Production of auto-antibodies against GCP-2/CXCL6, GMCSF, IL-17F, IL-17E/IL-25, TGF-β1 and MMP-9/gelatinase B.
Keywords: vaccine, interleukin, chemokine, cross-linking, antigen
Abstract
Anticytokine auto-vaccination is a powerful tool for the study of cytokine functions in vivo but has remained rather esoteric as a result of numerous technical difficulties. We here describe a two-step procedure based on the use of OVA multimers purified by size exclusion chromatography after incubation with glutaraldehyde at pH 6. When such polymers are incubated with a target protein at pH 8.5 to deprotonate reactive amines, complexes are formed that confer immunogenicity to self-antigens. The chemokine GCP-2/CXCL6, the cytokines GM-CSF, IL-17F, IL-17E/IL-25, IL-27, and TGF-β1, and the MMP-9/gelatinase B are discussed as examples. mAb, derived from such immunized mice, have obvious advantages for in vivo studies of the target proteins. Using a mAb against GCP-2, obtained by the method described here, we provide the first demonstration of the major role played by this chemokine in rapid neutrophil mobilization after Leishmania major infection. Pre-activated OVA multimers reactive with amine residues thus provide an efficient carrier for auto-vaccination against 9–90 kDa autologous proteins.
Introduction
Anticytokine auto-vaccination opens many perspectives for the study and modulation of cytokine function in vivo, as reviewed recently by Ratsimandresy et al. [1]. The procedure is based on the fact that self-cytokines, linked chemically to a nonself protein [2] or genetically associated to a defined foreign sequence [3] or viral-like particles [4], become immunogenic. The proposed rationale underlying this reaction is that the self-reactive B cell, which has captured the complex or fusion protein, will present foreign peptides on its MHC class II membrane proteins and thus, attract help from T cells reactive with the nonself structure [5]. Examples of successful application of this approach include TNF-α [3], IL-1α and IL-1β [6], IL-5 [7], IL-9 [8], IL-12 [9], IL-17A [10], VEGF [11], and IFN-α [12].
We recently noted a positive correlation between immunogenicity and immunogen size in a series of anti-IL-12–OVA vaccines that were fractionated according to size (unpublished results). This raises a dilemma, as the larger the complex, the greater the risk for structural alterations of the antigen.
To circumvent this problem, we tried a two-step procedure. We first made large OVA multimers by treating OVA with glutaraldehyde and after purifying the polymerized products by size exclusion chromatography, reacted these with the target cytokine before saturating remaining glutaraldehyde sites with PADRE [13] to maximize immunogenicity. Here, we show the efficacy of this procedure for mouse auto-vaccination against seven proteins involved in the control of immune and inflammatory responses. Using this methodology, we obtained mouse anti-mouse GCP-2 mAb that demonstrate the crucial role of GCP-2 in early neutrophil mobilization following Leishmania major infection.
MATERIALS AND METHODS
Reagents and mice
All vaccinations were performed in C57BL/6 mice maintained under specific pathogen-free conditions at our animal facility (Ludwig Institute for Cancer Research, Brussels Branch, Brussels, Belgium) under the supervision of Dr. Guy Warnier. The experimental protocol and animal handling were approved by the ethical committee of the Faculty of Medicine, Université Catholique de Louvain. The experimental procedures with L. major-infected mice were approved by the veterinary office regulation of the state of Vaud, Switzerland (Authorization Numbers 1266-4). IL-17A−/− mice on a C57BL/6 background were derived by Dr. Yoichiro Iwakura (University of Tokyo, Japan) [14].
Mouse IL-17F was from eBioscience (San Diego, CA, USA), mouse IL-17E/IL-25, IL-27 p28, and EBI3 from R&D Systems (Abingdon, UK) and Shenandoah Biotech (Warwick, PA, USA), and mouse GM-CSF from BioLegend (San Diego, CA, USA). Human TGF-β1, which differs from the mouse mature protein by a single amino acid (A in man and S in mouse at position 354 [15]), was from R&D Systems or was produced and purified by Dr. Peter Sun (Structural Immunology Section, Lab of Immunogenetics, National Institute of Allergy and Infectious Diseases/National Institutes of Health, Bethesda, MD, USA). Preparation of mouse rGCP-2/CXCL6 [16] and mouse MMP-9 [17] was as described. The GCP-2 form used was GCP-2(9–78).
Activated carrier production
OVA (Product A2512, Sigma-Aldrich, St. Louis, MO, USA), at a concentration of 0.22 mM, was polymerized by overnight incubation with 20 mM glutaraldehyde in 50 mM potassium phosphate buffer, pH 6, at 4°C. After dialysis against the same buffer, the soluble product was fractionated on a Superose 12 size exclusion column (GE Healthcare, Diegem, Belgium), equilibrated at pH 6 in 50 mM phosphate buffer. Large size products (>1000 kD), hereafter designated OVAglu, were collected and frozen at –80°C in aliquots.
