Abstract
A 44-year-old woman who is a new patient has no known current health problems and no family history of breast or ovarian cancer. Eighteen months ago, she had a normal screening mammogram. She recently read that mammograms may not help to prevent death from breast cancer and that “the patient should decide.” But she does not think she knows enough. She worries that there is a breast-cancer epidemic. What should her physician advise?
THE CLINICAL PROBLEM
In 1990, for the first time in 25 years, mortality from breast cancer in the United States began dropping; by 1999, the age-adjusted mortality rate was at its lowest level (27.0 per 100,000 population) since 1973.1 Meanwhile, by 1997, 71 percent of women in the United States who were 40 years of age or older reported having undergone mammography during the previous two years — an increase from 54 percent in 1989.2 Ironically, just as screening (or better treatment or both) seemed to be lowering mortality from breast cancer nationally, questions were raised about the validity of the studies that had led to widespread screening. For more than two decades, expert groups uniformly agreed that screening mammography reduces mortality from breast cancer among women in their 50s and 60s, even though they disagreed about other age groups.3 However, questions were raised in 2000, when two Danish investigators concluded that only three of eight randomized trials were of sufficient quality to determine the effectiveness of mammography and that the combined results of these three trials showed no benefit. This report led to confusion about the usefulness of screening mammography.
STRATEGIES, EVIDENCE, AND AREAS OF UNCERTAINTY
Women are interested in knowing about breast cancer and want information from their doctors.4,5 When women and their physicians are making decisions about screening, they need information about the underlying risk of the condition being screened for, the effectiveness of the procedure in preventing an untoward outcome such as death, and the potential ill effects of screening, such as false positive tests. (For policymakers and payers, cost effectiveness is an important factor in decisions about the allocation of finite resources.) Clinical information about each of these issues with regard to breast cancer and mammography is summarized below.
THE RISK OF DEVELOPMENT OF AND DEATH FROM BREAST CANCER
The average 10-year risk of the development of and death from breast cancer is shown in Table 1, along with the 10-year risk of death from any cause (in order to provide context).6,7 A computerized tool for calculating an individual woman's risk of breast cancer, the Breast Cancer Risk Assessment Tool (available at http://bcra.nci.nih.gov/brc/), can be used to calculate the five-year risk and the lifetime risk. The tool uses the woman's age, history of first-degree relatives with breast cancer (up to two relatives), number of previous breast biopsies (and whether any revealed atypical hyperplasia), age at menarche, and age at first delivery. It assumes regular screening and no history of breast cancer and does not include several known risk factors and several known protective factors (see Supplementary Appendix 1, available with the full text of this article at http://www.nejm.org).8 Overall, the tool has been found to predict breast cancer well, but its ability to discriminate at the individual level was not much better than that of predictions that would have occurred by chance,9 so its usefulness is similar to that of Table 1. For women with a strong family history of breast cancer, ovarian cancer, or both, a program that can be used to estimate the risk of genetic mutations in the BRCA1 and BRCA2 genes is available at http://astor.som.jhmi.edu/brcapro/. The program has been found to be effective in predicting risk on an individual level.10
Table 1.
Chances of the Development of and Death from Breast Cancer within the Next 10 Years.*
| Age | Cases of Invasive Breast Cancer | Death from Breast Cancer | Death from Any Cause |
|---|---|---|---|
| no./1000 women | |||
| 40 Yr | 15 | 2 | 21 |
| 50 Yr | 28 | 5 | 55 |
| 60 Yr | 37 | 7 | 126 |
| 70 Yr | 43 | 9 | 309 |
| 80 Yr | 35 | 11 | 670 |
MAMMOGRAPHY AND MORTALITY FROM BREAST CANCER
There have been eight randomized trials of the effectiveness of mammography: four trials in Sweden comparing mammography with no screening; one in Edinburgh, Scotland, one in New York, and one in Canada comparing the combination of mammography and clinical breast examination with no screening; and one in Canada evaluating the effect of the addition of mammography to a standardized, 10-to-15-minute clinical breast examination. The studies differed with respect to the years in which they were conducted, the type of mammography used, the interval between mammographic examinations, the method of assigning women to the screened and unscreened groups, the number of screening visits, the age of the women who were included, and the methods of analysis.3,11 For women between 50 and 69 years of age, all reports of studies comparing screening with no screening showed protective effects of screening, and meta-analyses that included all trials demonstrated statistically significant 20 to 35 percent reductions in mortality from breast cancer.
