Abstract
Purpose
The purpose of this study was to review the current literature on in vivo confocal microscopy of the cornea and to discuss the current clinical indications for its use in microbial keratitis.
Methods
Review of select recent literature on in vivo confocal microscopy and atypical microbial keratitis.
Results
Delayed diagnosis of Acanthamoeba and fungal keratitis is typical, resulting in significant vision loss. This is partially due to the low sensitivity and time delay of corneal cultures. In the hands of an experienced viewer, the confocal microscope has been found to have a sensitivity of up to 90% in the diagnosis of Acanthamoeba keratitis and close to 80% for fungal keratitis.
Conclusion
In vivo confocal microscopy is emerging as a tool for rapid diagnoses in severe infectious keratitis with high sensitivity. In addition, it can be used to monitor treatment response, allowing guidance to clinicians for medical or surgical management.
Keywords: acanthamoeba keratitis, cornea, fungal keratitis, in vivo confocal microscopy, infection
INTRODUCTION
Microbial keratitis can lead to irreversible complications, including blindness, and therefore its significance should not be understated. Microorganisms, including bacteria, viruses, fungi, and parasites trigger an immune response, leading to severe corneal inflammation, ulceration, scarring or even perforation, with devastating consequences. The incidence of microbial keratitis worldwide, ranges from 6.3 to 710 cases per 100,000 population per year, and is even more common in contact lens wearers.1
Studies have demonstrated that the key to prevention of permanent vision loss lies in early diagnosis and initiation of appropriate therapy targeted at the specific microbe.1 While patient history and slit-lamp examination are imperative in raising clinical suspicion for various organisms, microbiology remains the gold standard through stains, cultures, or pathology. However, corneal cultures are only positive in 52.5%–67% of cases.2 While these methods have been helpful in diagnosis, they are limited by many factors such as inadequate sample material, delay in culture, previous treatments with antimicrobials, and corneal staining. In addition, there is a significant delay in obtaining culture results, due to slow-growing organisms such as fungi and Acanthamoeba. Further, the false-negative rate is another important limitation to the diagnostic ability of corneal cultures.2
Given the importance of timing in diagnosis and initiation of anti-microbial therapy, there is an exciting emerging role for in vivo confocal microscopy (IVCM) evolving not only for the diagnosis of microbial keratitis, but also potentially in the management of this disease.
PRINCIPLES OF CONFOCAL MICROSCOPY
The confocal microscope was first described by Goldmann in 1940. In contrast to conventional light microscopes, which are limited by light scatter from structures outside of the focal plane, confocal microscopes create a point source of light by a pinhole aperture, focused by an objective lens on the tissue. The light reflected by the specimen is then collected by a parallel objective lens, focused onto a separate pinhole aperture, and collected by a detector. The illuminating point source and the observation aperture of the detector are conjugate with the same point in the tissue and are confocal. 3
In vivo confocal microscopy of the cornea was first described by Lemp et al. in 1985. Advantages of IVCM include the noninvasive and rapid examination of the cornea, as well as the possibility for longitudinal exams in the same patient. In addition to qualitative information, it also provides quantitative analysis of all corneal layers, nerves, and cells.
Currently there are 3 main microscopes in use: tandem scanning confocal microscope, slit-scanning confocal microscope, and laser scanning confocal microscope. The tandem scanning microscope (TSCM) is no longer produced. The slit scanning confocal (SSCM) uses two conjugate slits and is faster, with more light and higher resolution, as compared to the TSCM. The Confoscan 4 (Nidek Technologies, Italy) is an example of the SSCM. More recently developed is the third type of IVCM, the laser scanning confocal microscope Heidelberg Retinal Tomograph 3 (HRT3), in conjunction with the Rostock Cornea Module (RCM) (Heidelberg Engineering, Germany), which uses a 670 nm red wavelength diode laser. The HRT3/RCM offers up to 400 times magnification with an axial resolution of approximately 1 micrometer.4
ACANTHAMOEBA KERATITIS
Acanthamoeba is a ubiquitous protozoan found in water, air, and soil, with a 2-stage life cycle: active trophozoites and quiescent cysts. The infection rate is 1.2 per million adults and between 0.2 and 1 per 10,000 contact lens wearers per year in the United Kingdom, Hong Kong, Europe, and the USA.4 It typically presents in contact-lens wearers, especially silicone hydrogel lenses, with a history of water exposure. More recently, there has been a significant surge in the number of cases in contact lens wearers.4
The initial presentation is typically nonspecific, leading to initial misdiagnosis of patients as a bacterial corneal ulcer. Due to the lack of resolution, patients may subsequently be treated for a suspected herpetic keratitis, leading to further delay in diagnosis. A ring infiltrate and radial perineuritis may develop at later stages and patients complain typically of intense pain, at which point the diagnosis of Acanthamoeba keratitis is suspected. Patients diagnoses at these advanced stages have a fairly guarded prognosis. Current methods of identifying the organism include corneal smears, culture, and PCR. Unfortunately, cultures can take weeks to become positive and sensitivity ranges from 0 to 68%.4 Further, PCR is an emerging tool but is not yet available in all laboratories.