Coupling of cytokines with OVAglu was performed by incubation with stirring at 4°C with an equimolar amount of target protein at pH 8.5 in 0.1 M carbonate buffer to deprotonate amino groups of the cytokine and allow their reaction with free glutaraldehyde components. After a 6- to 12-h incubation, a tenfold molar excess of PADRE peptide (aKXVAAWTLKAAC) [13] was added, and the mixture was incubated further overnight before dialysis against 0.1 M glycine buffer, pH 5.8.
Immunizations
Immunizations were performed by four to five biweekly s.c. injections into the footpads of 2–5 μg complexes emulsified in GERBU100 adjuvant, according to the instructions of the supplier (GERBU Biochemicals, Gaiberg, Germany). Mice were bled 2 weeks after the last boost. After a 2- to 6-week rest, a combined i.v. and i.p. booster with 2–5 μg complexes was given for mAb production.
Antibody screening by ELISA.
Antibodies specific for the immunizing cytokines were tested by ELISA on Maxisorb immunoplates (Nunc, Roskild, Denmark), coated overnight at 4°C with the target antigen (20–200 ng/ml in 50 mM glycine buffer, pH 9) or coated first with Neutravidin (Pierce Biotechnology, Rockford, IL, USA) at 2 μg/ml in PBS, followed by biotinylated antigens (100 ng/ml). After plate saturation with 1% BSA, sera were processed as described [10].
Antibody screening in bioassays
The inhibitory activity of anti-GCP-2 mAb GCP-2 Ab4 was tested with human neutrophilic granulocytes loaded with fura-2/AM for monitoring the intracellular Ca2+ concentration [18]. Before addition to the cells, GCP-2 was preincubated with GCP-2 Ab4 or control antibody for 2 h at 37°C.
The effect of GCP-2 Ab4 was also tested in early neutrophil mobilization after L. major infection in C57BL/6 mice. GCP-2 Ab4, NIMP-R14, a mAb known to induce complete neutrophil depletion [19], or control antibody (500 μg) was injected i.p. 24 h prior to ear i.d. infection with 106 L. major (LV39 MRHO/Sv/59/P strain). Mice were killed 24 h later, and ear explants were prepared as described previously [20]. The leukocyte populations emigrating from the ear dermis were then counted and stained for FACS analysis (BD Biosciences, San Jose, CA, USA) with PE-conjugated anti-LY6G mAb (clone 1A8) from eΒioscience. Cells were analyzed with the program FlowJo (Tree Star Inc., Ashland, OR, USA).
IL-17F-neutralizing activity of mAb MM17F-8F5 was tested in supernatants of IL-17A−/− spleen cells activated for 72 h at 37°C with anti-CD3 (5 μg/ml) and anti-CD28 (2 μg/ml) under Th17-inducing conditions [anti-IFN-γ (10 μg/ml), anti-IL-4 (10 μg/ml), TGF-β (5 ng/ml), and IL-6 (20 ng/ml)] in the presence of MM17F-8F5 or isotype control. Following incubation, supernatants were set up in 48-well Boyden chemotaxis assay chambers. Briefly, the supernatants were diluted tenfold in RPMI + 1% FBS and loaded onto the bottom portion of the chamber. Peripheral blood leukocytes isolated from IL-17A−/− donors were loaded onto the top portion of the chamber (50,000/well), which was incubated at 37°C for 50 min before removal of the polycarbonate membranes (5 μm pore size) and stained with Wright Giemsa for counting of migrated neutrophils.
IL-17E/IL-25 activity was assessed by measuring IL-5 production (R&D Systems; ELISA) by bone marrow cells collected from RAG−/− BALB/c mice that were incubated (500,000 cells/well) with 10 ng/ml IL-17E/IL-25 (R&D Systems) for 96 h.
GM-CSF activity was tested on the myeloid cell line FDC-P1 [21], maintained in IMDM with 10% FCS and IL-3 [1/100 diluted supernatant of IL-3-producing CHO cells; a kind gift of Prof. Antony Burgess (Ludwig Institute for Cancer Research, Melbourne, Australia)]. After washing, 3000 cells were incubated with 125 pg/ml GM-CSF or IL-3 (30 U/ml) and serial serum dilutions for 3 days before quantifying cell growth by a hexosaminidase assay [22].
TGF-β activity was measured with TMLECs, as described by Abe et al. [23]. Briefly, 500 pg/ml human TGF-β1 was incubated with serial serum dilutions for 4 h at 37°C before transfer to an equal volume of culture medium (DMEM+10% FCS), wherein 50,000 TMLECs had been seeded 4 h earlier. After a further 24-h incubation, luciferase activity was measured with the Britelite luminescence kit (Perkin-Elmer, Wellesley, MA, USA).