A widely cited meta-analysis published in 200012 (updated in 200113) by Gotzsche and Olsen raised questions about the efficacy of mammography. The authors concluded that the methods used in five of the eight studies were so flawed that they had to be excluded from the meta-analysis. Appropriate randomization should lead to very similar groups, but for five of the studies (and part of a sixth, the Malmö II Trial), there were significant differences between the screened group and the control group in some of the characteristics; Gotzsche and Olsen suggested that these differences might bias the trial results. Also, numbers varied among different reports on the same trials. Finally, according to a combined analysis of the four Swedish studies, mortality from breast cancer, but not overall mortality, decreased in the screened group, raising the possibility of bias in determining the cause of death, as well as the possibility that treatments resulting from findings on screening could be dangerous. A meta-analysis of the remaining three studies showed no protective effect of mammography.
The investigators defended their trials. Several trials included some subjects who were later determined to be ineligible, and reports sometimes used the woman's age instead of the date of birth, accounting for differing numbers. The cluster randomization that was used in several trials probably led to small, unimportant base-line differences between groups.14,15 In an updated analysis of the four Swedish studies published after the critique, unadjusted overall mortality was lower in the screened group (relative risk, 0.98 [95 percent confidence interval, 0.96 to 1.00]).16 (Detailed responses to the criticisms of Gotzsche and Olsen are reviewed in Supplementary Appendix 2, available with the full text of this article at http://www.nejm.org.) In addition, Gotzsche and Olsen were criticized for not considering other methodologic aspects, such as the age of participants (one of the trials included only women in their 40s); the number, type, and quality of screenings and the intervals between them; compliance with the assigned strategy; and contamination (the degree to which women in control groups underwent screening mammography).14,15,17,18 Finally, they included a study that compared two methods of screening19 and had no unscreened control group.
In summary, criticisms of all but one of the trials excluded from the meta-analysis have been answered. In-depth independent reviews of the criticisms concluded that they do not negate the effectiveness of mammography, especially for women older than 50 years of age.3,18,20
WOMEN IN THEIR 40S
For many years, there has been controversy over the use of screening mammography for women in their 40s.21 In general, the effect of screening younger women has been slower to appear and less dramatic than the effect among women older than 50 years of age. These differences may result from mammographically denser breasts in younger women (leading to reduced sensitivity of mammography), faster spread of some cancers in younger women, or both. Meta-analyses show that screening in this age group decreased 15-year mortality from breast cancer by about 20 percent.3,22
Because trial results are presented according to women's ages at the time of entry into the studies, some women who entered in their late 40s received a diagnosis of breast cancer in their 50s; therefore, some of the benefit ascribed to the screening of women in their 40s would have occurred if the women had waited until 50 years of age to be screened.23,24 Also, although analyses are usually presented according to the decade of life, it is likely that a gradual change occurs as a woman ages. The latest analysis of the four Swedish trials — the first to examine screening effects according to five-year age increments — found that screening was most effective after 55 years of age.16
MAMMOGRAPHY IN WOMEN OLDER THAN 70 YEARS OF AGE
Too few women older than 70 years of age participated in randomized trials to permit conclusions to be drawn about the effects of mammographic screening in this age group. One case–control study in the Netherlands found that screening women between 65 and 74 years of age led to a 55 percent decrease in mortality from breast cancer (relative risk of death from breast cancer, 0.45 [95 percent confidence interval, 0.20 to 1.02]).25
RISKS ASSOCIATED WITH MAMMOGRAPHY
False Positive Mammograms
Because most women do not have breast cancer at the time of screening, there is potential to do harm with false positive results that necessitate further investigation before a woman can be declared to be free of disease. Nationally, an average of 11 percent of screening mammograms are read as abnormal and necessitate further diagnostic evaluation26; breast cancer is found in about 3 percent of women with an abnormal mammogram (representing 0.3 percent of all mammograms). Therefore, on average, a woman has about a 10.7 percent chance of a false positive result with each mammogram. Because women are screened repeatedly, a woman's risk of having a false positive mammogram increases over time. One study estimated that after 10 mammograms, about half of women (49 percent [95 percent confidence interval, 40 to 64]) will have had a false positive result, which will have led to a needle biopsy or an open biopsy in 19 percent (95 percent confidence interval, 10 to 41).27
False positive mammograms increase patients’ anxiety; the degree of anxiety is related to the intensity of the additional diagnostic procedures and the recency of the screening mammogram.20 One study found that in the 12 months after a false positive mammogram, women initiated more health care visits for both breast-related and non–breast-related problems.28 However, false positive mammograms increase women's adherence to further screening.29-31
The risk of a false positive mammogram varies according to characteristics of the woman and radiologic factors: a younger age, an increasing number of breast biopsies, a positive family history of breast cancer, estrogen use, an increasing interval between screenings, the lack of comparison with previous mammograms, and a tendency by the radiologist to consider mammograms abnormal (as determined by the percentage of mammograms read as abnormal) were independent risk factors for a false positive result in one study.32 Having mammographically dense breasts also increases the risk of false positive (or false negative) mammograms.33-35 Many characteristics of patients are immutable, but obtaining mammograms during the luteal phase of the menstrual cycle may decrease mammographic breast density.36 Also, a preliminary investigation found that stopping hormone-replacement therapy 10 to 30 days before a repeated mammogram eliminated or reduced mammographic abnormalities.37
Lowering the recall rate (the percentage of mammograms that result in recommendations for further tests) is likely to reduce the risk of false positive mammograms. Because of the trade-off between sensitivity and specificity, it is important not to lower the radiographic threshold for recall so much that cancers are missed. The Agency for Health Care Pol icy and Research recommends that the false positive rate be no more than 10 percent.38 The malpractice climate in this country may work against the lowering of the threshold, since failure to diagnose breast cancer is the leading reason for malpractice suits.39 Comparison of current and previous mammograms decreases the false positive rate, as does the use of screening intervals of 18 months or less.