Currently, treatment of Acanthamoeba keratitis includes topical polyhexamethylene biguanide, chlorhexidine, and propamidine. Typically an oral antifungal such as voriconazole may also be added to the regimen. Corticosteroid treatment, however, is controversial. In some cases where medical treatment fails, a therapeutic penetrating keratoplasty (TKP) is indicated. However, this procedure has a relatively high rate of failure due to the intense inflammation. Kitzmann et al. recently performed a retrospective, non-randomized, comparative, interventional case series of 31 eyes with Acanthamoeba keratitis.5 The authors found that 12 of the 22 eyes treated with a TKP required 2–6 keratoplasties, while only 1 of the 9 with optical keratoplasty (OKP) required a repeat graft. In their study, with at least 6 months of follow-up, only 50% of the patients who received a TKP had a final vision of 20/40 or better as compared to 89% of those with an OKP. Of note, a microbiological cure was achieved in all cases, demonstrating a surgical option for persistent infection.
The pharmacotherapy for Acanthamoeba keratitis is expensive, prolonged, and toxic, and thus ophthalmologists are hesitant to start empiric treatment. However, a retrospective case control study of 72 eyes of 65 patients, describing the prognostic factors affecting visual outcome in Acanthamoeba keratitis by Tu et al., recently revealed that late initiation of treatment, leading to deep stromal involvement or a ring infiltrate at presentation were associated with worse visual outcome.6 Therefore, there is great value in more rapid identification of the organism as compared to cornea culture.
In 1992, Chew et al. were the first to report images of Acanthamoeba by IVCM and indicated the potential use of this technique for rapid diagnosis.7 In addition, the classic keratoneuritis and intrastromal cavities seen in late stages of disease can be seen with IVCM. In 2004, the American Academy of Ophthalmology reviewed the evidence for the use of confocal microscopy for diagnosing Acanthamoeba keratitis and it was supported as an adjunctive modality.
Both the cyst and trophozoite form of this parasite are sufficient in size to be identifiable on IVCM exam, as compared to bacteria and viruses. The cysts are 15–28 micrometers with a double wall corresponding to an outer ecocyst and an inner endocyst. Acanthamoeba cysts typically have a higher reflectivity than other cells. They are usually spherical but may also sometimes appear ovoid. This double-wall may not always be apparent depending on the plane of the images. In this case, it may be difficult to differentiate between cysts, leukocytes, epithelial cells, and debris. The trophozoites are the active form and are usually 25–40 micrometers in diameter.4 They are hyper-reflective and surrounded by hypo-reflective edema, but are more difficult to distinguish and are controversial.
Tu et al. recently performed a study to determine the value of IVCM in diagnosis of acanthamoebal infection.8 The authors found that in patients with both clinical and objective evidence of Acanthamoeba keratitis, confocal microscopy had a sensitivity of 90.6% (95% confidence interval [CI]: 79.3%–96.9%) and a specificity of 100% (95% CI:95%–100%). Of interest is the fact that of the 53 patients with acanthamoeba keratitis, confocal microscopy was positive in 48, while corneal smears were positive in only 30 of 41 and cultures only in 23 of 42 patients.8
Acanthamoeba keratitis is, however, not just a diagnostic dilemma, but also a therapeutic dilemma. Throughout the prolonged course of treatment with toxic medications, it sometimes becomes difficult to distinguish toxicity from persistent infection. In this case, the confocal microscope is very helpful as it can demonstrate the presence or absence of cysts and trophozoites with a noninvasive, rapid test. Further, a recent study by the authors (unpublished observation) comparing outcome in patients diagnosed with conventional means vs. IVCM, demonstrates that the use of IVCM in the diagnosis of acanthamoeba keratitis reduces the number of days to diagnosis, expediting initiation of therapy, and leading to excellent visual outcome. In addition, confocal-guided treatment (patients followed by serial IVCM) resulted in decreased number of patients needing a therapeutic PK.