IL-27 activity was measured on the basis of inhibition of IL-17 or IL-9 production by spleen cells stimulated under Th17 [24] or Th9 conditions [25]. Briefly, WT BALB/c or DO11.10 OVA-TCR BALB/c spleen cells (500,000 cells/well) were stimulated, respectively, with staphylococcus enterotoxin B (20 ng/ml) under Th17 conditions {10 μg/ml anti-IFN-γ [26]; 10 ng/ml TGF-β plus IL-6 (10 ng/ml)} or with OVA (100 μg/ml) under Th9 conditions (IL-4 and TGF-β at 10 ng/ml). IL-17 and IL-9 productions were inhibited completely by addition of 30 ng/ml IL-27. This IL-27-suppressive activity was used for testing anti-IL-27 antibodies. IL-9 and IL-17 concentrations were determined after 4 days by ELISAs performed with homemade antibodies produced from mice immunized as described previously [10, 27].
For testing anti-MMP-9 antibodies, mouse MMP-9 (200 nM) was preincubated for 1 h at 37°C with mAb MMP-9.5G4 (4500 nM) and MMP-9.4A12 (7200 nM). Then, 400 nM NGCP-2 was added as a substrate. NGCP-2 is a peptide that resembles the N terminus of mouse GCP-2 and is labeled at the C terminus with Alexa fluor 488 as fluorophore [28]. Cleavage reactions by mouse MMP-9 in the presence or absence of anti-MMP-9 mAb were performed at 37°C. At different time-points, 8% of the reaction volume was diluted in water. Substrate variants were analyzed by multicapillary gel electrophoresis and confocal laser-induced fluorescence detection on a MegaBACE 1000 DNA sequencer (GE Healthcare). The relative percentage of intact substrate at different incubation times was measured.
Antibody titrations and statistics.
Inhibitory serum titers are defined as the serum dilutions giving 50% inhibition of cytokine biological activities. Statistical significance (*P<0.05; **P<0.01; ***P<0.001) was calculated by Mann-Whitney or Student's t test as indicated.
RESULTS AND DISCUSSION
Production of OVA-glutaraldehyde carrier
Glutaraldehyde cross-links proteins by reacting with nonprotonated NH2 residues and rapidly forms insoluble polymers when incubation is performed at neutral or alkaline pH [29]. We observed that OVA, incubated overnight with a 100-fold molar excess of glutaraldehyde at pH 6, formed large polymers, which in spite of their size, remained soluble. When fractionated by size exclusion chromatography, these conjugates (hereafter designated OVAglu) eluted in a broad peak >1000 kD in size (Fig. 1A). As glutaraldehyde is a multifunctional compound, we anticipated that OVA polymerized with an excess of cross-linking agent would be covered with free aldehyde groups that could react with free amines on other proteins. To favor this possibility, the reaction with a target protein was performed in 0.1 M carbonate buffer at pH 8.5 and incubated for at least 6 h. The remaining free glutaraldehyde sites were subsequently saturated with a tenfold molar excess of PADRE peptide to provide additional T cell help. As shown in Table 1, incubation of GCP-2 or GM-CSF with OVAglu at a 1:1 molar ratio resulted in the formation of conjugates that could be detected by ELISA using a combination of anti-OVA and anticytokine antibodies. Similar results were obtained for all complexes mentioned in this manuscript when specific antibodies against the target cytokine were available. These results prove that OVAglu multimers are highly reactive and stable. The major advantage of these polymers is that they minimize exposure of the target factor to the cross-linking agent as compared with reactions performed by mixing OVA, cytokine, and free cross-linker.
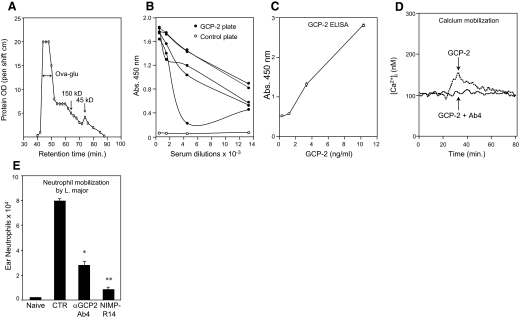
Figure 1. OVAglu polymers, anti-GCP-2–OVAglu auto-vaccination, and role of GCP-2 in neutrophil mobilization in L. major infection.
(A) OVAglu polymers fractionated by size exclusion chromatography. Size markers are mouse IgG1 (150 kD) and OVA (45 kD). (B) Detection by ELISA of anti-GCP-2 antibodies in sera from C57BL/6 mice vaccinated with GCP-2–OVAglu. (C) GCP-2 ELISA performed with two mAb derived from auto-vaccinated mice: GCP-2 Ab1 for coating and biotinylated GCP-2 Ab2 for detection. (D) Inhibition by GCP-2 Ab4 (0.5 mg/ml) of Ca2+ mobilization induced by GCP-2 (60 ng/ml) in human neutrophils. Ca2+ fluxes from one representative experiment out of three are shown. (E) Impairment of neutrophil mobilized 24 h after infection with L. major in the ear dermis of C57BL/6 mice treated with GCP-2 Ab4, neutrophil-depleting antibody NIMP-R14, or IgG2b control (CTR) antibody (mean±sem of six/group). These results are representative of three independent experiments. *P<0.05; **P<0.01 (Student's t test).