Possible Overdiagnosis — Ductal Carcinoma in Situ
Ductal carcinoma in situ was a relatively rare diagnosis before the introduction of mammography. In 1973, the incidence in the United States was 2.4 cases per 100,000 women; by 1998, it was 30.7 per 100,000 women, accounting for approximately 14 percent of all breast cancers diagnosed.1 With treatment, the prognosis is excellent. In one study, women given a diagnosis of ductal carcinoma in situ had a 9-year survival rate that was the same as or better than that in the general population,40 and in another study, the risk of death from breast cancer within 10 years after the diagnosis of ductal carcinoma in situ was 1.9 percent.41
Such an excellent prognosis could be attributable to the detection of lesions before they become invasive cancers, which could save lives. However, if ductal carcinoma in situ were the usual precursor to early invasive cancer, the incidence of early-stage invasive breast cancer should decrease as the incidence of in situ cancer increases, but the opposite is happening. Also, autopsy studies in women who died from causes unrelated to breast cancer have shown a substantial “reservoir” of ductal carcinoma in situ in such women.42 Therefore, detection of ductal carcinoma in situ may be an example of overdiagnosis — finding early neoplasms, many of which will never become invasive breast cancer.
Unfortunately, ductal carcinoma in situ can progress to invasive cancer. The eight-year rate of recurrence in one study of treatment with only surgical excision was 27 percent, and half the recurrences were invasive cancers.43 It is not clear who is at risk for recurrence and whether survival results would be the same if surgery were undertaken only after early invasive cancer had been diagnosed. In sum, women who undergo screening mammography are more likely than other women to be given a diagnosis of ductal carcinoma in situ. Whether finding it saves lives or merely increases the number of women who receive a diagnosis of breast cancer is not yet clear.
Other Risks
Many women have pain during mammography, but few report that pain deters them from obtaining subsequent mammograms.44-48 The risks associated with radiation are small. It has been estimated that 10 years’ worth of annual mammographic screenings in 10,000 women will cause one additional breast cancer.49 False negative interpretations are possible and are more common in younger women and in those with dense breasts.50-52
GUIDELINES
Recommendations from several leading groups regarding mammographic screening are summarized in Table 2.3 After the analysis by Gotzsche and Olsen, some, but not all, reconsidered and changed their recommendations. For example, the editorial board of the Physician Data Query data base of the National Cancer Institute (which does not issue recommendations, as such) backed away from concluding that mammography is effective; instead, the board now concludes that mammography “may” decrease mortality.23 The U.S. Preventive Services Task Force moved in the opposite direction and extended its recommendations for the use of screening to include women ranging from 40 years of age to more than 70 years of age.3
Table 2.