FUNGAL KERATITIS
Fungal keratitis is more commonly seen in developing countries with tropical climates. In India, half of all infectious ulcers are fungal.12 In these countries, filamentous fungi are more common, as opposed to more temperate climates where yeast such as Candida is more prevalent. In recent years, however, the rate of infection by filamentous fungi has increased. Jurkunas et al. reported an increase in the incidence of filamentous fungus at the Massachusetts Eye and Ear Infirmary from 30% (1999–2002) to 65% (2004–2007).9 In their series, from 2004–2007, of all fungal keratitis coses 41% of cases were associated with contact lens wear. Other risk factors for fungal keratitis include specific contact lens solutions, long-term steroid use, antibacterial use, trauma with vegetable matter, chronic ocular surface disease, and systemic immunosuppressive diseases. Interestingly, while a study in India reported that over 90% of fungal ulcers were caused by ocular trauma,10 only 11–44% of fungal ulcers in the United States are trauma-related.11
The clinical presentation of fungal keratitis is non-specific and indolent. Typically patients are initially treated for bacterial, viral or even amoebic infections. When therapy is delayed, the infection can develop into an endophthalmitis and the eye can be lost. Alternatively, the permanent scarring associated with the delay in treatment can lead to significant vision loss. The gold standard for diagnosis is corneal smear or culture. Unfortunately both have a limited sensitivity and cultures can take several days to weeks to obtain growth. Only 65%–75% of Gram and Giemsa stains are positive for fungal hyphae. The sensitivity of culture is even lower with only 25% becoming positive after 2 weeks.4
Treatment varies depending on the species of fungus involved. Typically Candida is sensitive to amphotericin B topical drops, while the filamentous fungi such as Aspergillus and Fusarium are sensitive to topical natamycin therapy. Voriconazole drops are occasionally used in combination with either natamycin or amphotericin; however their cost is sometimes prohibitory. It should be noted that epithelial scraping is usually performed to aid in the penetration of these drugs. Oral antifungal therapy is also administered in combination with topical treatment, most commonly voriconazole for filamentous or fluconazole for yeast. In the series reported by Jurkunas et al., 70% received additional oral antifungal therapy, and 40% required a TKP.9 Certain species of fungi such as Paecilomyces are multi-drug resistant and may need newer antifungals such as miconazole or posaconazole, and are also more likely to progress to TKP.
In vivo confocal microscopy is emerging as a tool for early identification of fungal elements. Filamentous fungal keratitis is most commonly caused by Aspergillus and Fusarium. Aspergillus hyphae are 5–10 micrometers in diameter and have septate hyphae with dichotomous branches at a 45 degree angle.4 In contrast, Fusarium typically branches at a 90 degree angle.4 The hyper-reflective elements seen on IVCM must be differentiated on the one hand from the basal corneal epithelial nerves, which have a more regular branching pattern. Stromal nerves, on the other hand, are much larger in diameter (25–50 micrometers). Both Aspergillus and Fusarium are 200–400 micrometers long. In addition, yeast such as Candida Albicans have round, budding bodies that may develop pseudohyphae. The structures are 10–40 micrometers in length and 5–10 micrometers in width.4
A recent study by Kanavi et al. in 133 patients with microbial keratitis demonstrated that confocal microscopy had a sensitivity of 94% and a specificity of 78% in patients with fungal keratitis.18 As with Acanthamoeba keratitis, confocal microscopy could be applied for monitoring and guidance of treatment. In fungal keratitis, the depth of invasion has been shown to be an important prognostic factor, with a very poor prognosis in cases where the organism has reached the anterior chamber. IVCM is the currently the only method that allows determination of the depth of infection. IVCM could aid the clinician in deciding the timing for surgical intervention when the disease is progressing despite medical treatment.
