Abstract
Thymic B cells represent a numerically minor cell population located primarily at the cortico-medullary junction. Their biological role is unclear. B cell-deficient μMT mice exhibited reduced medullary thymic epithelial cell (mTEC) numbers and reduced MOG and insulin mRNA expression. Lymphotoxin produced by B cells was critical for normal tissue restricted antigen (TRA) expression, suggesting that B cells regulate self-antigens through their production of LT. These results reveal an unexpected role of B cells in mTEC maintenance and expression of TRAs through their production of LT.
Keywords: Myelin oligodendrocyte glycoprotein, Insulin, B cells, Thymus, Self-Antigens
1. Introduction
B cells represent a numerically minor population in the thymus comprising 0.1–0.3% of total cells and express CD80, CD86 and CD40 as well as B220, CD19 and CD5 (Ferrero et al., 1999). This unique population was originally reported to arise in the thymus from local progenitors and to reside primarily in the cortico-medullary junction and the thymic medulla (Ferrero et al., 1999). Recently, it has been noted under conditions of Notch1 deficiency that B cells from the periphery can also populate the thymus (Feyerabend et al., 2009). The medulla plays an important role in the selection of developing T cells (Takahama, 2006), suggesting that B cells may play a role in T cell selection owing to their location. Supporting evidence for a role of B cells in T cell development derives from several sources. B cells are required for negative selection of T cells expressing TCR-Vβ chains reactive to various superantigens (Beutner et al., 1994; Ferrero et al., 1999; Mori et al., 1997), and B cell deficient μMT mice show impaired thymic function and reduced T cell receptor diversity ; the latter observation was previously attributed to a deficiency in B cell APC function (Ferrero et al., 1999; Kleindienst et al., 2000). Furthermore, reduced thymic B cell numbers have been correlated with increased susceptibility to type 1 diabetes mellitus in BB diabetic rats (Tullin et al., 1997) and lupus in mice (Andreu-Sanchez et al., 1990) suggesting that B cells could play a role in negative selection to tissue restricted antigens (TRAs) that are the targets in autoimmune diseases.
Self-antigen expression in the thymus is required for selection of a non-self-reactive T cell repertoire. Expression of TRAs, such as insulin and myelin oligodendrocyte glycoprotein (MOG), that are involved in the development of autoimmune diseases has been reported in the thymus and appears to be confined predominantly to medullary thymic epithelial cells (mTEC) (Kyewski and Derbinski, 2004). The discovery of autoimmune regulator (AIRE) shed light on the regulation of some TRA expression (Mathis and Benoist, 2007); however, expression of many other antigens, such as GAD67 (Anderson et al., 2002), α-fodrin (Kuroda et al., 2005) and collagen II (Chin et al., 2006), does not appear to be controlled by AIRE, suggesting that additional mechanisms may control TRA expression in the thymus
Lymphotoxin (LT) has been implicated in thymic development in several studies. Previous studies implicated LTα, LTαβ, and lymphotoxin β receptor (LTβR) in the direct and/or indirect control of mTEC development, TRAs and AIRE expression (Chin et al., 2003; Zhu et al., 2007). Recently, several groups have demonstrated that the mechanism by which LT deficiency influences TRA expression is by an overall reduction in mTEC numbers and not via an effect on AIRE or TRA expression in individual mTECS (Boehm et al., 2003; Rossi et al., 2007; Venanzi et al., 2007). Additional publications conclusively demonstrate that though LTβR does not regulate AIRE-dependent transcripts in individual mTECs, it does influence three dimensional architecture and chemokine expression in the thymus (Martins et al., 2008; Seach et al., 2008).
The presence of the unique B cell population, its location, and implied role in negative selection led us to hypothesize that thymic B cells contribute to expression of TRAs in the thymus. We concentrated on MOG because of our long standing interest in its importance in experimental autoimmune encephalomyelitis (Juedes et al., 2000; Juedes and Ruddle, 2001; Oliver et al., 2003) and multiple sclerosis and compared thymic MOG and insulin because of the extensive literature analyzing regulation of expression of the latter autoantigen in the thymus. In this report, we show that B cells contribute to thymic MOG and insulin expression in the thymus via their expression of LT. These findings shed a new light on the role of this small yet significant thymic cell population.
2. Materials and Methods
2.1 Mice
Mice were maintained at Yale University’s animal facility. 4–8 week old female wild type, B cell deficient μMT mice, RAG−/− mice, CD154−/− mice, and B6.Cg-Foxn1nu/J all on a C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, ME). Female LTα−/− and LTβ−/− mice were previously described (De Togni et al., 1994; Koni et al., 1997) and are maintained in our colony. Mice that are conditionally deleted for LTβ in B cells (B LTβ−/−) by means of the CD19 promoter driven Cre that have been previously characterized (Tumanov et al., 2002) were obtained from Alexei Tumanov (University of Chicago). All animal use and husbandry protocols were approved by the Yale University Institutional Animal Care and Use Committee.
2.2 Thymic cell isolation
Examination of distinct cell numbers in the thymus was done by magnetic cell isolation and staining. The following cell surface markers were used for magnetic cell isolation: CD11c for dendritic cells, CD11b for macrophages, CDR1 for cortical thymic epithelial cells CDR1, and EP-CAM (G8.8) for medullary epithelial cells.