North American Recommendations for Routine Mammographic Screening in Women at Average Risk Who Are 40 Years of Age or Older.*
| Group (Date) | Frequency of Screening | Initiation of Screening | ||
|---|---|---|---|---|
| 40–49 Yr of Age | 50–69 Yr of Age | ≥70 Yr of Age | ||
| Government-sponsored and private groups | yr | |||
| U.S. Preventive Services Task Force (2002)† | 1–2 | Yes | Yes | Yes‡ |
| Canadian Task Force on Preventive Health Care (1998, 1999, 2001) | 1–2 | No | Yes | No |
| National Institutes of Health consensus conference (1997) | No§ | — | — | |
| American Cancer Society (1997) | 1 | Yes | Yes | Yes |
| National Cancer Institute (2002) | 1–2 | Yes | Yes | Yes |
| Medical societies | ||||
| American College of Obstetricians and Gynecologists (2000) | 1–2 if 40–49 yr old 1 yr if ≥50 yr old |
Yes | Yes | Yes |
| American Medical Association (1999) | 1 | Yes | Yes | Yes |
| American College of Radiology (1998) | 1 | Yes | Yes | Yes |
| American College of Preventive Medicine (1996) | 1–2 | No¶ | Yes | Yes |
| American Academy of Family Physicians (2001) | 1–2 | No§¶ | Yes | No |
| American Geriatrics Society (1999) | 1–2 | — | — | Yes‡ |
| Advocacy groups | ||||
| National Breast Cancer Coalition (2000) | No | — § | No | |
| National Alliance of Breast Cancer Organizations (2002) | 1 | Yes | Yes | Yes |
| Susan B. Komen Foundation (2002) | 1 | Yes | Yes | Yes |
Adapted from the U.S. Preventive Services Task Force.3 A “no” recommendation may be a statement that there is insufficient evidence for a positive recommendation.
Recommendations are for mammography with or without clinical breast examination.
There is an explicit recommendation to screen women older than 70 years of age.
Recommendations note that women should be counseled about the risks and benefits of mammography.
Recommendations note that women at high risk should be screened beginning at 40 years of age.
Recommendations from expert groups with regard to screening women in their 40s have long varied, but over time, more groups have moved toward endorsing the same approach for this age group as for older women. Most groups have not issued explicit recommendations for women older than 70 years and merely recommend that screening begin at a certain age. More groups have begun calling for shared decision making about breast-cancer screening, but the information to be shared has not been specified.
CONCLUSIONS AND RECOMMENDATIONS
GENERAL CONCLUSIONS
Breast cancer is common, but when viewed over a 10-year period, the risk for the average woman is relatively small. During the past few years, scientific controversy about the benefits of screening mammography has increased. As with most screening tests, there are hazards — primarily, risks of false positive mammograms, with associated anxiety and unnecessary biopsies, and perhaps a risk of overdiagnosis.
When the benefits of medical interventions are controversial and when hazards exist, shared decision making is needed, with the clinician providing facts and the patient assessing her situation from the vantage point of her personal values. In addition, the climate in the United States with regard to malpractice makes discussions between clinician and patient about breast-cancer screening essential for all women beginning at 40 years of age. To save time, information can be provided by handouts and an office practice that is organized to address the concerns of patients.
Women vary in terms of how much they want to participate in decisions about screening. In one survey of women younger than 50 years of age, 49 percent wanted to share in decision making, 44 percent wanted to make the decision themselves, and 7 percent wanted the physician to decide.5 However, 79 percent wanted information from the doctor. Because of varying individual values, and because women have a good deal of fear about breast cancer,53 physicians should be prepared for a decision different from the one they would recommend.
A woman needs some knowledge of her risk of breast cancer and the benefits and hazards of screening — specifically, her risks of the development of and death from breast cancer and her chances of successful treatment with screening and without screening, of having a false positive mammogram or an invasive breast procedure, and of having ductal carcinoma in situ diagnosed. Numerical risks may be best explained with the use of pictures or graphs, with discussion of absolute as well as relative risks (occurring over meaningful periods), and through comparisons with other risks.54
SPECIFIC RECOMMENDATIONS
All women, regardless of age, should be asked whether they have a family history of breast cancer, ovarian cancer, or both (Table 3).55,56 For women without strong family histories, discussions about breast-cancer screening should begin at 40 years of age and continue until life expectancy is less than 10 years. Evidence supporting the usefulness of mammographic screening is strongest for women between 50 and 69 years of age, and screening should be routinely recommended for women in this age group. For women 40 to 49 years of age (such as the patient described in the vignette), shared decision making is especially important, because the absolute benefit of screening is smaller and the risks associated with it are greater. Screening should be routinely discussed, and the patient and clinician should decide together according to the woman's values.
Table 3.