Table 1. Detection of Cytokine–OVA Complexes.
| Plate coating | Sample | Antibodies 450 nm |
|---|---|---|
| Goat anti-mouse GCP-2 | GCP-2 | 0.162 |
| OVA | 0.092 | |
| GCP-2–OVA | 1.236 | |
| Rat anti-mouse GM-CSF | GM-CSF | 0.114 |
| OVA | 0.134 | |
| GM-CSF–OVA | 1.764 |
ELISA plates were coated with the indicated antibodies, and after blocking with 1% BSA, they were incubated with OVA, cytokines, or complexes at 10 ng/ml, followed by rabbit anti-OVA antibodies and finally, with HRP-conjugated goat anti-rabbit IgG. All incubations were carried out at 37°C for 1–2 h. HRP activity was evaluated by a 10-min incubation with tetramethylbenzidine substrate.
Anti-GCP-2 chemokine auto-vaccination and demonstration of the role of GCP-2 in neutrophil mobilization during infection with L. major
To test whether OVAglu could be used as a carrier for auto-vaccination against a small self-protein, we used the mouse chemokine GCP-2, which is a major chemotactic factor for neutrophils in this species [30]. Sera from C57BL/6 mice immunized with GCP-2(9-78)–OVAglu complexes (5 μg complexes/mouse) gave a positive and specific signal on GCP-2(9–78)-coated ELISA plates (Fig. 1B). Four anti-GCP-2 mAb were derived from these mice. Two of these—GCP-2 Ab1 (IgG2a-κ) and GCP-2 Ab2 (IgG2b-κ)—recognized distinct epitopes, as they could be combined in an ELISA detecting GCP-2 (Fig. 1C) but did not inhibit GCP-2 activity, and the two others—GCP-2 Ab4 (IgG2b-κ) and Ab5 (IgG2a-κ)—inhibited GCP-2-triggered calcium mobilization in human neutrophils, as shown for GCP-2 Ab4 in Fig. 1D.
The capacity of anti-GCP-2 Ab4 to inhibit neutrophil mobilization in vivo was tested in mice infected with L. major, where neutrophils are recruited within hours of parasite infection (reviewed in ref. [31]). C57BL/6 mice were given an i.p. injection of 0.5 mg GCP-2 Ab4 24 h before i.d. injection with 106 L. major parasites. The number of neutrophils migrating out of infected ear dermis 24 h after infection was inhibited (±65%; P<0.05) in mice treated with this antibody (Fig. 1E). This inhibition was significant but nevertheless, weaker than that obtained with the NIMP-R14 mAb, which causes a total disappearance of blood neutrophils for 3 days [19]. Similar results were obtained in two repeat experiments and in one performed in BALB/c mice (data not shown). The factors involved in neutrophil migration in response to L. major infection are still not well defined and may involve several chemokines and cytokines such as MIP-2(CXCL2), keratinocyte-derived chemokine (CXCL1), and IL-17 [20]. The inhibition of neutrophil recruitment by anti-GCP-2 Ab4 reported here is, to our knowledge, the first demonstration of the role played by this chemokine in the early neutrophil mobilization in this infection.
Anticytokine auto-vaccination
To further evaluate the efficacy of OVAglu in breaking tolerance against self-antigens, attempts were made to vaccinate mice with OVAglu conjugated to various cytokines: GM-CSF, IL-17F, IL-17E/IL-25, IL-27p28, EBI3, and TGF-β1. In all cases, specific antibodies could be detected by ELISA at serum dilutions of 104–106 (Figs. 2A–C, 3A and B, and 4A).
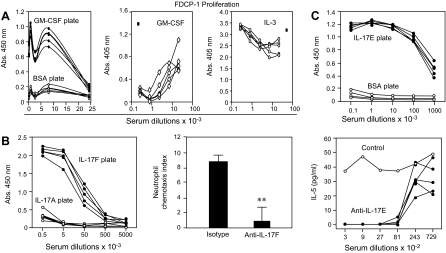
Figure 2. Anti-GM-CSF, anti-IL-17F, and anti-IL-17E/IL-25 auto-vaccination.
(A) Sera from control and GM-CSF–OVAglu-immunized C57BL/6 mice tested by ELISA on biotinylated mouse GM-CSF or control cytokine (human IL-9) bound to neutravidin-coated plates and by inhibition of FDC-P1 cell proliferation induced by GM-CSF (●, no antiserum) or IL-3 (■, no antiserum). (B) Anti-IL-17F antibodies in sera of C57BL/6 mice vaccinated with IL-17F–OVAglu on IL-17F-coated ELISA plates (IL-17A as control) and inhibition by MM17F-8F5, a mAb derived from these mice, of IL-17F-induced neutrophil chemotactic activity produced by IL-17A−/− splenocytes activated under Th17 conditions and tested on IL-17A−/− splenic leukocytes as responders. Bar graph shows mean chemotactic index (±sem) for each group compiled from six measurements. (C) Anti-IL-17E/IL-25 antibody detection by ELISA in sera from C57BL/6 mice vaccinated with IL-17E/IL-25–OVAglu and by inhibition of IL-5 induction by IL-17E/IL-25 in bone marrow cells from RAG−/− BALB/c mice incubated with 10 ng/ml IL-17E/IL-25. **P<0.01 (Student's t test).
