Abstract
Neurodegenration is a pathological hallmark of Alzheimer's disease (AD), but the underlying molecular mechanism remains elusive. Here, we present evidence that reveals a crucial role of Wnt5a signaling in this process. We showed that Wnt5a and its receptor Frizzled-5 (Fz5) were up-regulated in the AD mouse brain, and that beta-amyloid peptide (Aβ), a major constituent of amyloid plaques, stimulated Wnt5a and Fz5 expression in primary cortical cultures; these observations indicate that Wnt5a signaling could be aberrantly activated during AD pathogenesis. In support of such a possibility, we observed that inhibition of Wnt5a signaling attenuated while activation of Wnt5a signaling enhanced Aβ-evoked neurotoxicity, suggesting a role of Wnt5a signaling in AD-related neurodegeneration. Furthermore, we also demonstrated that Aβ-induced neurotoxicity depends on inflammatory processes, and that activation of Wnt5a signaling elicited the expression of proinflammatory cytokines IL-1β and TNF-α whereas inhibition of Wnt5a signaling attenuated the Aβ-induced expression of the cytokines in cortical cultures. Our findings collectively suggest that aberrantly up-regulated Wnt5a signaling is a crucial pathological step that contributes to AD-related neurodegeneration by regulating neuroinflammation.
Introduction
Beta-Amyloid (Aβ) peptide is a dominant candidate of the causative agents for Alzheimer's disease (AD) [1], [2]. According to the widely-held amyloid hypothesis of AD, Aβ initiates an array of molecular and cellular cascades that eventually lead to progressive neuronal dysfunction and degeneration [1], [2]. However, mechanistic molecular processes that link Aβ and neurodegeneration remain to be firmly established.
Chronic neuroinflammation associated with persistent glial activation is a major disease process evoked by Aβ and intimately associated with the progress of AD pathologies [3], [4]. Previous studies suggest that neuroinflammation contributes to the development of neurodegenerative hallmarks in AD brains, including Aβ plaques [4] and tau tangles [5], [6], [7]. AD therapeutic approaches that target neuroinflammation are under development [8], [9], [10], [11]. AD neuroinflammation is likely triggered by Aβ-mediated activation of microglia and astrocytes [3], [4], [12],[13],[14]. It was reported that Aβ induces the expression of cytokines (including IL-1β, TNF-α, IL-6, and IL-8) in cultured astrocytes and microglia [15], [16], [17], [18]. Mounting evidence suggests that Aβ may activate glial cells via specific sensor receptors such as toll-like receptors (TLR), receptors for advanced glycoxidation end-products (RAGE) and NOD-like receptors (NLR) [4]. Despite the significant understandings on the induction of AD neuroinflammation, the downstream molecular processes that are elicited by Aβ and regulate the inflammation remain poorly understood.
Wnts are secreted signaling proteins that play important roles in neural development and plasticity [19], [20], [21], [22]. Multiple lines of evidence indicate a critical role of Wnt signaling in AD [22]. β-catenin, a key downstream effector protein in the canonical Wnt signaling pathway, interacts with and is regulated by presenilin [23], [24], [25]. Glycogen synthase kinase (GSK)-3, a central serine/threnine kinase in the canonical Wnt signaling pathway, plays a critical role in the regulation of Aβ production [26] and aggregation [27] and in tau phosphorylation [28]. Genetic studies revealed that LRP6 polymorphisms are causally linked to AD [29]. In AD brains, canonical Wnt signaling is impaired [30], and DKK1, an antagonist of Wnt signaling, is upregulated [31], [32]. Importantly, Aβ was reported to inhibit Wnt signaling by directly binding to the Frizzled receptors [33]. The impairment of canonical Wnt signaling is likely etiologically significant, because forced up-regulation of the canonical Wnt signaling pathway has rescuing effects on the development of AD-related phenotypes in both neuron cultures and animal models [27], [30], [34], [35]. In contrast to the canonical pathway, the involvement of non-canonical Wnt signaling pathways in the regulation of AD pathogenesis is less clear. A recent study indicates that Wnt5a-activated non-canonical Wnt signaling antagonizes Aβ synaptotoxicity [36].
In this paper, we report an important role of Wnt5a signaling in the regulation of Aβ-evoked neurotoxicity and neuroinflammation. We observed that (1) Wnt5a/CaMKII signaling is up-regulated at the early stages of AD development in an APPswe/PSEN1ΔE9 transgenic mouse model, (2) Aβ activates Wnt5a signaling in primary cortical cultures, (3) Aβ-induced Wnt5a up-regulation is a critical molecular step leading to the development of Aβ neurotoxicity in cultures, (4) Wnt5a stimulates inflammatory processes, and (5) Wnt5a is critical for Aβ-induced inflammatory response. Our results suggest that abnormally up-regulated non-canonical Wnt5a signaling may regulate chronic neuroinflammation in AD brains.