Recommendations Regarding Breast-Cancer Screening in Women.
| Age | Recommendations |
|---|---|
| Any | Ask about family history of breast cancer, ovarian cancer, or both on both maternal and paternal sides. Consider referral or counseling for possible genetic testing if risk of a BRCA1 or BRCA2 gene mutation is at least 10 percent (to calculate risk, see http://astor.som.jhmi.edu/brcapro/) or the patient has one of the following: a first-degree relative with a known deleterious mutation for breast cancer; ≥2 relatives given a diagnosis of breast cancer before 50 yr of age, ≥1 of them a first-degree relative; ≥3 relatives given a diagnosis of breast cancer, ≥1 of them before 50 yr of age; ≥2 relatives given a diagnosis of ovarian cancer; ≥1 relative given a diagnosis of breast cancer and ≥1 relative given a diagnosis of ovarian cancer. |
| 40–70 Yr | Begin discussions about breast-cancer screening at 40 yr of age. Recommend screening mammography every 1–2 yr between 50 and 69 yr of age. Use information on the chances of development of or death from breast cancer within the next 5 yr (as given in the National Cancer Institute Breast Cancer Assessment Tool) or 10 yr.* Also give information on benefits and hazards of mammography.† Emphasize the increasing risk of breast cancer, increasing benefit of screening, and decreased harms associated with screening with increasing age. Record decision about screening in the medical record. |
| >70 Yr | For women with life expectancy of ≥10 yr, consider screening as above, making clear that risks of breast cancer are known but less is known about the benefits and harms of screening. Record decision about screening in the medical record. |
For women who want more information, Table 1, the Breast Cancer Risk Assessment Tool, or both can be used to estimate the individual risk of breast cancer. Women should be reminded that the risk of breast cancer increases with age and that the one-in-eight risk is a lifetime risk for a newborn who lives for 90 years.
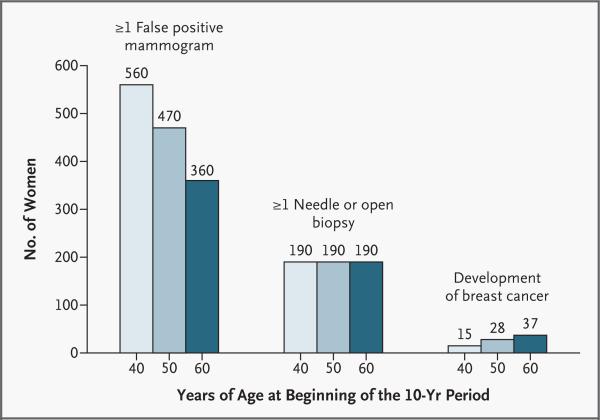
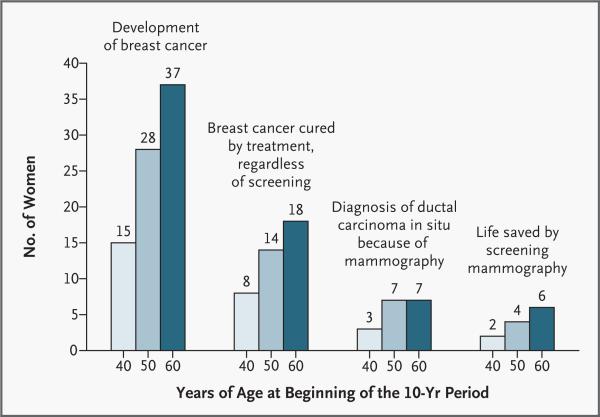
The chances of being helped or harmed by screening mammography are summarized in Figures 1 and 2, which contain information that may be useful to patients. These figures show the chances that yearly screening mammography in women of different ages will result in a false positive mammogram, an invasive breast procedure, or a diagnosis of ductal carcinoma in situ or invasive breast cancer. Women should be made aware that at least half the patients given a diagnosis of breast cancer survive regardless of the use or nonuse of screening — a fact that many women do not understand.57,58 Recently, survival rates have been improving, but how much of this improvement is attributable to treatment itself and how much to earlier diagnosis due to screening are difficult to determine. The number of women “saved” is calculated according to the estimates that screening of women in their 40s reduces mortality from breast cancer by about 20 percent and screening of women in their 50s or 60s reduces it by about 30 percent. It should be emphasized that these numbers may vary, depending on the efficacy of mammography in reducing mortality. Individual women will interpret these numbers differently depending on their own values.
Figure 1. Chances of False Positive Mammograms, Need for Biopsies, and Development of Breast Cancer among 1000 Women Who Undergo Annual Mammography for 10 Years.
All numbers are rounded. The numbers for 10-year rates of false positive mammograms and breast biopsies come from a single study in which, overall, the rate of false positive mammograms was 6.5 percent,27 and the rate may be different in other settings. Data on the development of breast cancer are broken down further in Figure 2.
Figure 2. Chances of Breast-Cancer–Related Outcomes among 1000 Women Who Undergo Annual Mammography for 10 Years.