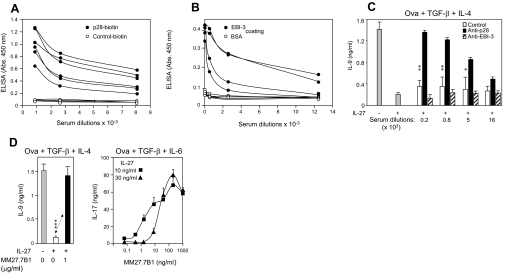
Figure 3. Anti-IL-27 p28 and EBI3 auto-vaccines.
Anti-p28 (A) and anti-EBI3 (B) antibodies detected on IL-27 p28- or EBI3-coated ELISA plates in sera of C57BL/6 mice immunized with p28–OVAglu or EBI3–OVAglu vaccines. (C) Inhibition by sera from p28 (not by EBI-3)-vaccinated mice of IL-27 (30 ng/ml)-mediated suppression of IL-9 production by spleen cells from DO11.10 mice stimulated in vitro with OVA in the presence of TGF-β and IL-4. Results represent mean ± sem (five mice each in triplicate). (D) Prevention of IL-27-mediated suppression of IL-27 of IL-9 or IL-17 production by a mAb specific for p28, MM27.7B1 (mean±sem of triplicate cultures). *P<0.05; **P<0.01; **P<0.001 (Mann-Whitney test).
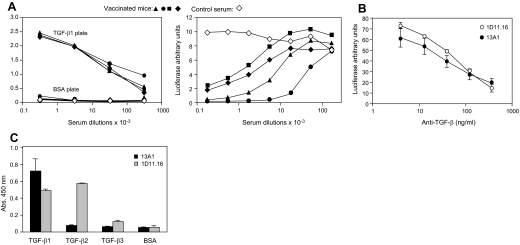
Figure 4. Anti-TGF-β1 vaccine.
(A) Anti-human TGF-β1 antibodies in C57BL/6 mice vaccinated with human TGF-β1–OVAglu complexes and detected by ELISA on human TGF-β1-coated plates and by inhibition of TGF-β1-induced luciferase activity in TMLECs. (B) Comparison of 1D11.16 and MTGF-β1.13A1 mAb for inhibition of TGF-β1 activity in TMLECs (mean of 4) and (C) for binding to different TGF-β isoforms (mean of 3).
With the exception of anti-EBI3 (see below), sera or mAb derived from the vaccinated mice inhibited the biological activities of these factors in their respective in vitro tests.
For GM-CSF, this was demonstrated with the myeloid cell line FDC-P1. As shown in Fig. 2A, the proliferation induced by GM-CSF was inhibited by the anti-GM-CSF-vaccinated mouse serum, and that induced by IL-3 was slightly enhanced at high serum concentration. No inhibition was seen with control serum (data not shown).
Anti-IL-17F sera could not be tested as such, because of interference of mouse serum in the IL-17F bioassay. However, a mAb MM17F-8F5, derived from IL-17F-vaccinated mice, was able to completely abrogate the neutrophil chemotactic activity of Th17-polarized spleen cells obtained from IL-17A−/− mice [14] and tested in Boyden chemotaxis chambers loaded with IL-17A−/− leukocytes (Fig. 2B). Incidentally, these results indicate that in the absence of IL-17A, IL-17F is the predominant inducer of neutrophil-mobilizing agents produced by Th17 cells.
IL-17E/IL-25 has been reported to induce Th2 cytokine production by accessory cells [32]. We observed that it induced massive IL-5 production by bone marrow cells from RAG−/− BALB/c mice and took advantage of this activity to test anti-IL-17E/IL-25 sera. As shown in Fig. 2C, the latter completely abrogated this IL-5 induction.
Blocking of IL-27 by sera from p28–OVAglu-immunized mice was tested by evaluating IL-9 production by T cells cultured under Th9 conditions (TGF-β+IL-4) [25], which we have found to be inhibited by IL-27 (unpublished results). These sera restored IL-9 production in the presence of IL-27, which also indicates that they reacted with p28, even when combined to EBI3 (Fig. 3C). A mAb, designated MM27.7B1, was derived from another set of mice that was immunized with a p28–EBI3 fusion protein linked to OVAglu. This antibody restored IL-9 and also IL-17A production in the presence of IL-27 (Fig. 3D). Anti-EBI3 sera, however, failed to inhibit IL-27-suppressive activity (Fig. 3C). As ELISA anti-p28 and anti-EBI3 antibody titers were similar (Fig. 3A and B), this result suggests that the repertoire of self-reactive anti-EBI3 B cells is restricted to certain epitopes not required for IL-27 binding to its receptor.