LIMITATIONS
While the IVCM has introduced a powerful new tool for diagnosing acanthamoebal and fungal infections, it remains extremely user-dependent, as the testing requires a skilled operator, and interpretation requires an experienced viewer. Therefore, cornea cultures remain the gold standard and are required. In addition, confocal examinations can be technically limited by patient collaboration and motion artifacts. Further, depending on the confocal machine used, dense corneal infiltrates or scarring could preclude proper tissue penetration and visualization. Moreover, the TSCM and SSCM are generally limited to scans of the central cornea. Although the new laser scanning confocal microscope now allows for imaging of the corneal periphery as well, it is not widely available. Finally, currently smaller organisms, including bacteria and viruses, are not visible on examination, and the use of IVCM in these cases is not helpful.
CONCLUSION
In vivo confocal microscopy is an emerging non-invasive tool that allows for rapid diagnoses of potentially blinding atypical infections, such as Acanthamoeba and fungal keratitis. It has been demonstrated that the prognosis and visual outcome improve greatly with earlier diagnosis and initiation of treatment. Given the low sensitivity and delay for traditional methods such as corneal smears and culture, this new diagnostic modality, with a very high sensitivity and specificity, allows for rapid diagnosis and prompt initiation of treatment, leading to improved patient outcome. In the near future, it also may provide the unique advantage of following the disease course and guiding treatment.
FIGURE 1.
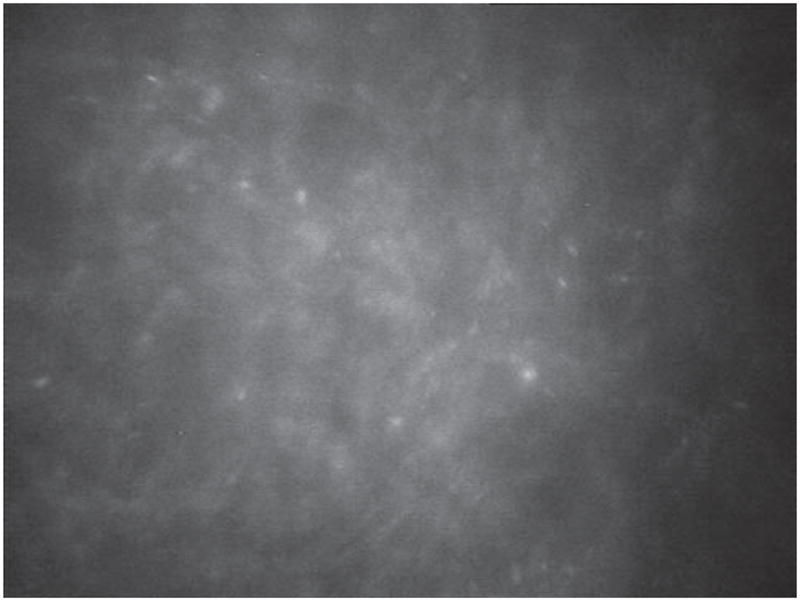
Acanthamoeba cysts taken with Nidek Confoscan 4.
FIGURE 2.

Acanthamoeba cysts taken with Heidelberg Retina Tomograph 3 Rostock Cornea Module (HRT3-RCM).
FIGURE 3.