All numbers are rounded. The numbers for the incidence of invasive breast cancer and ductal carcinoma in situ, as well as the number of women whose lives are saved by treatment (those surviving at least 20 years after the first diagnosis of breast cancer) regardless of screening, were calculated on the basis of data from the Surveillance, Epidemiology, and End Results program.1,57 The numbers of women whose lives are saved because of screening (those surviving at least 15 years after diagnosis) were calculated on the assumption of a reduction of 20 percent in mortality from breast cancer among women 40 to 49 years of age and a reduction of 30 percent among women 50 to 69 years of age; these numbers are approximate.
To decrease the risk of false positive results, patients should be referred to experienced mammographers with recall rates of no more than 10 percent. They should be encouraged to obtain previous mammograms for comparison and should undergo screening more frequently than every 18 months.
Women often are unaware of the difference between screening and diagnostic examinations to evaluate a breast symptom or abnormal finding. In one study, cancer was diagnosed in about 10 percent of women older than 40 years of age who reported a breast mass and in almost 5 percent of those with any breast-related problem.59 Clinicians and women should not be falsely reassured by a previously normal screening mammogram in the case of a new breast-related problem.
Footnotes
This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the authors’ clinical recommendations.
references
- 1.Ries LAG, Eisner MP, Kosary CL, et al., editors. SEER cancer statistics review, 1973-1999. National Cancer Institute; Bethesda, Md.: 2002. [April 1, 2003]. (at http://seer.cancer.gov/csr/1973_1999/.) [Google Scholar]
- 2.Blackman DK, Bennett EM, Miller DS. Trends in self-reported use of mammograms (1989–1997) and Papanicolaou tests (1991–1997) —behavioral risk factor surveillance system. MMWR CDC Surveill Summ. 1999;48(SS-6):1–22. [PubMed] [Google Scholar]
- 3.Preventive Services Task Force Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137:344–6. doi: 10.7326/0003-4819-137-5_part_1-200209030-00011. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JD, Meishcke H. Differences in evaluations of communication channels for cancer-related information. J Behav Med. 1992;15:429–45. doi: 10.1007/BF00844940. [DOI] [PubMed] [Google Scholar]
- 5.Kinsinger L, Harris R, Karnitschnig J. Interest in decision-making about breast cancer screening in younger women. J Gen Intern Med. 1998;13(Suppl):98. abstract. [Google Scholar]
- 6.Feuer EJ, Wun LM. DevCan: probability of developing or dying of cancer. 4.1. National Cancer Institute; Bethesda, Md.: 1999. [Google Scholar]
- 7.Anderson RN, DeTurk PB. United States life tables, 1999. National vital statistics reports. 6. Vol. 50. National Center for Health Statistics; Hyattsville, Md.: 2002. (DHHS publication no. (PHS) 2002-1120 02-0141.) [PubMed] [Google Scholar]
- 8.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–85. doi: 10.1056/NEJM200101253440407. [Erratum, N Engl J Med 2001;344: 1804.] [DOI] [PubMed] [Google Scholar]
- 9.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93:358–66. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 10.Berry DA, Iversen ES, Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20:2701–12. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S. Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst. 1993;85:1644–56. doi: 10.1093/jnci/85.20.1644. [DOI] [PubMed] [Google Scholar]
- 12.Gotzsche PC, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet. 2000;355:129–34. doi: 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- 13.Olsen O, Gotzsche PC. Cochrane review on screening for breast cancer with mammography. Lancet. 2001;358:1340–2. doi: 10.1016/S0140-6736(01)06449-2. [DOI] [PubMed] [Google Scholar]
- 14.Screening mammography re-evaluated. Lancet. 2000;355:747–8, 752. doi: 10.1016/S0140-6736(05)72152-8. [DOI] [PubMed] [Google Scholar]
- 15.Screening for breast cancer with mammography. Lancet. 2001;358:2164–8. doi: 10.1016/s0140-6736(01)07190-2. [DOI] [PubMed] [Google Scholar]
- 16.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359:909–19. doi: 10.1016/S0140-6736(02)08020-0. [Erratum, Lancet 2002;360:724.] [DOI] [PubMed] [Google Scholar]
- 17.Basinski A. Commentary on “Review: High-quality studies do not show reductions in breast cancer mortality for women screened with mammography.”. ACP J Club. 2000;133:67. [Comment on: Gotzsche PC, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet 2000; 355:129-34.]