Anti-TGF-β1 activity of TGF-β1–OVAglu-vaccinated mice was tested in a TMLEC luciferase assay. Inhibitory titers ranged from 103 to 104 (Fig. 4A). From such vaccinated mice, anti-TGF-β1 mAb were produced. One, MTGF-β1.13A1, was a potent inhibitor of TGF-β1 bioactivity (Fig. 4B), similar or slightly more potent than 1D11.16, the established reference anti-TGF-β antibody [33]. Contrary to 1D11.16, which recognizes TGF-β1 and TGF-β2, 13A1 reacted exclusively with TGF-β1 (Fig. 4C). Formal proof that 13A1 recognizes and inhibits mouse TGF-β1 was obtained in TMLECs incubated with acid-treated mouse serum as a source of mouse TGF-β1 (data not shown).
Anti-gelatinase B/MMP-9 auto-vaccination
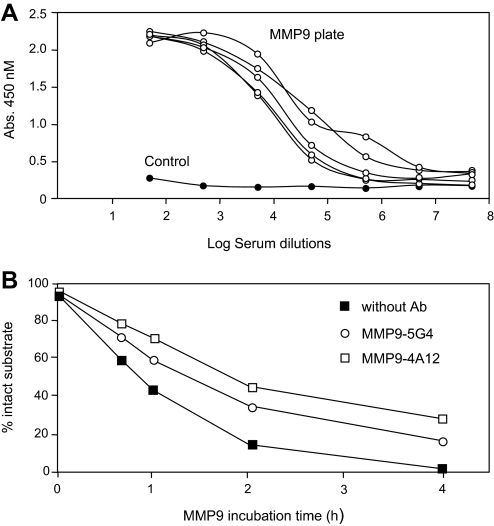
The results reported so far were obtained with proteins smaller in size than OVA monomers. We wondered whether OVAglu would also confer immunogenicity to a larger protein and one that does not belong to the chemokine/cytokine families. MMP-9/gelatinase B, a 92-kD homodimeric proteinase that plays multiple roles in inflammatory processes, including the processing of chemokines [34], and is structurally different from chemokines and cytokines, seemed a good candidate. MMP-9–OVAglu conjugates were produced and used for immunization of C57BL/6 mice. These complexes induced significant and specific antibodies binding to MMP-9-coated ELISA plates, which remained positive at serum dilutions in the range of 103–104 (Fig. 5A). As a result of interference with the enzymatic activity of MMP-9 by gelatinase present in mouse serum, it was not possible to test inhibition of MMP-9-mediated substrate degradation by mouse sera. However, two mAb (MMP-9.5G4 and MMP-9.4A12) derived from the vaccinated mice inhibited the cleavage by MMP-9 of a GCP-2 peptide, a natural substrate of this protease (Fig. 5B). After a 4-h incubation, a five- to tenfold increase in the concentration of uncleaved substrate was observed. These inhibitory effects were confirmed in another experiment.
Figure 5. Anti-MMP-9 auto-vaccination.
(A) Anti-MMP-9 antibodies in C57BL/6 mice immunized with mouse MMP-9–OVAglu complexes detected by ELISA on a MMP-9-coated ELISA plate. (B) Inhibition of MMP-9 protease activity by two mAb derived from MMP-9-vaccinated mice. Kinetics of the degradation of a GCP-2 peptide labeled with Alexa fluor 488 (NGCP-2) by MMP-9 was measured as a function of time in the presence of anti-MMP-9 mAb MMP-9-5G4 and MMP-9-4A12.
Summary and conclusion
OVAglu conjugates provide a strong stimulus for auto-vaccination, as illustrated by several examples described in this manuscript. However, some attempts were not successful. In one case (IL-21; not shown), no antibodies were detected at all, and for others, EBI3 (Fig. 3B) and IL-33 (not shown), we obtained antibodies that failed to neutralize the biological activity of the cytokine. The reasons for these failures are not clear, although it is interesting to note that all failures involved monomeric cytokines. It is possible that critical antigenic epitopes remain more accessible when a dimeric cytokine is conjugated to OVAglu. This is, however, not an absolute requirement, as we were successful for p28.
Pre-activated OVA multimers reactive with amine residues thus provided an efficient carrier for auto-vaccination against autologous proteins ranging from 9 to 90 kDa in size and belonging to distinct families. In addition to the production of mouse mAb against mouse factors, which changes the perspectives of their prolonged use in vivo, auto-vaccination offers an alternative to gene inactivation in studies of in vivo cytokine function as discussed by Zagury et al. [35]. In principle, it should also allow for concomitant inhibition of several cytokines in the same animal.