2.3 Bone marrow chimeras
Mixed BM chimeras were generated by transplanting donor BM into sublethally irradiated (500 rads) 4–8 week old recipient mice. Chimeras were generated by transplanting μMT mice with BM (107 cells) from μMT (μMT→μMT) or WT (WT→μMT) mice. Animals were then maintained for 60 days. During the resting period animals were bled every two weeks and circulating B cell levels (CD19+B220+) were determined by FACS (Fig. S1). Two months following transplantation, animals were sacrificed and lymph nodes and thymi were collected.
2.4 T cell transfer into nu/nu mice
T cells were prepared from WT C57BL/6 or μMT spleens by negative selection on MACS bead columns using anti-Class II and anti-B220 magnetic beads. The resulting WT T cells (7 ×106; greater than 98% purity) were injected into 5 wk old female B6.Cg-Foxn1nu/J (nu/nu) mice. An identical number of μMT T cells were injected into nu/nu or C57BL/6 mice. Recipients were weighed at 3-day intervals for 30 days. At termination of the experiment, ear skin, neck skin, LN, spleen, pancreas, intestine, liver, lung, kidney, heart, and brain were removed and processed for paraffin and frozen sections.
2.5 FACS analysis
The following antibodies were obtained from BD Biosciences – Pharmingen: anti-CD45RB/B220, CD19, CD4, CD154, CD40, CD5, I-Ab, CD80, CD86. Data were collected on a BD FACScan™ flow cytometer and analyzed using FlowJo software.
2.6 Immunohistochemistry and immunofluorescence
Thymi were harvested and immediately frozen in OCT compound (Tissue-Tek) on dry ice. Sections of 7 μm were fixed in 100% cold acetone, air dried and blocked with 5% mouse serum/BSA for 1 hr at room temperature (RT).
Immunohistochemistry staining for B cells was done with 2 μg/ml of anti-B220 (cloneRA3-6B2, BD Biosciences.) Slides were treated with streptavidin-conjugated alkaline phosphatase VectaStain reagent (Vector Laboratories), according to the manufacturer’s protocol (Vector Laboratories. Sections were counterstained with Harris’ hematoxylin (Sigma Diagnostics) and mounted in Crystal/Mount (Biomeda).
For immunofluorescence staining, the following primary antibodies/reagents were used for staining B220 (clone RA3-6B2), EP-CAM (clone G8.8), and biotinylated-UEA-1 (Sigma). Secondary antibodies/reagents were Cy2-conjugated streptavidin for UEA-1 staining, and staining Cy3-conjugated streptavidin for EP-CAM. In all experiments, species-specific isotype-matched irrelevant antibody was used as a control. Sections were counterstained with 20% Harris’ hematoxylin (Sigma Diagnostics). Slides were analyzed by fluorescence microscopy using a Carl Zeiss Microimaging, Inc. Axioskop microscope. For histomorphic analysis, 5 μm-thick sections were collected on every 15 μm of tissue and stained with UEA-1 and anti-B220 as described above followed by analysis using ImageJ software.
2.7 Histomorphic image analysis
Image analysis and post processing were performed using ImageJ (http://rsb.info.nih.gov/ij/). Numbers of single- and dual-color-labeled cells were counted using functions in ImageJ (colocalization, watershed, and analyze particles). For an review of ImageJ see Collins et al. (Collins, 2007).
2.8 Real time PCR
Individual thymi were isolated and parathymic LNs carefully removed. Thymi were homogenized and total RNA was isolated using totallyRNA kit (Ambion). cDNA was generated using SuperScript II (Invitrogen). Insulin, MOG, AIRE, LTα, and LTβ mRNA was quantitated by real-time RT-PCR using SYBR Green (Quiagen) on the Stratagene Mx3000P PCR system. All components of the PCR mix were purchased from Bio-Rad and used according to the manufacturer’s instructions. All genes were normalized to GAPDH expression. Data analysis was performed using Stratagene Mx3000P analyzing software. PCR primer pairs were as follows. GAPDH: F-CTGCACCACCAACTGCTTAG, R-GATGGCATGGACTGTGGTCAT; Insulin: F-TTCAGACCTTGGCGTTGGA, R-ATGCTGGTGCAGCACTGATC; MOG: F-GTGCCCTGCTGGAAGATAACA, R-GGGTCCAAGAACAGGCACAA; LTα: F-AACCCAAGAATTGGATTCCAGG R- TGTGACCCTTGAAACAACGGT; LTβ: F-TGTCTCCAGCTGCGGATTCTA, R-TTTGGCAGCTGTTGAACCC.
2.9 Statistical analysis
Graphs were generated using Prizm V4.0 (GraphPad Software Inc.) and Microsoft Excel (Microsoft Inc.). Statistical analysis (student’s t test and correlation) were done using Prizm V4.0 and Statistica 6.0 (Statsoft Inc.).
