus, Serratia marcescens, Clostridium sporogenes, E. coli, Klebsiella pneumoniae, Bacillus cereus, and Pseudomonas fluorescence. Cowan38 suggested the antimicrobial action may be attributed to the intrinsic properties that are related to the permeability of their cell surface to the extracts.
Plants have an almost limitless ability to synthesize aromatic substances. Most of them are secondary metabolites, of which at least 12,000 have been isolated. In many cases, these substances serve as plant defense mechanisms against predation by microorganisms, insects, and herbivores.40 The potential for developing antimicrobials from higher plants appears rewarding, as it will lead to the development of a phytomedicine to act against microbes. Many plants have been used because of their antimicrobial traits, which are due to compounds synthesized in the secondary metabolism of the plant, such as phenols,41 essential oils,42 terpenoids,43,44 alkaloids,24 and flavonoids.45
Conclusions
Our study confirmed that H. sabdariffa was effective at all levels in inhibiting E. coli O157:H7 isolates from food, veterinary, and clinical sources. This shows that plant extracts possess antimicrobial activity and hold great promise as antimicrobial agents against foodborne pathogens, including E. coli O157:H7. In addition, disk diffusion provided data that reliably predicted the effectiveness of antimicrobial activity of the extracts. Plant extracts may be used as possible sources to obtain new and effective herbal medicines to treat foodborne infections, as they may be an excellent alternative to combat the further spread of multi-drug-resistant microorganisms. It is important, however, to determine toxicity of the active constituents, their side effects, and pharmacokinetic properties. Future studies need to address these phenolic compounds from various plant extracts, which must be subjected to animal and human studies to determine their effectiveness in whole-organism systems, including, in particular, toxicity studies as well as an examination of their effects on beneficial normal microbiota. Therefore, the use of natural antimicrobials such as sorrel (H. sabdariffa) in combination with other food preservation techniques such as low temperature, high pressure processing, and pulsed ultraviolet-light technology to create a synergistic effect would be a novel alternative.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rangel JM. Sparling PH. Crowe C. Griffin PM. Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. E. coli O157 Outbreak Summaries 2007. Centers for Disease Control and Prevention; Atlanta: 2007. [Google Scholar]
- 3.Bell C. Approach to the control of entero-haemorrhagic Escherichia coli (EHEC) Int J Food Microbiol. 2002;78:197–216. doi: 10.1016/s0168-1605(02)00188-5. [DOI] [PubMed] [Google Scholar]
- 4.Food Safety & Inspection Service, U.S. Department of Agriculture. 2008. www.fsis.usda.gov/PDF/Recall-005-2008_Release.pdf www.fsis.usda.gov/PDF/Recall-005-2008_Release.pdf
- 5.Centers for Disease Control and Prevention. Investigation of Multistate Outbreak of E. coli O157:H7 Infections. Centers for Disease Control and Prevention; Atlanta: 2008. [Google Scholar]
- 6.Braga LC. Leite AM. Xavier KGS. Takahashi JA. Bemquerer MP. Chartone-Souza E. Nascimento AMA. Synergic interaction between pomegranate extracts and antibiotics against Staphylococcus aureus. Can J Microbiol. 2005;51:541–547. doi: 10.1139/w05-022. [DOI] [PubMed] [Google Scholar]