Materials and Methods
Preparation of Aβ42 peptides
Recombinant human Aβ (1–42) (Chemicon) was used to prepare monomers (Aβ-mon), oligomers (Aβ-olig) and fibrils (Aβ-fib), as described [37]. For monomer preparation, 5 mM Aβ42 in DMSO was diluted directly with cell culture media. For oligomer preparation, 5 mM Aβ42 in DMSO was diluted to 100 µM in ice-cold D-MEM/F-12 (Invitrogen), vortexed for 30″, centrifugated (10,000 g; room temperature) for 1′, and incubated at 4°C for 24 h. For fibril preparation, 10 mM HCl was added to the Aβ42 solution (5 mM in DMSO) to bring Aβ to a final concentration of 100 µM, vortexed for 30″, centrifugated (10,000 g, room temperature) for 1′, and incubated with shaking at 37°C for 24 h. Products from such preparations are mixtures that contain Aβ monomers and the intended aggregates [37]. The quality of the preparations was confirmed by Western blotting analysis with the 6E10 (Covance; 1∶5000), and the molecular mass was estimated by rainbow pre-stained protein markers (GE Healthcare).
Animals and hippocampus dissection
All procedures were approved by Animal Care and Use Committees of the University of Texas Medical Branch. Male APP (amyloid precursor protein)/presenilin-1 double transgenic (APPswe/PSEN1ΔE9, 2×Tg) mice [38] and wild-type littermates at 3.5 months of age were anesthetized and then decapitated. The hippocampi were rapidly collected on ice for Western blotting, immunohistochemistry, or fluorescent immunostaining..
Neuron cultures
C57BL E18 cortical cultures were prepared, as described [39]. The cells were plated on poly-D-lysine (30,000–70,000; Sigma) -coated dishes at a density of 1.5×105 cells/cm2. Immunostaining with cell-type markers indicate that the cultures are mixtures of neuron (∼60%) and glial cells (∼40%). Cultures at 12–14 DIV were used in this study. All experimental treatments were carried out by adding the administrated agents into the freshly changed culture media. Only morphologically healthy neuronal cultures were used for drug treatments.
Western blotting and antibodies
Western blotting was performed as described [40]. The intensity of non-saturated bands on Western blots was quantified by densitometry analysis with NIH ImageJ. GAPDH or β-actin were used as loading controls. Rabbit-anti-APP antibody was from Sigma-Aldrich (1∶3000); rabbit-anti-Wnt5a (1∶1000) and rabbit-anti-Fz5 (1∶1000) antibodies were from Abcam; rabbit-anti-p-αCaMKII(Thr286) (1∶1000), rabbit-anti-NIK (1∶1000), and rabbit-anti-IκB-α (1∶1000) antibodies were from Cell Signaling Technology; and mouse-anti-GAPDH (1∶1000) and rabbit-anti-β-actin (1∶1000) antibodies were from Santa Cruz Biotechnology.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed on 5 µm-thick paraffin-embedded sections. The sections were deparaffined, dehydrated and treated with 1% H2O2 as decribed [41] Antigen retrieval was carried out using citrate buffer (0.01 M; pH 6.0). The sections were then incubated with the primary antibody recognizing Aβ (1–40/42) (Millipore; 1∶100) overnight at 4°C followed by incubation with biotinylated goat anti-rabbit IgG. After treatment with streptavidin-HRP and staining with DAB, the sections were stained with hematoxylin and mounted. Indirect fluorescence immunostaining was performed, as described [42].with rabbit anti-Wnt5a (abcam; 5 µg/ml) and rabbit anti-Fz5 (abcam; 1 µg/ml) primary antibodies and Cy3-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch; 1∶200).
Measurement of secreted cytokines
Cortical cultures (12–14 DIV) were treated with Wnt5a (200 ng/ml), and media were collected at 0.5, 1, 2, 6, 12 and 24 h after Wnt5a application. Tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) in collected media were quantified using Mouse TNF-α or IL-1β Quantikine ELISA Kits (R&D Systems), respectively.
MTT assay and trypan blue staining
The MTT assay and trypan blue staining were performed as described in [43]. Three independent experiments were performed with each experiment including 4–6 replicates for each treatment. Reagents: 3% hydrogen peroxide solution (Sigma-Aldrich); recombinant mouse Wnt-5a (R&D Systems); anti-mouse Wnt-5a antibody (R&D Systems); anti-Fz5 antibody (against the extracellular cysteine-rich domain of human Fz5) (R&D Systems); Foxy5 peptide (Inbiolabs); Box5 peptide (Inbiolabs); recombinant mouse IL-10 (R&D Systems); and activated protein C (APC) (Sigma-Aldrich).
Statistical analysis
Data were expressed as the means ± SEM. Statistical analysis was performed with the Prism software (GraphPad). We used the Student's two-tailed t-test for statistical comparisons between any two groups, and one-way ANOVA analyses with a Bonferroni post-hoc test for comparisons between multiple groups.