ACKNOWLEDGMENTS
This study was supported by the Fonds National de la Recherche Scientifique Médicale (FRSM, Belgique), the Concerted Research Actions (G.O.A.) of the Regional Government of Flanders, and an Interuniversity Attraction Pole of the Belgian Federal Science Policy. Part of this work was supported by the Swiss National Foundation (310030-129852/1 to F.T-C.). R.G.M. is a Haas-Teichen Fellow of the de Duve Institute, Université Catholique de Louvain, Brussels, Belgium. S.G. is a research associate of the F.R.S-FNRS, Belgium. The authors thank Dominique Donckers and Isabelle Minnoy for excellent technical assistance and Suzanne Depelchin for figure editing.
Footnotes
- EBI3
- EBV-induced gene 3
- FDC-P1
- factor-dependent cell progenitor 1
- GCP-2
- granulocyte chemotactic protein 2
- GCP-2(9-78)
- granulocyte chemotactic protein 2 lacking 8 N terminal amino acids
- MMP-9
- matrix metalloproteinase 9
- NGCP-2
- Alexa Fluor 488-labeled GCP2 peptide
- OVAglu
- OVA polymers covered with glutaraldehyde
- PADRE
- pan HLA-DR epitope peptide
- TMLEC
- TGF-βR-transfected mink lung epithelial cell line
AUTHORSHIP
C.U. directed all vaccinations, tested immunizations, and produced mAb with R.G.M., S.G., and D.S. F.T-C., M.C., R.R.C., J.M.D., J.V.D., S.S., and G.O. performed functional analyses of antibodies. J.V.S. produced immunogens and wrote the manuscript.
REFERENCES
- 1. Ratsimandresy R. A., Rappaport J., Zagury J. F. (2009) Anti-cytokine therapeutics: history and update. Curr. Pharm. Des. 15, 1998–2025 [DOI] [PubMed] [Google Scholar]
- 2. Johnson H. E., DeAvila D. M., Chang C. F., Reeves J. J. (1988) Active immunization of heifers against luteinizing hormone-releasing hormone, human chorionic gonadotropin and bovine luteinizing hormone. J. Anim. Sci. 66, 719–726 [DOI] [PubMed] [Google Scholar]
- 3. Dalum I., Butler D. M., Jensen M. R., Hindersson P., Steinaa L., Waterston A. M., Grell S. N., Feldmann M., Elsner H. I., Mouritsen S. (1999) Therapeutic antibodies elicited by immunization against TNF-α. Nat. Biotechnol. 17, 666–669 [DOI] [PubMed] [Google Scholar]
- 4. Chackerian B., Lowy D. R., Schiller J. T. (2001) Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J. Clin. Invest. 108, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalum I., Jensen M. R., Hindersson P., Elsner H. I., Mouritsen I. (1996) Breaking of B cell tolerance toward a highly conserved self protein. J. Immunol. 157, 4796–4804 [PubMed] [Google Scholar]
- 6. Spohn G., Keller I., Beck M., Grest P., Jennings G. T., Bachmann M. F. (2008) Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis. Eur. J. Immunol. 38, 877–887 [DOI] [PubMed] [Google Scholar]
- 7. Hertz M., Mahalingam S., Dalum I., Klysner S., Mattes J., Neisig A., Mouritsen S., Foster P. S., Gautam A. (2001) Active vaccination against IL-5 bypasses immunological tolerance and ameliorates experimental asthma. J. Immunol. 167, 3792–3799 [DOI] [PubMed] [Google Scholar]
- 8. (2000) Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc. Natl. Acad. Sci. USA 97, 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uyttenhove C., Arendse B., Stroobant V., Brombacher F., Van Snick J. (2004) Development of an anti-IL-12 p40 auto-vaccine: protection in experimental autoimmune encephalomyelitis at the expense of increased sensitivity to infection. Eur. J. Immunol. 34, 3572–3581 [DOI] [PubMed] [Google Scholar]
- 10. Uyttenhove C., Van Snick J. (2006) Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur. J. Immunol. 36, 2868–2874 [DOI] [PubMed] [Google Scholar]
- 11. Rad F. H., Le Buanec H., Paturance S., Larcier P., Genne P., Ryffel B., Bensussan A., Bizzini B., Gallo R. C., Zagury D., Uzan G. (2007) VEGF kinoid vaccine, a therapeutic approach against tumor angiogenesis and metastases. Proc. Natl. Acad. Sci. USA 104, 2837–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zagury D., Le Buanec H., Mathian A., Larcier P., Burnett R., Amoura Z., Emilie D., Peltre G., Bensussan A., Bizzini B., Gallo R. C., Koutouzov S. (2009) IFNα kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proc. Natl. Acad. Sci. USA 106, 5294–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexander J., Sidney J., Southwood S., Ruppert J., Oseroff C., Maewal A., Snoke K., Serra H. M., Kubo R. T., Sette A., et al. (1994) Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1, 751–761 [DOI] [PubMed] [Google Scholar]
- 14. Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. (2002) Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 [DOI] [PubMed] [Google Scholar]
- 15. Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. (1986) The murine transforming growth factor-β precursor. J. Biol. Chem. 261, 4377–4379 [PubMed] [Google Scholar]