- 18.The benefit of population screening for breast cancer with mammography. Health Council of the Netherlands; The Hague, the Netherlands: 2002. (Publication no. 2002/03E.) [Google Scholar]
- 19.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study. 2. Breast cancer detection and death rates among women 50 to 59 years. CMAJ. 1992;147:1477–88. [Erratum, CMAJ 1993;148: 718.] [PMC free article] [PubMed] [Google Scholar]
- 20.Vainio H, Bianchini F. Breast cancer screening. IARC Press; Lyons, France: 2002. [Google Scholar]
- 21.Breast cancer screening for women ages 40-49. 1. Vol. 15. National Institutes of Health Consensus Development Program; Bethesda, Md.: 1997. [April 1, 2003]. (at http://consensus.nih.gov/cons/103/103_intro.htm.) [Google Scholar]
- 22.Berry DA. Benefits and risks of screening mammography for women in their forties: a statistical appraisal. J Natl Cancer Inst. 1998;90:1431–9. doi: 10.1093/jnci/90.19.1431. [DOI] [PubMed] [Google Scholar]
- 23.Breast Cancer (PDQ): screening. National Cancer Institute; Bethesda, Md.: 2003. [April 1, 2003]. (at http://www.cancer.gov/cancerinfo/pdq/screening/ breast/.) [Google Scholar]
- 24.Fletcher SW. Journal of the National Cancer Institute Monographs. 22. Vol. 22. National Cancer Institute; Bethesda, Md.: 1997. Breast cancer screening among women in their forties: an overview of the issues. In: National Institutes of Health Consensus Conference on breast cancer screening for women ages 40–49. pp. 5–9. [DOI] [PubMed] [Google Scholar]
- 25.van Dijck JA, Verbeek AL, Beex L, et al. Mammographic screening after the age of 65 years: evidence for a reduction in breast cancer mortality. Int J Cancer. 1996;66:727–31. doi: 10.1002/(SICI)1097-0215(19960611)66:6<727::AID-IJC3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Brown ML, Houn F, Sickles EA, Kessler LG. Screening mammography in community practice: positive predictive value of abnormal findings and yield of follow-up diagnostic procedures. AJR Am J Roentgenol. 1995;165:1373–7. doi: 10.2214/ajr.165.6.7484568. [DOI] [PubMed] [Google Scholar]
- 27.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089–96. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]
- 28.Barton MB, Moore SM, Polk S, Shtat-land E, Elmore JG, Fletcher SW. Increased patient concern after false-positive mammo-grams: clinician documentation and subsequent ambulatory visits. J Gen Intern Med. 2001;16:150–6. doi: 10.1111/j.1525-1497.2001.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burman ML, Taplin SH, Herta DF, Elmore JG. Effect of false-positive mammograms on interval breast cancer screening in a health maintenance organization. Ann Intern Med. 1999;131:1–6. doi: 10.7326/0003-4819-131-1-199907060-00002. [DOI] [PubMed] [Google Scholar]
- 30.Pisano ED, Earp JA, Schell M, Vokaty K, Denham A. Screening behavior of women after a false-positive mammogram. Radiology. 1998;208:245–9. doi: 10.1148/radiology.208.1.9646820. [DOI] [PubMed] [Google Scholar]
- 31.Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med. 1991;114:657–61. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen CL, Wang F, Barton MB, et al. Predicting the cumulative risk of false-positive mammograms. J Natl Cancer Inst. 2000;92:1657–66. doi: 10.1093/jnci/92.20.1657. [DOI] [PubMed] [Google Scholar]
- 33.Fajardo LL, Hillman BJ, Frey C. Correlation between breast parenchymal patterns and mammographers’ certainty of diagnosis. Invest Radiol. 1988;23:505–8. doi: 10.1097/00004424-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Laya MB, Gallagher JC, Schreiman JS, Larson EB, Watson P, Weinstein L. Effect of postmenopausal hormonal replacement therapy on mammographic density and parenchymal pattern. Radiology. 1995;196:433–7. doi: 10.1148/radiology.196.2.7617857. [DOI] [PubMed] [Google Scholar]
- 35.Laya MB, Larson EB, Taplin SH, White E. Effect of estrogen replacement therapy on the specificity and sensitivity of screening mammography. J Natl Cancer Inst. 1996;88:643–9. doi: 10.1093/jnci/88.10.643. [DOI] [PubMed] [Google Scholar]
- 36.White E, Velentgas P, Mandelson MT, et al. Variation in mammographic breast density by time in menstrual cycle among women aged 40-49 years. J Natl Cancer Inst. 1998;90:906–10. doi: 10.1093/jnci/90.12.906. [DOI] [PubMed] [Google Scholar]