Results
Increased expression of key proteins in the non-canonical Wnt signaling pathway in the hippocampus of AD mouse models
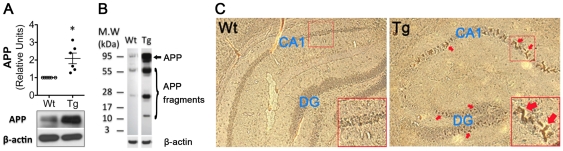
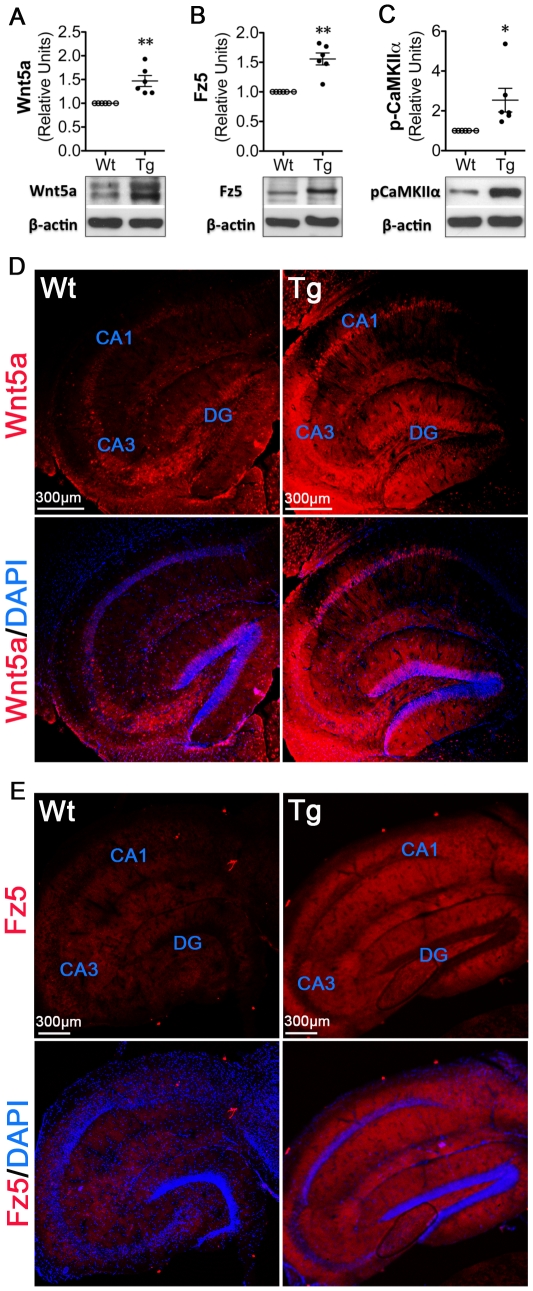
Previous studies revealed down-regulation of canonical Wnt signaling in the brain of APPswe/PSEN1ΔE9 AD mouse models [27]. Consistent with these findings, we observed decreased expression of proteins in the canonical pathway, including Wnt3a, Fz1 and β-catenin (data not shown). In contrast, in preliminary studies, we also observed up-regulated expression of several proteins implicated in the non-canonical Wnt signaling pathway, including Wnt5a and its receptor Fz5, at various postnatal stage (3.5–9.5 months). At 3.5 months of age, APP transgene was clearly expressed (Fig. 1A). APP processing expected to generate Aβ was evident in the transgenic hippocampi (Fig. 1B). In addition, immunohistochemistry staining with an antibody that recognized Aβ revealed clustered signals in the cell body layer (Fig. 1C), which may include Aβ that starts accumulating. Because memory deficits have not manifested at this stage [44], we reasoned that the up-regulation of non-canonical Wnt signaling is potentially an early molecular abnormality during AD pathogenesis. Therefore, we performed more detailed analysis on the non-canonical Wnt signaling in mice at 3.5 months of age. Western blotting analysis showed that, compared with wild-type controls, Wnt5a protein increased 1.5 fold in the hippocampus of the 2×Tg mouse (p<0.01; Fig. 2A). Similarly, Fz5 protein was also significantly up-regulated in the 2×Tg hippocampus (1.6 fold; p<0.01; Fig. 2B). Because activation of non-canonical Wnt signaling can cause αCaMKII phosphorylation [45], we next determined the protein level of phosphorylated αCaMKII (pT286-αCaMKII). pT286-αCaMKII was increased 2.6 fold (p<0.05; Fig. 2C). Fluorescent immunostaining showed that, in comparison with control, the increase of Wnt5a (Fig. 2D) and Fz5 (Fig. 2E) occurred in all hippocampal fields, including CA1, CA2/CA3 and dentate gyrus (DG), of the 2×Tg mouse, which resembled the spatial pattern of Aβ plaques (Fig. 1C). These data together suggest that non-canonical Wnt signaling is up-regulated in the 2×Tg AD hippocampus.
Figure 1. Aβ increases significantly in the hippocampus of the APPswe/PSEN1ΔE9 (2×Tg) mouse at the age of 3.5 months.
A, APP in the wild-type and 2×Tg hippocampus (3.5-months). B. APP processing in the hippocampus revealed by immunoblotting with the monoclonal antibody 6E10 (recognizing residues 1–16 of Aβ). C. Immunohistochemical staining of the hippocampus with 6E10. Note the clustered Aβ signals (arrows) in the cell body layer of the 2×Tg hippocampus. Similar results were obtained from 6 pairs of wild-type and 2×Tg mice.
Figure 2. Wnt5a/CaMKII signaling is up-regulated in the hippocampus of the 2×Tg AD mouse model.