- 7.Lewis K. Ausubel FM. Prospects of plant derived antibacterials. Nat Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 8.Bonjar S. Evaluation of antibacterial properties of some medicinal plants used in Iran. J Ethnopharmacol. 2004;94:301–305. doi: 10.1016/j.jep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Islam B. Khan SN. Haque I. Alam M. Mushfiq M. Khan AU. Novel anti-adherence activity of mulberry leaves: inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J Antimicrob Chemother. 2008;62:751–757. doi: 10.1093/jac/dkn253. [DOI] [PubMed] [Google Scholar]
- 10.de Boer HJ. Kool A. Broberg A. Mziray WR. Hedberg I. Levenfors JJ. Antifungal and antibacterial activity of some herbal remedies from Tanzania. J Ethnopharmacol. 2005;96:461–469. doi: 10.1016/j.jep.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Brantner A. Grein E. Antibacterial activity of plant extracts used externally in traditional medicine. J Ethnopharmacol. 1994;44:35–40. doi: 10.1016/0378-8741(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 12.Yin MC. Chao CY. Anti-Campylobacter, anti-aerobic, and anti-oxidative effects of roselle calyx extract and protocatechuic acid in ground beef. Int J Food Microbiol. 2008;127:73–77. doi: 10.1016/j.ijfoodmicro.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Moreira MR. Ponce AG. del Valle CE. Roura SI. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT Food Sci Technol. 2005;38:565–570. [Google Scholar]
- 14.Sekeroglu NS. Deveci MBuruk CK. Gurbuz B. Ipek A. Chemical composition and antimicrobial activity of Anzer tea essential oil. J Sci Food Agric. 2007;87:1424–1426. [Google Scholar]
- 15.Majhenic L. Skerget M. Knez Z. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chem. 2007;104:1258–1268. [Google Scholar]
- 16.Raj G. George V. Pradeep NS. Sethuraman MG. Chemical composition, antimicrobial activity of the leaf oil from Syzygium gardneri Thw. J Essent Oil Res. 2008;20:72–74. [Google Scholar]
- 17.Esmaeili A. Rustaiyan A. Nadimi M. Larijani K. Nadjafi F. Tabrizi L. Chalabian F. Amiri H. Chemical composition and antibacterial activity of essential oils from leaves, stems and flowers of Salvia reuterana Boiss. grown in Iran. Nat Prod Res. 2008;22:516–520. doi: 10.1080/14786410701592067. [DOI] [PubMed] [Google Scholar]
- 18.Fisher K. Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol. 2008;19:156–164. [Google Scholar]
- 19.Somchit MN. Reezal I. Nur IE. Mutalib AR. In vitro antimicrobial activity of ethanol and water extracts of Cassia alata. J Ethnopharmacol. 2003;84:1–4. doi: 10.1016/s0378-8741(02)00146-0. [DOI] [PubMed] [Google Scholar]
- 20.Nair R. Chanda S. Activity of some medicinal plants against certain pathogenic bacterial strains. Indian J Pharmacol. 2006;38:142–144. [Google Scholar]
- 21.Dalziel JM. The Useful Plants of West Tropical Africa. The Crown Agents; London: 1973. pp. 314–315. [Google Scholar]
- 22.Wang CJ. Wang JM. Lin WL. Chu CY. Chou FP. Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide–induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–416. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 23.Odigie IP. Ettarh RR. Adigun SA. Chronic administration of aqueous extract of Hibscus sabdariffa attenuates hypertension and reverses cardiac hyperytrophy in 2K-1C hypertensive rats. J Ethnopharmacol. 2003;86:181–185. doi: 10.1016/s0378-8741(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 24.Akindahunsi AA. Olaleye MT. Toxicological investigation of aqueous methanolic extract of Hibscus sabdariffa L. J Ethnopharmacol. 2003;89:161–164. doi: 10.1016/s0378-8741(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 25.Okasha MAM. Abubakar MS. Bako IG. Study of the effect aqueous Hibiscus sabdariffa L. seed extract on serum prolactin level in lactating albino rats. Eur J Sci Res. 2008;22:575–583. [Google Scholar]