3. Results
Thymi of B cell deficient μMT mice show reduced TRA expression
Examination of thymi from WT mice revealed a small (~0.2%) but reproducible population of CD19+B220+ B cells by both IHC staining and FACS analysis (Fig. 1A and 1B). IHC revealed that the presence of B220+ cells predominantly at the cortico-medullary junction in WT mice with fewer cells staining in both medulla and cortex (Fig. 1A). In contrast, only a few B220+ cells were found in the thymi of B cell deficient μMT mice (Fig. 1A). Accordingly, no CD19+B220+ B cells were detected by FACS analysis in the thymi of B cell deficient μMT mice (Fig. 1B). Given the evidence for a role for thymic B cells in T cell selection in the thymus we examined the effects of B cell deficiency on mTEC numbers in μMT mice. We confirmed the previous observations showing a reduction in mTECs in the thymi of LTα−/− and LTβ−/− mice compared to WT mice and extended these findings to μMT mice showing a reduction in both mTEC (G8.8+) and cTEC (CDR1+) cells (Table 1). Next, we conducted a histomorphic analysis of UEA+ cells in thymic sections of LTα−/−, LTβ−/−, and μMT mice. While UEA-1 is expressed primarily on mTECs in the thymus (Gray et al., 2007), this cell surface marker is also found on cortical epithelial cells (Gray et al., 2002). To avoid the inclusion of cTECs, we calculated (using histomorphic analysis with ImageJ) the percentage of UEA-1 positive cells present only in the medulla. An overall reduction in UEA-1+ cells was noted in μMT mice lacking B cells (Fig 1C). A quantification of UEA-1+ cells revealed a reduction in mTECs similar to that observed in LTα−/− and LTβ−/− thymi (Fig 1D and Table 1). Previous reports conclusively demonstrated that LT influences TRA expression through its effect on mTEC numbers (Venanzi et al., 2007) and their 3-dimensional organization (Boehm et al., 2003). Given the similar drop in mTEC numbers in μMT and LT deficient mice, we proceeded to analyze total thymic extracts for the expression of MOG and insulin mRNA by quantitative RT-PCR. Consistent with a reduction in mTECs, μMT mice showed an 80% reduction in insulin and MOG mRNA compared to WT mice (Fig. 1E). Levels of AIRE mRNA were somewhat (40%) reduced but not nearly to the extent of TRAs, suggesting that though B cells affect TRAs, they affect AIRE to a considerably lesser extent. These results demonstrate an unexpected drop in mTEC numbers and TRA levels in μMT mice similar to that of LTα−/− and LTβ−/− mice.
Figure 1.
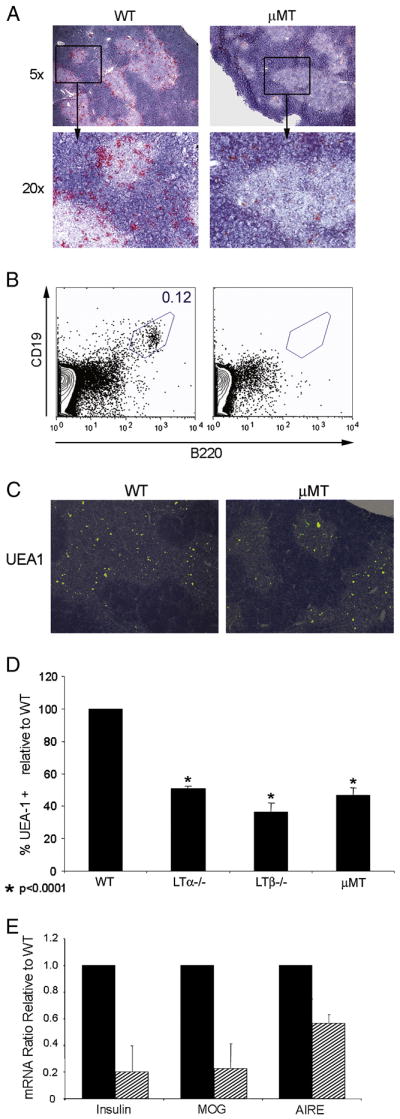
mTECs and TRA expression is reduced in μMT mice. (A) Immunohistochemistry staining for B220 (red) in the thymi of WT and μMT mice. (B) FACS analysis of CD19 and B220 double positive thymic B cells. (C) Merge of hematoxylin and mTEC (UEA-1) (green) staining in thymi of WT, LTα−/−, LTβ−/− and μMT mice. (D) Histomorphic analysis of UEA-1+ cells in the thymic medullae of WT, LTα−/−, LTβ−/− and μMT mice (E) Real time RT-PCR measurement of insulin, MOG, and AIRE mRNA in μMT (n=3) mice relative to WT mice (n=3). Solid bars- WT; hatched bars- μMT. Results are expressed relative to WT mice.
Table 1.