A, B, and C. Protein levels of Wnt5a (A), Fz5 (B) and pT286-αCaMKII (C) in the wild-type and 2×Tg hippocampus. In summary graphs of quantitative data, the levels of target proteins were normalized with the β-actin loading control and expressed as relative units to the wild-type controls. Data presented in graphs are means ± SEM from six pairs of mice. * p<0.05. D and E. Distribution of Wnt5a (D) and Fz5 (E) protein in the wild-type and 2×Tg hippocampus. DAPI staining was performed to visualize the cell bodies.
Rapid up-regulation of non-canonical Wnt signaling by Aβ oligomers in primary neuron culture
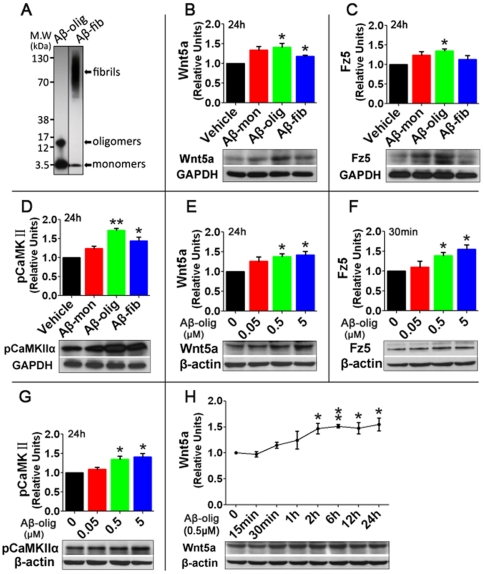
We next sought to understand the mechanism by which non-canonical Wnt signaling is activated in the AD mouse brain. Because Aβ is a key pathological agent for AD, we hypothesized that it causes the up-regulated expression of Wnt5a and other relevant proteins measured in 2×Tg mice. To test this hypothesis, we determined the effect of Aβ on the expression of these proteins in mouse cortical cultures (12–14 DIV). A wide range of Aβ concentrations (100 nM - 15 µM) have been used in previous in vitro studies. We chose to use a relatively low Aβ concentration (500 nM) to better reflect the fact that the proteins are up-regulated at early AD stages when Aβ begins to accumulate (Fig. 1C). Prior works have shown that 500 nM Aβ is efficient to initiate some of the earliest and reversible AD-relevant pathologies [46], [47]. More recent studies have indicated that different forms of Aβ (monomer, oligomer and fibril) vary in their AD-related toxicity, with the oligomer being considered the most toxic form [37], [48]. We prepared the oligomers and fibrils of Aβ (1–42) (Fig. 3A) and compared the effect of Aβ monomers, oligomers and fibrils on Wnt5a, Fz5 and pT286-αCaMKII protein expression in cortical cultures. As shown in Fig. 3B–D, Aβ oligomer treatment for 24 h caused significant increases in Wnt5a (1.4 fold; p<0.05), Fz5 (1.4 fold; p<0.01) and pT286-αCaMKII (1.7 fold; p<0.01). Under the same experimental conditions, preparations of monomers and fibrils caused changes with lower magnitudes (Fig. 3B–D). Next, we investigated the dose effects of the oligomer. As shown in Fig. 3E–G, the oligomer increased expression of Wnt5a, Fz5, and pT286-αCaMKII proteins in a concentration-dependent manner (50 nM–5 µM). Although Wnt5a and p-CaMKII displayed a clear dose-effect at 24 hours after treatment, Fz5 showed this effect at 30 min. In addition, 500 nM of Aβ oligomer caused a time-dependent increase in Wnt5a (Fig. 3H). These results demonstrated that the non-canonical Wnt5a signaling is up-regulated by Aβ peptide, especially the oligomers.
Figure 3. Up-regulation of proteins in the non-canonical Wnt signaling pathway by Aβ peptide in cortical neuron cultures.
A. Western blot analysis of the preparations of Aβ (1–42) oligomers and fibrils. Aβ preparations were, separated on SDS–PAGE (4–12% NuPAGE bis-Tris gel), and probed with the monoclonal antibody 6E10.. B, C, and D. Protein levels of Wnt5a (B), Fz5 (C) and pT286-αCaMKII (D) in cortical cultures treated with 500 nM of monomeric, oligomeric, and fibrillar Aβ peptide for 24 hrs. E, F, and G. Concentration-dependent effects of Aβ oligomer on the protein levels of Wnt5a (E), Fz5 (F) and pT286-αCaMKII (G). Although the dose effect of Aβ on Wnt5a was most clearly observed at 24 hours post Aβ administration, the effect on Fz5 could be observed at 30 min. H. Time course of the Aβ oligomer effects (500 nM) on Wnt5a. In summary graphs of quantitative data, the levels of target proteins were normalized against GAPDH or β-actin and expressed as relative units to the vehicle-treated controls. Data in summary graphs are means ± SEM from three independent experiments. * p<0.05; ** p<0.01.