- 26.Jonadet M. Bastide J. Bastide P. Boyer B. Carnat AP. Lamaison JL. In vitro enzyme inhibitory and in vivo cardio-protective activities of Hibiscus sabdariffa L. J Pharmacol Belgium. 1990;45:120–124. [PubMed] [Google Scholar]
- 27.Chen CC. Hsu JD. Wang SF. Chrang HC. Yang MY. Kao ES. Ho YO. Wang CJ. Hibscus sabdariffa extract inhibit the development of atherosclerosis in cholesterol-fed rabbits. J Agric Food Chem. 2003;51:5472–5477. doi: 10.1021/jf030065w. [DOI] [PubMed] [Google Scholar]
- 28.Olatunji LA. Adebayo JO. Adesokan AA. Olatunji AO. Soladote OA. Chronic administration of aqueous extract of Hibiscus sabdariffa enhances Na+-K+-ATPase and Ca2+-Mg2+-ATPase activities of rat heart. Pharm Biol. 2005;44:213–216. [Google Scholar]
- 29.Liu C. Wang J. Chu C. Cheng M. Tseng T. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem Toxicol. 2002;40:635–641. doi: 10.1016/s0278-6915(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 30.Roselle. Hibiscus sabdariffa L. www.hort.purdue.edu/newcrop/morton/roselle.html. [Nov;2010 ]. www.hort.purdue.edu/newcrop/morton/roselle.html
- 31.Morton JF. Roselle. In: Dowling CF, editor. Fruits of Warm Climate. Media, Inc.; Greensboro, NC: 1987. pp. 281–286. [Google Scholar]
- 32.Fullerton MSC. Verghese M. Walker LT. Shackelford LA. Cebert E. Boateng J. Ogutu S. Khatiwada J. Guyton M. Jones J. Chawan CB. Determination of antioxidant contents in red sorrel and its anticarcinogenic potential in azoxymethane-induced colonic aberrant crypt foci. J Res Phytochem. 2008;2:69–76. [Google Scholar]
- 33.Kim D. Jeong SW. Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. [Google Scholar]
- 34.Lee SB. Cha KH. Kim SN. Altantsetseg S. Shatar S. Sarangerel O. Nho CW. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. J Microbiol. 2007;45:53–57. [PubMed] [Google Scholar]
- 35.Krasaekoopt W. Kongkarnchanatip A. Antimicrobial properties of Thai traditional flower vegetable extracts. Au J Tech. 2005;9:71–74. [Google Scholar]
- 36.Rabe T. van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol. 1997;56:81–87. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 37.Siriponputikorn S. Thummaratwasik P. Huang Y. Antimicrobial and antioxidation effects of Thai seasoning, Tom-Yum. LWT Food Sci Technol. 2005;38:347–352. [Google Scholar]
- 38.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh SE. Maillard JY. Russel AD. Catrenich CE. Charbonneau AL. Bartolo RG. Activity and mechanism of action of selected biocidal agents on Gram -positive and -negative bacteria. J Appl Microbiol. 2003;94:240–247. doi: 10.1046/j.1365-2672.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- 40.Wink M. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor Appl Genet. 1998;75:225–233. [Google Scholar]
- 41.Kazmi MH. Malik A. Hameed S. Akhtar N. Ali SN. An anthraquinone derivative from Cassia italica. Phytochemistry. 1994;36:761–763. [Google Scholar]
- 42.Daferera DJ. Ziogas BN. Polissiou MG. The effectiveness of plant essential oils in the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Protect. 2003;22:39–44. [Google Scholar]
- 43.Habtemariam S. Gray AI. Waterman PG. A new antibacterial sesquiterpene from Premna oligotricha. J Nat Prod. 1993;56:140–143. doi: 10.1021/np50091a022. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RSL. Edel F. Manandhar NP. Towers GHN. Antimicrobial activities of southern Nepalese medicinal plants. J Ethnopharmacol. 1996;50:97–102. doi: 10.1016/0378-8741(95)01335-0. [DOI] [PubMed] [Google Scholar]
- 45.Batista O. Duarte A. Nascimento J. Simones MF. Structure and antimicrobial activity of diterpenes from the roots of Plectranthus hereroensis. J Nat Prod. 1994;57:858–861. doi: 10.1021/np50108a031. [DOI] [PubMed] [Google Scholar]