mTEC and cTEC numbers are reduced in the thymi of B cell deficient and lymphotoxin deficient mice
| Animal Model | Cell Number per thymus × 106 |
|||
|---|---|---|---|---|
| DC (CD11c+) | M„U (F4/80+) | mTECs (G8.8+) | cTECs (CDR1+) | |
| WT | 1.0±0.10 | 0.3±0.13 | 2.5±1.2 | 3.0±1.8 |
| μMT | 0.4±0.01 | 0.3±0.07 | 1.5±0.3 | 1.0±0.4 |
| LTα−/− | 0.4±0.06 | 0.2±0.05 | 1.5±0.2 | 1.2±0.3 |
| LTβ−/− | 0.6±0.04 | 0.2±0.02 | 1.0±0.2 | 0.9±0.05 |
Isolated thymi from various models were analyzed for the presence of dendritic cells (DCs) macrophages (Mφ) medullary thymic epithelial cells (mTECs) and cortical thymic epithelial cells (cTECs)
3.1 B cell reconstitution restores TRAs in the thymus of μMT mice
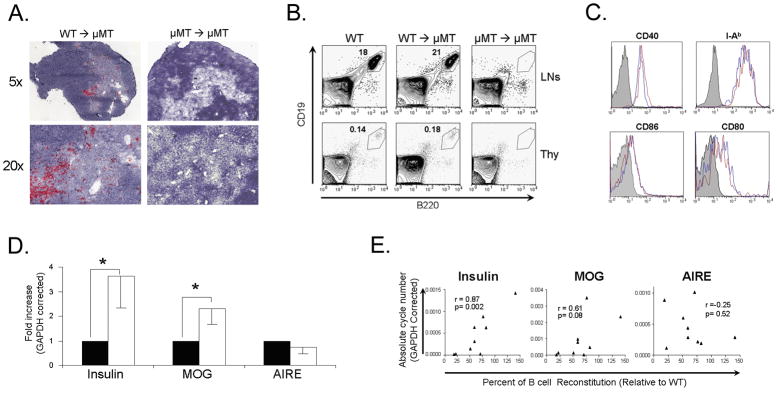
To determine whether B cell restoration in μMT mice could overcome the TRA expression defect, 107 BM cells from WT or μMT donor mice were transplanted into sublethally irradiated μMT recipients. Peripheral blood of treated mice was monitored biweekly for B cell reconstitution over the course of two months. CD19+B220+ B cells were readily detected in the blood of μMT recipients of WT BM as early as 2 weeks post transplantation. In contrast, no B cells were detected in the blood of μMT mice recipients of μMT BM (Fig. S1A). Analysis of thymic and LN B cells in μMT mice recipients of WT BM revealed normal B cell positioning in the thymus. These B cells were confined to the thymic cortico-medullary border and the thymic medulla (Fig. 2A). This reconstitution was confirmed by FACS analysis of CD19+B220+ cells in the thymus (Fig. 2B). Such restoration was not observed in recipients of μMT BM (Fig. 2A and 2B). B cells in the thymi of WT BM-reconstituted μMT mice expressed CD40, MHC II I-Ab, CD80, and CD86 at levels similar to those of thymic B cells from unmanipulated WT mice (Fig. 2C). The restoration of thymic B cells resulted in a significant increase in the expression of both MOG and insulin mRNA (Fig. 2D). This increase was not observed in μMT mice that received μMT BM (Fig. 2D). Despite statistically significant increases in insulin and MOG mRNAs, no increase was detected in AIRE expression, further supporting our previous observation of only a mild effect of B cell deficiency on AIRE expression in the thymus (Fig. 1C). Additional analysis of these data revealed a positive correlation between the extent of B cell reconstitution and levels of insulin (r=0.87, p=0.002) and MOG (r=0.61, p=0.08) expression in the thymus (Fig. 2E). That is, at low levels of reconstitution, MOG and insulin were comparably low, and at higher levels, there was more TRA expression. Examination of thymi from μMT mice receiving WT BM cells revealed a normalization of UEA-1+ cells to levels which were indistinguishable from those of unmanipulated WT mice (Figure S1B) These results demonstrate that restoration of thymic B cells correlates with restoration of MOG and insulin mRNA levels.
Figure 2.
WT BM restores B cells and thymic insulin and MOG expression in B cell deficient μMT mice. (A) Immunohistochemistry staining for B220 (red) in the thymi of μMT mice receiving either WT BM or μMT BM two months following BM transfer. (B) FACS analysis of CD19+B220+ cells in LNs and thymi of BM recipient mice two months following BM transfer. (C) FACS analysis of B cells in thymi of WT and B cell restored μMT mice two months following BM transfer. Shaded histogram- isotype control, blue histogram- WT, red histogram- B cell restored μMT mice. (D) Real-time RT-PCR analysis of insulin, MOG, and AIRE in thymi of μMT receipt mice transplanted with μMT (n=5) or WT (n=7) BM two months following BM transfer are expressed relative to μMT BM recipients. Solid bars- μMT BM; open bars- WT BM. * p<0.05. (E) Correlation analysis between degree of B cell reconstitution and insulin, MOG, and AIRE expression in thymi of μMT mice receiving WT BM.
3.2 Thymic B cells constitutively express LTα and LTβ and are required for normal TRA expression
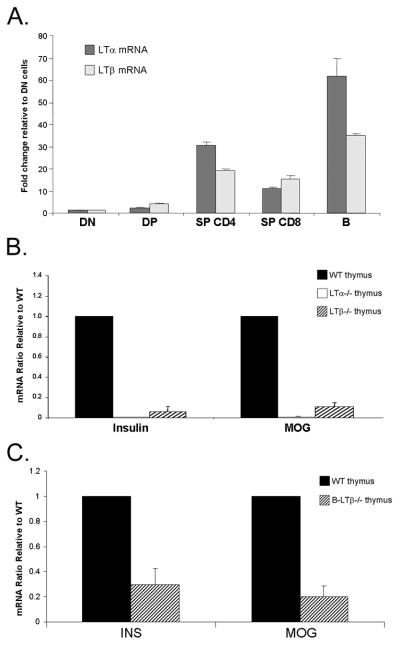
The above data demonstrating a role for LT in mTEC numbers, suggest that LT production by a resident thymic cell may contribute to its availability in the thymic microenvironment. Though several populations have been demonstrated to produce LT or LTβR ligands, the ability of thymic B cells to make these molecules had not been evaluated. Cell sorting of various thymocyte subtypes and thymic CD19+B220+ B cells revealed LTα and LTβ mRNA expression by several T cell subsets and also thymic B cells (Fig. 3A). In fact, on a per cell basis, thymic B cells produced the highest levels of LTα and LTβ mRNA compared with other individual thymic populations. Examination of individual thymi from LTα−/− and LTβ−/− mice showed an overall reduction in the expression of insulin and MOG mRNA (Fig. 3B), owing to the fact that mTEC numbers are reduced in these mice (Fig. 1B). To answer the question of whether LTβ expression in B cells is required for normal TRA expression in the thymus, we took advantage of the availability of mice that lack LTβ expression specifically in B cells. Conditional deletion of LTβ expression in B cells (Tumanov et al., 2002) resulted in a significant reduction in TRA expression when compared with WT mice (Fig. 3C). These data place B cells as important regulators of TRAs and suggest that despite their relative low number, thymic B cells may act as an important source of LT in the thymus.