Suppression of Wnt5a signaling alleviates Aβ neurotoxicity
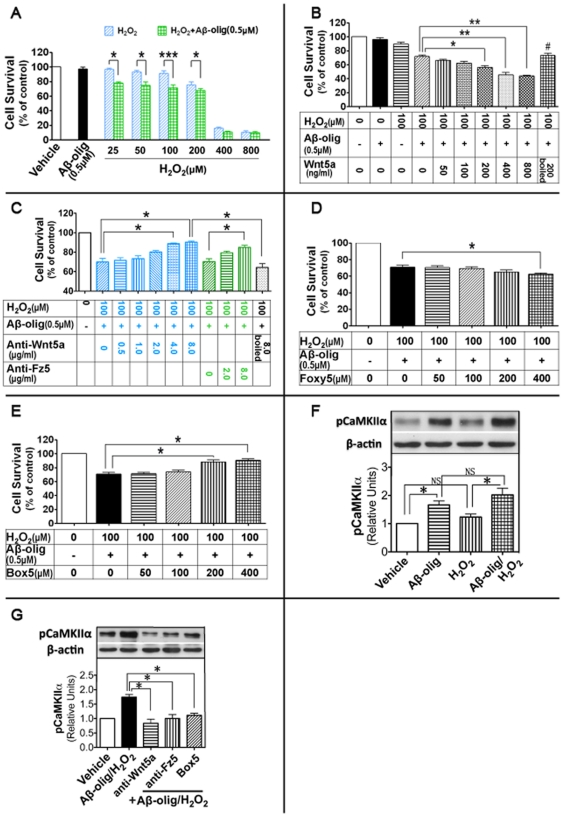
The observations of the upregulation of the Wnt5a signaling in early AD mouse brains and by Aβ in cortical cultures suggest a potential role of Wnt5a signaling in AD pathogenesis. We next sought to directly test this idea by determining the significance of Aβ-mediated Wnt5a up-regulation on Aβ cytotoxicity in cortical cultures. Previous work revealed concentration-dependent neurotoxicity of Aβ peptides [37], [49], [50], with high concentrations of Aβ (>5.0 µM) causing neuron death in cultures and low concentrations of Aβ (0.1∼1.0 µM) sensitizing neurons to become vulnerable to further stresses [50]. To mimic the early AD brain condition when the non-canonical Wnt signaling proteins are aberrantly upregulated, in these experiments we used a relatively low concentration (500 nM) of Aβ that can upregulate Wnt5a and Fz5 (Fig. 3). Aβ oligomer at this concentration did not cause cell death, as measured by MTT assays (Fig. 4A). We also used trypan blue staining to confirm this observation (Fig. S1). This finding is consistent with the idea that in the early stages of AD pathogenesis Aβ may not cause neuron death. Next, we wanted to investigate if Aβ at this concentration causes the cells to become more vulnerable to detrimental stresses that are relevant to AD pathogenesis. To this end, we tested the effect of Aβ on the sensitivity of the cultures to hydrogen peroxide (H2O2) stresses. Hydrogen peroxide was chosen because it is generated during the very early stages of aggregation of the amyloid peptides [51] and is critically involved in AD pathogenesis [49], [52]. At low concentrations (25–100 µM), hydrogen peroxide did not cause cell death by itself; however, when co-administrated with Aβ oligomers (500 nM) it caused marked cell death (Fig. 4A, Fig. S1). These results indicate that neurons exposed to 500 nM Aβ are more vulnerable to other detrimental factors. We also showed that H2O2 neither stimulated αCaMKII phosphorylation by itself nor significantly enhanced the Aβ-induced phsphorylation (Fig. 4F), indicating that H2O2 did not activate the non-canonical pathway.
Figure 4. Wnt5a signaling regulates Aβ neurotoxicity.
The MTT assay was used to measure cell viability in cortical cultures at 24 hrs after indicated treatments. A. Cell survival rates in cultures challenged with Aβ oligomers, H2O2, or a combination of Aβ oligomers and H2O2. B. Cultures challenged with Aβ (500 nM) and H2O2 (100 µM) in the presence of Wnt5a at different concentrations. C. Cultures challenged with Aβ and H2O2 in the presence of anti-Wnt5a or ant-Fz5 antibodies. D. Effects of Foxy5 on the neurotoxicity of Aβ/H2O2. E. Effects of Box5 on the neurotoxicity of Aβ/H2O2. Data are expressed as mean ± SEM (n≥12 from three independent experiments). * p<0.05; ** p<0.01; *** p<0.001. # p<0.05 (vs. the group of Wnt5a (200 ng/ml)+Aβ/H2O2). F. Protein levels of pT286-αCaMKII in cortical cultures treated with Aβ (500 nM), H2O2 (100 µM), or the combination of Aβ and H2O2. G. Protein levels of pT286-αCaMKII in cortical cultures treated with anti-Wnt5a (4 µg/ml), anti-Fz5 (4 µg/ml) antibodies or Box5 (200 µM), in the presence of Aβ (500 nM) and H2O2 (100 µM). * p<0.05; NS, not significant.