- 37.Harvey JA, Pinkerton JV, Herman CR. Short-term cessation of hormone replacement therapy and improvement of mammographic specificity. J Natl Cancer Inst. 1997;89:1623–5. doi: 10.1093/jnci/89.21.1623. [DOI] [PubMed] [Google Scholar]
- 38.Bassett LW, Hendrick RE, Bassford TL, et al. Clinical practice guideline. 13. Agency for Health Care Policy and Research; Rockville, Md.: Oct, 1994. Quality determinants of mammography. (AHCPR publication no. 95-0632.) [Google Scholar]
- 39.Breast cancer study . Physician Insurers Association of America; Washington, D.C.: Jun, 1995. [Google Scholar]
- 40.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–8. [PubMed] [Google Scholar]
- 41.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based Surveillance, Epidemiology and End Results program. Arch Intern Med. 2000;160:953–8. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 42.Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med. 1997;127:1023–8. doi: 10.7326/0003-4819-127-11-199712010-00014. [DOI] [PubMed] [Google Scholar]
- 43.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–6. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 44.Andrews FJ. Pain during mammography: implications for breast screening programmes. Australas Radiol. 2001;45:113–7. doi: 10.1046/j.1440-1673.2001.00889.x. [DOI] [PubMed] [Google Scholar]
- 45.Kemmers-Gels ME, Groenendijk RP, van den Heuvel JH, Boetes C, Peer PG, Wobbes TH. Pain experienced by women attending breast cancer screening. Breast Cancer Res Treat. 2000;60:235–40. doi: 10.1023/a:1006457520996. [DOI] [PubMed] [Google Scholar]
- 46.Dullum JR, Lewis EC, Mayer JA. Rates and correlates of discomfort associated with mammography. Radiology. 2000;214:547–52. doi: 10.1148/radiology.214.2.r00fe23547. [DOI] [PubMed] [Google Scholar]
- 47.Kornguth PJ, Keefe FJ, Conaway MR. Pain during mammography: characteristics and relationship to demographic and medical variables. Pain. 1996;66:187–94. doi: 10.1016/0304-3959(96)03057-6. [DOI] [PubMed] [Google Scholar]
- 48.Rutter DR, Calnan M, Vaile MS, Field S, Wade KA. Discomfort and pain during mammography: description, prediction, and prevention. BMJ. 1992;305:443–5. doi: 10.1136/bmj.305.6851.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feig SA. Assessment of radiation risk from screening mammography. Cancer. 1996;77:818–22. [PubMed] [Google Scholar]
- 50.Ma L, Fishell EK, Wright B, Hanna W, Allan S, Boyd NF. Case-control study of factors associated with failure to detect breast cancer by mammography. J Natl Cancer Inst. 1992;84:781–5. doi: 10.1093/jnci/84.10.781. [DOI] [PubMed] [Google Scholar]
- 51.Holland R, Hendriks JH, Mravunac M. Mammographically occult breast cancer: a pathologic and radiologic study. Cancer. 1983;52:1810–9. doi: 10.1002/1097-0142(19831115)52:10<1810::aid-cncr2820521009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 52.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA. 1996;276:33–8. [PubMed] [Google Scholar]
- 53.Black WC, Nease RF, Jr, Tosteson ANA. Perceptions of breast cancer risk and screening effectiveness in women younger than 50 years of age. J Natl Cancer Inst. 1995;87:720–31. doi: 10.1093/jnci/87.10.720. [DOI] [PubMed] [Google Scholar]
- 54.Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827–30. doi: 10.1136/bmj.324.7341.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, adopted on February 20, 1996. J Clin Oncol. 1996;14:1730–6. doi: 10.1200/JCO.1996.14.5.1730. [DOI] [PubMed] [Google Scholar]
- 56.Guideline NGC-0461. Kaiser Permanente; Rockville, Md.: 1998. BRCA1 genetic screening. [Google Scholar]
- 57.Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK, editors. SEER cancer statistics review, 1973-1994. National Cancer Institute; Bethesda, Md.: 1997. (NIH publication no. 97-2789.) [Google Scholar]
- 58.Silverman E, Woloshin S, Schwartz LM, Byram SJ, Welch HG, Fischhoff B. Women's views on breast cancer risk and screening mammography: a qualitative interview study. Med Decis Making. 2001;21:231–40. doi: 10.1177/0272989X0102100308. [DOI] [PubMed] [Google Scholar]
- 59.Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med. 1999;130:651–7. doi: 10.7326/0003-4819-130-8-199904200-00005. [DOI] [PubMed] [Google Scholar]