Figure 3.
Lymphotoxin producing B cells are required for normal TRA expression. (A) Real-time RT-PCR analysis of FACS sorted thymocytes and thymic B cells (n=3) for LTα and LTβ mRNA expression. Results are GAPDH corrected and are expressed relative to double negative (DN) cell. DP- double positive; SP- single positive. (B) Real time RT-PCR analysis of insulin, MOG and AIRE mRNA in thymi of WT (n=3), LTα−/− (n=4), and LTβ−/− (n=3) mice. Results are GAPDH corrected and are expressed relative to WT mice. Solid bars-WT; open bars- LTα−/−; hatched bars- LTβ−/−. (C) Real-time RT-PCR analysis of insulin and MOG mRNA in thymi of WT and B LTβ−/− mice. Results are expressed relative to WT mice. Solid bars-WT thymus; hatched bars-B LTβ−/− thymus.
3.3 TRA expression is reduced in other mouse models showing decreased B cell numbers in the thymus
An alternation in normal T cell development and TRA presentation has been reported in other mouse models lacking key immunological factors. Several groups have demonstrated a role for CD40-CD40L interactions in mediating normal thymic function. CD40L has been implicated in mediating T cell selection to self- and super-antigens (Foy et al., 1995; Williams et al., 2002) and a dependency of mTECs on CD40 signaling for their maturation (Akiyama et al., 2008). Therefore, we investigated whether, in addition to these defects, a reduction in thymic B cell numbers also occurred. CD40L−/− mice showed an overall tendency towards reduced thymic numbers when compared with WT controls (WT=112×106 vs. CD40L−/−=57×106 cells; p<0.06). IHC staining showed a reduction in B220 positive cells in the thymus of CD40L−/− mice (Fig. 4A). In addition, there was a 90% reduction in the frequency of thymic but not LN CD19+B220+ cells from CD40L−/− mice when compared with WT mice (Fig. 4B). Reduced B cell numbers in the thymi of CD40L−/− mice were associated with decreased expression of insulin and MOG mRNA (Fig. 4C).
Figure 4.
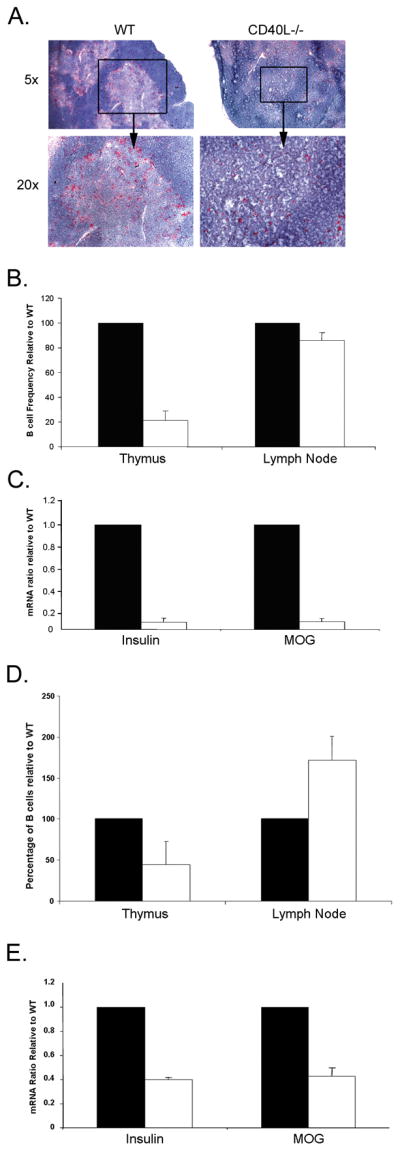
Reduced B cell numbers in CD40L and CCR7 deficient mice is associated with TRA expression. (A) Immunohistochemistry staining for B220 (red) in the thymi of WT and CD40L−/− mice. (B) Frequencies of B220+CD19+ cells as obtained by FACS analysis of LNs and thymi from 4–6 week old WT and CD40L−/− mice. Solid bars-WT; open bars- CD40L−/−. (C) Real-time PCR analysis of TRA and AIRE in thymi of WT (n=3) and CD40L−/− (n=3) mice. Results are expressed relative to WT mice. Solid bars-WT; open bars- CD40L−/−. (D) Frequencies of B220+CD19+ cells as obtained by FACS analysis of LNs and thymi from 4–6 week old WT and CCR7−/− mice. Solid bars-WT; open bars- CCR7−/−. (E) Real-time PCR analysis of TRA and AIRE in thymi of WT (n=3) and CCR7−/− (n=3) mice. Results are expressed relative to WT mice. Solid bars-WT; open bars- CCR7−/−.