To determine the potential role of Wnt5a up-regulation in Aβ-induced sensitization to H2O2 stress, we neutralized Wnt5a in media with specific anti-Wnt5a antibody. As shown in Fig. 4C, the antibody (0.5–8.0 µg/ml) displayed a rescue effect on cell death in a concentration-dependent manner, which reached statistical significance after 4.0 µg/ml. Recent studies identified a modified Wnt5a-derived hexapeptide (Box5) that can specifically antagonize Wnt5a in malignant melanoma cells [53]. Similar to the anti-Wnt5a antibody, Box5 also had a rescue effect on Aβ/H2O2-induced cell death in a concentration-dependent manner (Fig. 4E). Because Fz-5 is a Wnt5a receptor [54], we further tested the effect of anti-Fz5 antibody (against the extracellular cystein-rich domain). Consistent with the findings with anti-Wnt5a antibody and Box5, the anti-Fz5 antibody also had a rescue effect (Fig. 4C). We further tested the effect of, anti-Wnt5a, anti-Fz5 antibodies and Box5 on the phosphorylation of αCaMKII, a molecular marker of the activation of the non-canonical Wnt/Ca2+ pathway. The results showed that all these reagents significantly blocked Aβ/H2O2-induced up-regulation of p-αCaMKII (Fig. 4G), and thus provide an independent molecular confirmation of the activity of anti-Wnt5a, anti-Fz5 antibodies and Box5. These results together strongly suggest that Aβ-induced Wnt5a up-regulation at least partially mediates Aβ neurotoxicity.
Exogenous Wnt5a potentiates Aβ neurotoxicity
Because Aβ-induced Wnt5a up-regulation is critical for Aβ neurotoxicity (Fig. 4C and E), we next investigated if exogenous Wnt5a can potentiate Aβ/H2O2-induced cell injury. To this end, we tested the effect of recombinant Wnt5a on Aβ/H2O2 neurotoxicity. As shown in Fig. 4B, compared with Aβ/H2O2-treated cultures, addition of purified recombinant Wnt5a protein (50–800 ng/ml) increased cell death in a concentration-dependent manner. This potentiation activity of Wnt5a was completely abolished when the protein was heat-inactivated (Fig. 4B). This observation indicates that the biochemical activity of Wnt5a rather than the mere presence of Wnt5a peptide is essential for the potentiation. In addition, Foxy5, a Wnt5a-derived hexapeptide (the same peptide as Box5 but differently modified) that can mimic Wnt5a-induced activities in malignant melanoma and in breast cancer cells [55], was also able to potentiate the neurotoxicity of Aβ/H2O2 (Fig. 4D). These results indicate that Wnt5a is sufficient to elicit neurotoxicity.
Anti-inflammation factors attenuate Aβ neurotoxicity
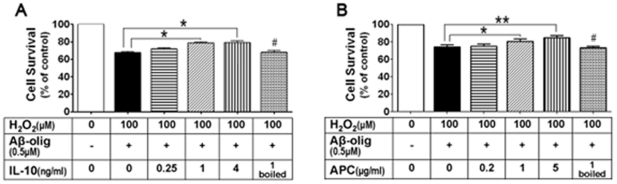
The results described above indicate that up-regulated Wnt5a may mediate Aβ neurotoxicity. We then sought to understand the mechanism by which Wnt5a contributes to Aβ neurotoxicity. Because recent studies revealed a critical role of Wnt5a in the regulation of inflammatory responses in peripheral systems, we were interested in determining if Wnt5a contributes to Aβ neurotoxicity by regulating inflammation. To this end, we first investigated the contribution of neuroinflammation to the Aβ-induced sensitization of cortical cultures to H2O2 stress. We found that IL-10, a prototypic anti-inflammatory cytokine, caused concentration-dependent rescue effects on Aβ toxicity (Fig. 5). IL-10 started to rescue Aβ/H2O2-induced cell death at 0.25 ng/ml and reached a plateau effect at 1 ng/ml (Fig. 5A). Heat-inactivated IL-10 did not have any rescuing effect (Fig. 5A). To further confirm the involvement of inflammation in the Aβ toxicity, we determined the effect of activated protein C (APC), another well established anti-inflammatory protein. The results showed that APC displayed a similar rescue effect as IL-10 (Fig. 5B). These results indicate that neuroinflammatory response likely contributes to Aβ neurotoxicity.
Figure 5. Anti-inflammatory factors alleviate Aβ neurotoxicity.
A. Effects of IL-10 on cell viability of cortical cultures challenged with Aβ/H2O2 for 24 hrs. B. Effects of APC. Data (means ± SEM) are from three independent experiments. * p<0.05; ** p<0.01; # p<0.05 (vs. the group treated with active IL-10 (1 ng/ml) or APC (1 µg/ml) in the presence of Aβ/H2O2).