- 16. Van Coillie E., Van Aelst I., Wuyts A., Vercauteren R., Devos R., De Wolf-Peeters C., Van Damme J., Opdenakker G. (2001) Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. Am. J. Pathol. 159, 1405–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masure S., Nys G., Fiten P., Van Damme J., Opdenakker G. (1993) Mouse gelatinase B. cDNA cloning, regulation of expression and glycosylation in WEHI-3 macrophages and gene organization. Eur. J. Biochem. 218, 129–141 [DOI] [PubMed] [Google Scholar]
- 18. Struyf S., Menten P., Lenaerts J. P., Put W., D′Haese A., De Clercq E., Schols D., Proost P., Van Damme J. (2001) Diverging binding capacities of natural LD78β isoforms of macrophage inflammatory protein-1α to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur. J. Immunol. 31, 2170–2178 [DOI] [PubMed] [Google Scholar]
- 19. Lopez A. F., Strath M., Sanderson C. J. (1984) Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br. J. Haematol. 57, 489–494 [DOI] [PubMed] [Google Scholar]
- 20. Charmoy M., Auderset F., Allenbach C., Tacchini-Cottier F. (2010) The prominent role of neutrophils during the initial phase of infection by Leishmania parasites. J. Biomed. Biotechnol. 2010, 719361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. (1980) Growth of factor-dependent hemopoietic precursor cell lines. J. Exp. Med. 152, 1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landegren U. (1984) Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Application to detection of lymphokines and cell surface antigens. J. Immunol. Methods 67, 379–388 [DOI] [PubMed] [Google Scholar]
- 23. Abe M., Harpel J. G., Metz C. N., Nunes I., Loskutoff D. J., Rifkin D. B. (1994) An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 [DOI] [PubMed] [Google Scholar]
- 24. Batten M., Li J., Yi S., Kljavin N. M., Danilenko D. M., Lucas S., Lee J., de Sauvage F. J., Ghilardi N. (2006) Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7, 929–936 [DOI] [PubMed] [Google Scholar]
- 25. Veldhoen M., Uyttenhove C., van Snick J., Helmby H., Westendorf A., Buer J., Martin B., Wilhelm C., Stockinger B. (2008) Transforming growth factor-β ″reprograms″ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9, 1341–1346 [DOI] [PubMed] [Google Scholar]
- 26. Billiau A., Heremans H., Vandekerckhove F., Dillen C. (1987) Anti-interferon-γ antibody protects mice against the generalized Shwartzman reaction. Eur. J. Immunol. 17, 1851–1854 [DOI] [PubMed] [Google Scholar]
- 27. Uyttenhove C., Brombacher F., Van Snick J. (2010) TGF-β interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur. J. Immunol. 40, 2230–2235 [DOI] [PubMed] [Google Scholar]
- 28. Piccard H., Hu J., Fiten P., Proost P., Martens E., Van den Steen P. E., Van Damme J., Opdenakker G. (2009) ″Reverse degradomics″, monitoring of proteolytic trimming by multi-CE and confocal detection of fluorescent substrates and reaction products. Electrophoresis 30, 2366–2377 [DOI] [PubMed] [Google Scholar]
- 29. Avrameas S., Ternynck T. (1969) The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry 6, 53–66 [DOI] [PubMed] [Google Scholar]
- 30. Wuyts A., Haelens A., Proost P., Lenaerts J. P., Conings R., Opdenakker G., Van Damme J. (1996) Identification of mouse granulocyte chemotactic protein-2 from fibroblasts and epithelial cells. Functional comparison with natural KC and macrophage inflammatory protein-2. J. Immunol. 157, 1736–1743 [PubMed] [Google Scholar]
- 31. Charmoy M., Brunner-Agten S., Aebischer D., Auderset F., Launois P., Milon G., Proudfoot A. E., Tacchini-Cottier F. (2010) Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 6, e1000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fort M. M., Cheung J., Yen D., Li J., Zurawski S. M., Lo S., Menon S., Clifford T., Hunte B., Lesley R., Muchamuel T., Hurst S. D., Zurawski G., Leach M. W., Gorman D. M., Rennick D. M. (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 [DOI] [PubMed] [Google Scholar]
- 33. Dasch J. R., Pace D. R., Waegell W., Inenaga D., Ellingsworth L. (1989) Monoclonal antibodies recognizing transforming growth factor-β. Bioactivity neutralization and transforming growth factor β 2 affinity purification. J. Immunol. 142, 1536–1541 [PubMed] [Google Scholar]
- 34. Opdenakker G., Nelissen I., Van Damme J. (2003) Functional roles and therapeutic targeting of gelatinase B and chemokines in multiple sclerosis. Lancet Neurol. 2, 747–756 [DOI] [PubMed] [Google Scholar]
- 35. Zagury D., Le Buanec H., Bizzini B., Burny A., Lewis G., Gallo R. C. (2003) Active versus passive anti-cytokine antibody therapy against cytokine-associated chronic diseases. Cytokine Growth Factor Rev. 14, 123–137 [DOI] [PubMed] [Google Scholar]