CCL19 and CCL21 play important roles in mediating normal T cell selection as CCR7−/− mice show reduced medullae size and impaired single positive T cell development (Ueno et al., 2004). Previous reports demonstrated the ability of activated and memory B cells to respond to CCL19 and CCL21 gradient via their expression of CCR7 (Reif et al., 2002). Therefore, we examined B cell frequency and TRA expression in CCR7−/− mice. Examination of total thymic cell number revealed a significant increase in total thymocytes in CCR7−/− mice when compared with WT controls (WT=112×106 vs. CD40L−/−=206×106 cells; p<0.037). Despite this overall increase in cell number, CCR7−/− mice showed a reduction in the percentage of B cells in the thymus but not lymph nodes (Fig. 4D). In addition, CCR7−/− thymi were associated with a decrease in insulin and MOG expression (Fig. 4E). While the above models hold many developmental and functional defects, these data are consistent with the suggestion that reduced B cell recruitment may further contribute to the drop in insulin and MOG expression in the thymus.
3.4 Consequences of B cell deficiency on autoimmunity
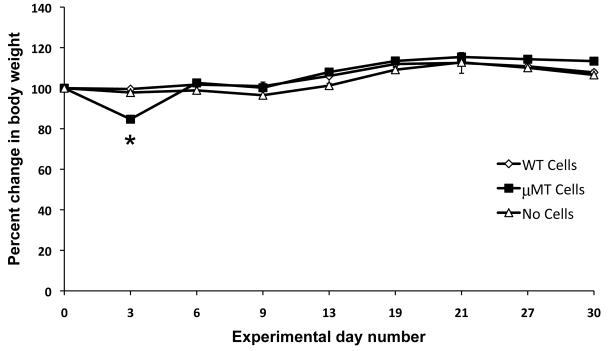
One prediction of the data presented here is that T cells from B cell-deficient mice that have not undergone negative selection due to reduced thymic TRA expression should be more likely to affect autoimmunity. To test this hypothesis, we isolated splenic T cells from WT and μMT mice and injected them into T cell deficient nu/nu mice. Recipient mice were weighed every 3 days and euthanized on day 30 post injection, at which time histological analyses were performed. Recipients of μMT T cells experienced a dramatic, though transient weight loss on day 3 post injection (Figure 5; WT T cells vs. μMT T cells p<0.0001). Body weights were restored to those of control mice (Figure 5). The recipients of WT cells experienced no such fluctuations in weight. Cellular infiltration in the examined organs (skin, LN, pancreas, spleen, small intestine, liver, lung, heart, and brain) was minimal in recipients of WT or μMT mice.
Figure 5.
Adoptive transfer of T cells from μMT but not WT mice induces an acute yet transient body weight loss. Purified T cells from spleens of μMT or WT donors were intraperitonealy injected into T cell deficient nu/nu mice. Animals were weighted every 3 days for a period of 30 days. p<0.0001
4. Discussion
The data presented in this paper demonstrate, for the first time, a role for thymic B cells in mTEC cellularity and in the expression of self-antigens. They show that B cells influence the number of mTECs. In addition, these data identify thymic B cells as a critical source of LT in thymus directly affecting TRA expression. Finally, these data demonstrate a similar decrease in TRA expression in additional mouse models with reduced thymic B cells. These broad effects of B cells on the number of mTECs support previous observations that the predominant cell that produces TRAs is the mTEC.
The apparent disconnect between TRA and AIRE expression noted in our study has been reported by others. Martins et al (Martins et al., 2008) and Venanzi et al (Venanzi et al., 2007) show an architectural defect in LTβR−/− mice, while both Chin et al. and Boehm et al. demonstrated reduced TRAs (Chin et al., 2003) and altered thymic structure (Boehm et al., 2003) in LTα and LTβR deficient mice, respectively. However, neither Boehm et al. nor Seach et al. observed any reduction in AIRE expression in LTβR, LTα, or LTβ deficient mice, respectively (Boehm et al., 2003; Seach et al., 2008). Rossi et al. showed that isolated mTECs from LTα−/− mice exhibit normal AIRE expression though RANKL was required for normal expression of AIRE and the TRAs, salivary protein 1 and 2 (Rossi et al., 2007). These seemingly contradictory results may stem from several factors. While Chin et al. described AIRE and TRA expression in complete thymic extracts, Boehm et al. and Rossi et al. studied isolated mTECs, raising the possibility that an overall reduction in mTEC numbers may be reflected as reduced TRA expression in the whole thymus of LT deficient mice. Our data showed reduced mTEC numbers in both LTα−/−, LTβ−/−, and μMT. Such a reduction ultimately results in an overall reduction in TRA expression in these mice as described here. The effects of reduced B cells on overall mTEC numbers may derive from either increased mTEC death or reduced mTEC proliferation. The normalization of UEA-1+ numbers following B cell reconstitution in μMT mice support these possibilities. Moreover, the fact that the degree of B cell reconstitution in μMT mice was positively correlated with increased thymic expression of insulin and MOG, suggests that the increase in mTEC number following B cell reconstitution results in increased TSA expression. The effect of B cell deficiency on cTEC numbers may derive from the fact that some B cells were detected in the cortex as well as in the medulla. B cells in the cortex may promote cTEC proliferation or prevent cTEC death; however; the exact role of this cell interaction remains to be determined.