Wnt5a regulates proteins that involve in inflammatory response in cortical cultures
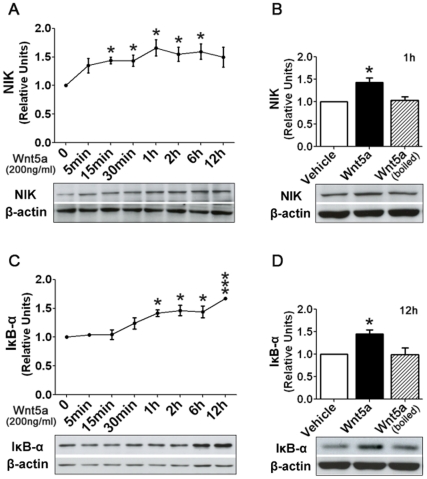
Recent studies reveal an important role of Wnt5a in control of inflammatory response in non-neuronal peripheral tissues [56], [57], [58], but its involvement in regulation of inflammatory response in nervous systems has not been reported. To investigate the potential contribution Wnt5a signaling to neuroinflammation, we examined the effect of Wnt5a on the protein expression of NF-κB-inducing kinase (NIK), a key positive regulator of inflammation that controls NF-κB activity by promoting the processing of p100, the inactive NF-κB2 precursor, to produce the functional p52 subunit [59]. We observed that NIK protein began to increase 5 min after Wnt5a administration (200 ng/ml), and this increase continued gradually, peaked at 60 min. and stayed at a higher level afterward (Fig. 6A). Heat-inactivated Wnt5a did not induce a NIK increase (Fig. 6B), indicating that Wnt5a protein activates inflammatory pathways in cortical cultures. We also investigated the effect of Wnt5a on the expression of IκB-α protein, an inhibitory regulator of inflammation response by sequestering NF-κB in the cytosol [60]. Interestingly, Wnt5a also induced a gradual increase of IκB-α (Fig. 6C). In contrast to the NIK increase, the IκB-α up-regulation did not begin until 30–60 min after Wnt5a treatment but continued for the rest of the time that measurements were recorded (Fig. 6C). It is possible that the lag in up-regulation of IκB-α functions to resolve the Wnt5a-initiated inflammatory response.
Figure 6. Wnt5a activates molecular pathways critical for inflammatory response.
A. The time course of NIK protein dynamics after Wnt5a (200 ng/ml) treatment. B. Heat-inactivated Wnt5a cannot induce NIK. C. The time course of IκB-α protein dynamics after Wnt5a (200 ng/ml) treatment. D. Heat-inactivated Wnt5a cannot induce of IκB-α protein. * p<0.05; *** p<0.001 (vs. 0 h in A and C, or vs. vehicle in B and D).
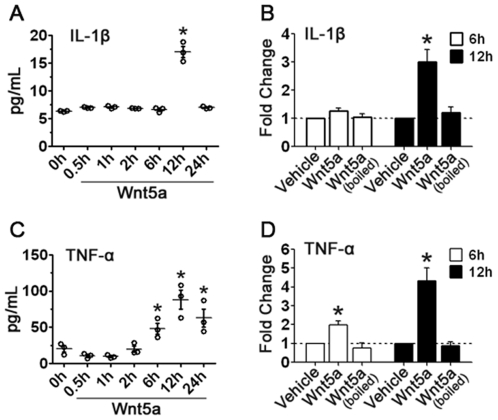
Inflammatory response culminates with the secretion of an array of inflammatory cytokines. If Wnt5a indeed induces the inflammatory response, specific inflammatory cytokines must be secreted after Wnt5a administration. Thus, we used ELISA to measure IL-1β and TNF-α in culture media after Wnt5a treatment for 0.5, 1, 2, 6, 12, or 24 hrs. We observed that Wnt5a stimulation caused a 2.7-fold increase in IL-1β at 12 h, while no change was detected at other time points (Fig. 7A). On the other hand, TNF-α increased significantly at 6 h (2.3 fold), 12 h (4.3 fold) and 24 h (3.1 fold) (Fig. 7C). However, this effect of Wnt5a was completely abolished when the protein was heat-inactivated (Fig. 7B and D). These results together strongly suggest that Wnt5a can elicit the inflammatory response in neuron cultures.
Figure 7. Wnt5a evokes cytokine release.
A. The temporal profiles of IL-1β in media of Wnt5a-treated cortical cultures measured by ELISA. B. Heat-inactivation abolished the activity of Wnt5a in eliciting IL-1β release. C. The temporal profiles of TNF-α in media after Wnt5a treatment. D. Effects of heat-inactivation on the Wnt5a activity in eliciting TNF-α release. * p<0.05 (vs. 0 h in A and C, or vs. vehicle in B and D).
Blockage of Wnt5a signaling impairs Aβ-induced up-regulation of proinflammatory cytokines
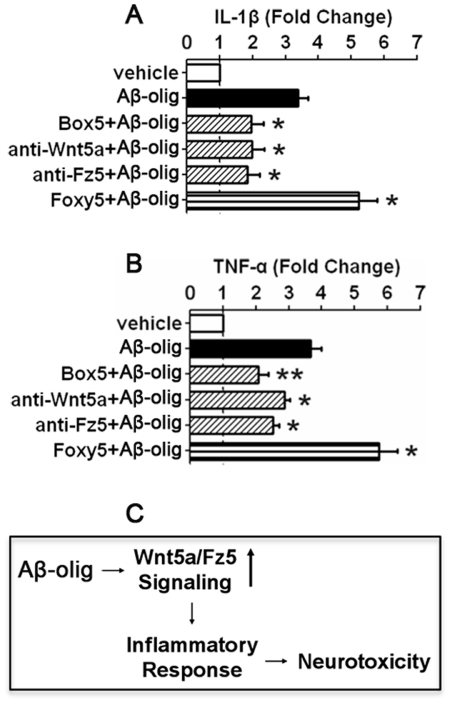
Because Aβ evoked inflammation-mediated neurotoxicity, we next investigated the effect of Wnt5a signaling inhibition on the Aβ-elicited inflammatory response. As shown in Fig. 8A, Aβ oligomers (500 nM) significantly increased IL-1β release (3.4 fold; p<0.05). Box5 peptide (200 µM), anti-Wnt5a (2 µg/ml) or anti-Fz5 (2 ug/ml) antibody suppressed the increase of IL-1β induced by Aβ, by 58%, 59% and 55%, respectively (all 3 with p<0.05). In contrast, Foxy5 (200 µM), an agonist of Wnt5a, increased IL-1β release (1.5 fold, compared with Aβ alone; p<0.05; Fig. 8A). Box5, anti-Wnt5a and anti-Fz5 antibodies caused similar effects on Aβ-induced TNF-α release (Fig. 8B). These results together demonstrate that the suppression of Wnt5a signaling inhibits Aβ-evoked inflammatory responses in cortical neuron cultures.