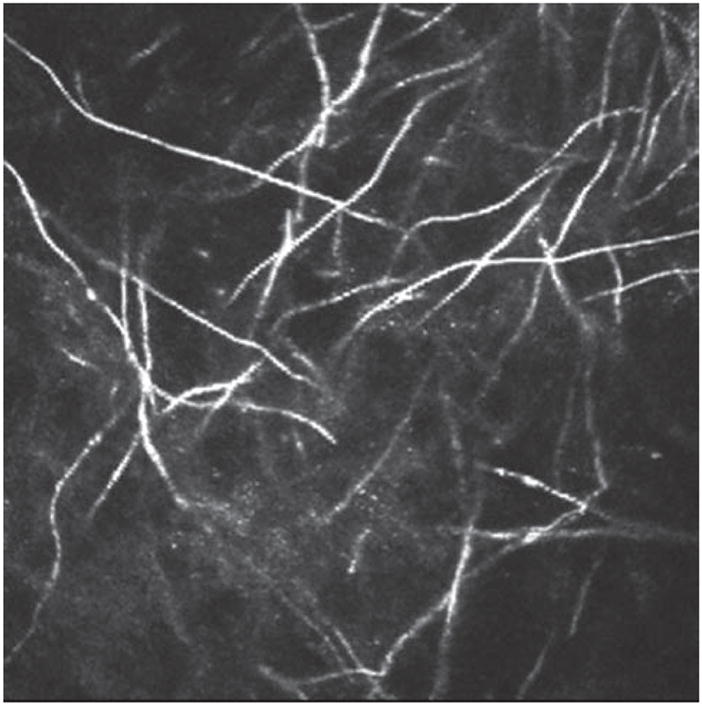
Fusarium Solani hyphae taken with Heidelberg Retina Tomograph 3 Rostock Cornea Module (HRT3-RCM). Courtesy of Drs. Antoine Labbé and Christophe Baudouin, Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts, Paris, France.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129–141. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 2.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Erie J, McLaren J, Patel S. Confocal microscopy in ophthalmology. American Journal of Ophthalmology. 2009;148:639–646. doi: 10.1016/j.ajo.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Labbe A, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. The Ocular Surface. 2009;7:41–52. doi: 10.1016/s1542-0124(12)70291-4. [DOI] [PubMed] [Google Scholar]
- 5.Kitzmann A, Goins K, Sutphin J, Wagoner M. Keratoplasty for treatment of Acanthamoeba keratitis. Ophthalmology. 2009;16:864–869. doi: 10.1016/j.ophtha.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Tu E, et al. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008;115:1998–2003. doi: 10.1016/j.ophtha.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew SJ, Feuerman RW, Assouline M, et al. Early diagnosis of infectious keratitis with in vivo real time confocal microscopy. CLAO J. 1992;18:197–201. [PubMed] [Google Scholar]
- 8.Tu E, et al. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of acanthamoeba keratitis. Cornea. 2008;27:764–772. doi: 10.1097/ICO.0b013e31816f27bf. [DOI] [PubMed] [Google Scholar]
- 9.Jurkunas U, Behlau I, Colby K. Fungal keratitis: changing pathogens and risk factors. Cornea. 2009;28:638–643. doi: 10.1097/ICO.0b013e318191695b. [DOI] [PubMed] [Google Scholar]
- 10.Ou JI, Acharya NR. Epidemiology and treatment of fungal corneal ulcers. Int Ophthalmol Clin. 2007;47:7–16. doi: 10.1097/IIO.0b013e318074e727. [DOI] [PubMed] [Google Scholar]
- 11.Patel A, Hammersmith K. Contact lens-related microbial keratitis: recent outbreaks. Curr Opin Ophthalmol. 2008;19:302–306. doi: 10.1097/ICU.0b013e3283045e74. [DOI] [PubMed] [Google Scholar]
- 12.Loh A, Hong K, Lee S, et al. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28:856–859. doi: 10.1097/ICO.0b013e318199fa77. [DOI] [PubMed] [Google Scholar]
- 13.Patel D, McGhee C. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 14.Guthoff R, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea – a major review. Clinical and Experimental Ophthalmology. 2008;37:100–117. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 15.Niederer RL, McGhee CNJ. Clinical in vivo confocal microscopy of the human cornea in health and disease. Progress in Retinal and Eye Research. 2009 doi: 10.1016/j.preteyeres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Thebpatiphat N, Hammersmith K, Rocha F. Acanthamoeba Keratitis: a parasite on the rise. Cornea. 2007;26:701–706. doi: 10.1097/ICO.0b013e31805b7e63. [DOI] [PubMed] [Google Scholar]
- 17.Hammersmith K. Diagnosis and management of acanthamoeba keratitis. Current Opinion in Ophthalmology. 2006;17:327–331. doi: 10.1097/01.icu.0000233949.56229.7d. [DOI] [PubMed] [Google Scholar]
- 18.Kanavi MR, Javadi M, Yazdani S, Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007 Aug;26(7):782–786. doi: 10.1097/ICO.0b013e318064582d. [DOI] [PubMed] [Google Scholar]