There have been surprisingly few studies that investigated the cellular origin of LT in the thymus. One publication indicated that CD4+CD8+ thymocytes produce both LTα and LTβ; other cells, including B cells, were not analyzed (Silva-Santos et al., 2005). Our demonstration of LT production by thymic B cells is a novel observation, though consistent with the known LT production by peripheral blood, splenic, and lymph node B cells (Ware, 2005). In fact, in our study the production of LT by B cells was critical for TRA expression since the deletion of LT in B cells resulted in a similar reduction in TRAs. Thymic B cells may produce additional cytokines of the TNF family, including RANKL, that are also important in TRA expression. Although RANKL is expressed by CD4+CD3− lymphoid tissue inducer cells (Rossi et al., 2007), single positive T cells and γδ+ cells (Hikosaka et al., 2008) in the thymus, its production by thymic B cells has not been studied. The presence of similar defects in μMT and LT deficient mice support the concept that B cells produce LT and that cytokine contributes to the defects in TRA, but not necessarily AIRE expression.
In addition to LT and RANKL, a report showing mTEC dependency on CD40 signaling (Akiyama et al., 2008) suggests that CD40-CD40L acts directly on mTECs, and thus contributes to mTEC development and TRA expression. Our observations showing a reduction in thymic B cell numbers in CD40L−/− mice reveal a previously unappreciated defect in these mice which may further contribute to reduce TRA expression. Similarly to CD40l, CCR7−/− mice show reduced B cell number exclusively in the thymus. CCR7−/− mice show reduced medullae and impaired single positive T cell development (Ueno et al., 2004), and it has been suggested that reduced expression of TRAs in the thymus of CCR7−/− mice could result from a defect in DP to SP transition in migration from the cortex to the medulla (Kurobe et al., 2006). Activated and memory B cells respond to a CCL19 and CCL21 gradient via their expression of CCR7 (Reif et al., 2002), making it possible that reduced CCL21 and CCL19 can also affect B cell recruitment to the thymus, thus accounting for the reduced B cell numbers in CCR7 deficient mice. In addition the production of LT by thymic B cells may further potentiate the expression of CCL19 and CCL21 in the thymus allowing for appropriate T cell and DC positioning in the thymus, as we have previously shown that LT production results in expression of lymphoid chemokines (Drayton et al., 2003; Hjelmstrom et al., 2000) and LT elimination results in reduced CCL19 and CCL21 expression in the spleen (Ngo et al., 1999). Our data showing a reduction in thymic B cell numbers in the thymi of CCR7−/− mice highlight once again the critical role of these cells in mediating normal TRA expression. While the above models hold many developmental and functional defects, they suggest that reduced B cell recruitment may further exacerbate the drop in insulin and MOG expression in the thymus. Additional studies examining TRA expression and B cell numbers in mice deficient in CD40L and CCR7 exclusively in B cells would shed new light on the role of these two molecules in controlling B and mTEC numbers in the thymus.
The data presented here predict that an absence of LT or B cells would result in an increase in autoimmunity. Increased cellular infiltration in several organs was previously reported (Chin et al., 2003). The data showing an acute weight loss in recipient of μMT but not WT T cells is consistent with this prediction. There are several possible explanations to account for the absence of further manifestations of autoimmunity in these mice such as the presence of Tregs in the preparations. Additional experiments will address these possibilities.
In summary, the findings presented here demonstrate a novel and unappreciated interaction between thymic B cells and mTECs and offer new insight into the mechanisms that control TRA expression. This appears to be mediated in part by LT, which is produced by B cells. This contribution to overall LT levels may play a role in mediating normal thymic structure via the production of thymic chemokines. Normal B cell numbers are dependent on CD40-CD40L and CCR7-CCL21/19 interactions, which when absent can lead to reduced B cell numbers and impaired TRA expression. The delicate balance between thymic B cells and their interactions with the thymic epithelium emphasize the importance of quantitatively minor cell subsets in mediating normal TRA expression and their putative roles in the process of negative selection. Additional studies examining the implication of TRA loss and its effect on T cell repertoire in the context of specific thymic B cell loss may provide additional insight into the role of these cells in controlling autoimmunity.
Supplementary Material
Restoration of B cells in peripheral blood following BM reconstitution results in increased UEA-1+ cells in the thymux. (A) Irradiated μMT mice received 107 BM cells from WT or μMT donors. Peripheral blood was collected every two weeks over a period of 60 days and analyzed for CD19+B220+ cells. Efficacy of B cell restoration is expressed as a ratio relative to B cell percentages in unmanipulated WT thymus. Diamonds WT → μMT; Squares μMT → μMT mice. (B) Histomorphic analysis of UEA-1+ cells in the thymic medullae of WT and WT-BM receipients μMT mice.
Acknowledgments
We thank Myriam Hill, Cheryl Bergman, and Rawad Mounzer for their assistance, and Alexei Tumanov and Yan-Xin Fu (University of Chicago) for the generous gift of B LTβ−/− mice. This work was supported by the National Multiple Sclerosis Society Grants NMSS RG2394, CA 1009A10, and RG 4126-A-7, and NIH R01 CA 16885.
Abbreviations used
- AIRE
autoimmune regulator
- LT
lymphotoxin
- LTβR
lymphotoxin β receptor
- MOG
myelin oligodendrocyte glycoprotein
- mTEC
medullary thymic epithelial cell
- TRA
tissue restricted antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Andreu-Sanchez JL, Faro J, Alonso JM, Paige CJ, Martinez C, Marcos MA. Ontogenic characterization of thymic B lymphocytes. Analysis in different mouse strains. Eur J Immunol. 1990;20:1767–1773. doi: 10.1002/eji.1830200822. [DOI] [PubMed] [Google Scholar]
- Beutner U, Kraus E, Kitamura D, Rajewsky K, Huber BT. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med. 1994;179:1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- Chin RK, Zhu M, Christiansen PA, Liu W, Ware C, Peltonen L, Zhang X, Guo L, Han S, Zheng B, Fu YX. Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. J Immunol. 2006;177:290–297. doi: 10.4049/jimmunol.177.1.290. [DOI] [PubMed] [Google Scholar]
- Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero I, Anjuere F, Martin P, Martinez del Hoyo G, Fraga ML, Wright N, Varona R, Marquez G, Ardavin C. Functional and phenotypic analysis of thymic B cells: role in the induction of T cell negative selection. Eur J Immunol. 1999;29:1598–1609. doi: 10.1002/(SICI)1521-4141(199905)29:05<1598::AID-IMMU1598>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Feyerabend TB, Terszowski G, Tietz A, Blum C, Luche H, Gossler A, Gale NW, Radtke F, Fehling HJ, Rodewald HR. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Foy TM, Page DM, Waldschmidt TJ, Schoneveld A, Laman JD, Masters SR, Tygrett L, Ledbetter JA, Aruffo A, Claassen E, Xu JC, Flavell RA, Oehen S, Hedrick SM, Noelle RJ. An essential role for gp39, the ligand for CD40, in thymic selection. J Exp Med. 1995;182:1377–1388. doi: 10.1084/jem.182.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DH, Chidgey AP, Boyd RL. Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
- Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juedes AE, Hjelmstrom P, Bergman CM, Neild AL, Ruddle NH. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2000;164:419–426. doi: 10.4049/jimmunol.164.1.419. [DOI] [PubMed] [Google Scholar]
- Juedes AE, Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- Kleindienst P, Chretien I, Winkler T, Brocker T. Functional comparison of thymic B cells and dendritic cells in vivo. Blood. 2000;95:2610–2616. [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta in lymphotoxi-beta deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, Takahama Y. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, Sakaguchi S, Ueno T, Takahama Y, Uchida D, Sun S, Kajiura F, Mouri Y, Han H, Matsushima A, Yamada G, Matsumoto M. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- Kyewski B, Derbinski J. Self-representation in the thymus: an extended view. Nat Rev Immunol. 2004;4:688–698. doi: 10.1038/nri1436. [DOI] [PubMed] [Google Scholar]
- Martins VC, Boehm T, Bleul CC. Ltbetar signaling does not regulate Aire-dependent transcripts in medullary thymic epithelial cells. J Immunol. 2008;181:400–407. doi: 10.4049/jimmunol.181.1.400. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- Mori S, Inaba M, Sugihara A, Taketani S, Doi H, Fukuba Y, Yamamoto Y, Adachi Y, Inaba K, Fukuhara S, Ikehara S. Presence of B cell progenitors in the thymus. J Immunol. 1997;158:4193–4199. [PubMed] [Google Scholar]
- Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJ, Anderson G. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, Scott HS, Ware CF, Chidgey AP, Gray DH, Boyd RL. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol. 2008;180:5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of gammadelta cell differentiation by alphabeta T cell progenitors. Science. 2005;307:925–928. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Tullin S, Farris P, Petersen JS, Hornum L, Jackerott M, Markholst H. A pronounced thymic B cell deficiency in the spontaneously diabetic BB rat. J Immunol. 1997;158:5554–5559. [PubMed] [Google Scholar]
- Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venanzi ES, Gray DH, Benoist C, Mathis D. Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J Immunol. 2007;179:5693–5700. doi: 10.4049/jimmunol.179.9.5693. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Williams JA, Sharrow SO, Adams AJ, Hodes RJ. CD40 ligand functions non-cell autonomously to promote deletion of self-reactive thymocytes. J Immunol. 2002;168:2759–2765. doi: 10.4049/jimmunol.168.6.2759. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chin RK, Tumanov AV, Liu X, Fu YX. Lymphotoxin beta receptor is required for the migration and selection of autoreactive T cells in thymic medulla. J Immunol. 2007;179:8069–8075. doi: 10.4049/jimmunol.179.12.8069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Restoration of B cells in peripheral blood following BM reconstitution results in increased UEA-1+ cells in the thymux. (A) Irradiated μMT mice received 107 BM cells from WT or μMT donors. Peripheral blood was collected every two weeks over a period of 60 days and analyzed for CD19+B220+ cells. Efficacy of B cell restoration is expressed as a ratio relative to B cell percentages in unmanipulated WT thymus. Diamonds WT → μMT; Squares μMT → μMT mice. (B) Histomorphic analysis of UEA-1+ cells in the thymic medullae of WT and WT-BM receipients μMT mice.