Figure 8. Wnt5a is critical for Aβ-elicited cytokine release.
A. Inhibition of Wnt5a/Fz5 signaling impaired Aβ-evoked IL-1β increase in media. B. Inhibition of Wnt5a/Fz5 signaling impaired Aβ-evoked TNF-α increase in media. ELISA assay of IL-1β (A) and TNF-α (B) secreted in cortical cultures treated for 12 hrs with Aβ (500 nM) or Aβ and Box5 (200 µM), anti-Wnt5a (2 µg/ml) or anti-Fz5 (2 µg/ml) antibody. Foxy5, a Wnt5a agonist, potentiates the cytokine release. * p<0.05; ** p<0.01 (vs. vehicle). C. A working model for the role of Wnt5a signaling in AD pathogenesis.
Discussion
In this study, we found that non-canonical Wnt5a signaling is up-regulated in mouse brains prior to AD phenotypes and by Aβ peptide in cortical neuron cultures. The up-regulated Wnt5a signaling contributes to the inflammation-dependent Aβ neurotoxicity in cultures. We also found that Wnt5a up-regulates inflammation regulatory proteins and proinflammatory cytokines and that Wnt5a is required for the Aβ-induced proinflammatory cytokines. These observations collectively suggest the following working model (Fig. 8C): accumulation of Aβ in the brain aberrantly up-regulates Wnt5a signaling, which in turn evokes an inflammatory response that causes neurodegeneration or cell death in AD brains.
The observed up-regulation of Wnt5a signaling is probably an early etiologically relevant event during AD development. Both Wnt5a and Fz5 proteins significantly increase in the APPswe/PSEN1ΔE9 hippocampus at the age of 3.5 months (Fig. 2). Previous studies showed that this AD mouse model started to accumulate Aβ plaques after 4 months of age [61] and did not develop cognitive impairments until 5–7 months of age [44]. Thus, the observed Wnt5a and Fz5 up-regulation at 3.5 months of age is likely prior to the development of major AD phenotypes. This notion is consistent with the finding that a relatively low concentration of Aβ is able to up-regulate Wnt5a and Fz5 (Fig. 3), suggesting that Wnt5a signaling is a potential target for slowing or blocking early AD pathogenesis.
Converging lines of evidence support a critical role of the down-regulation of the canonical Wnt/β-catenin pathway in AD pathogenesis. In contrast, the involvement of non-canonical Wnt signaling is less clear. Our findings reveal an early up-regulation of Wnt5a signaling in the hippocampus of 2×Tg AD mice. Etiological significance of this dysregulation is suggested by the observation that Wnt5a signaling is necessary for Aβ to fully induce neurotoxicity in cortical cultures. Previous studies demonstrated that down-regulation of canonical signaling contributed to Aβ neurotoxicity [27], [30], [34], [62]. It is possible that Aβ causes parallel up-regulation of the non-canonical Wnt signaling and down-regulation of the canonical signaling to initiate neurotoxicity cascades. The Wnt canonical and non-canonical pathways often antagonize one another [63]. Thus, another possible scenario is that Aβ may directly down-regulate the canonical pathway, as suggested by a recent study [33], which consequently causes the up-regulation of the non-canonical pathway.
Our results reveal a neurotoxic activity of Wnt5a signaling, and this Wnt5a activity contributes to Aβ toxicity in neuron cultures. Cerpa et al. recently reported that acute administration of exogenous Wnt5a (500 nM) prevented Aβ-induced synaptotoxicity within 40 min after Aβ oligomer application [36]. Their results indicate a synapto-protective activity of Wnt5a signaling soon after Aβ exposure. Because Aβ-up-regulated Wnt5a does not occur by 1 hour after Aβ treatment in cultures (Fig. 3H) and 500 nM Aβ itself does not induce obvious cell death in this period (Fig. 4A and S1), we reason that basal Wnt5a has a synapto-protective activity. On the other hand, sustained up-regulation of Wnt5a, which occurs at 2 hours after Aβ treatment (Fig. 3H), probably potentiates neurotoxicity.
We further found that activation of Wnt5a signaling stimulates the expression of proinflmmatory cytokine in cortical cultures (Figs. 6 and 7). This finding indicates that up-regulation of Wnt5a may mediate Aβ-induced neuroinflammation in AD brains (Fig. 8C). Because the Aβ-elicited inflammatory response (Fig. 8A, 8B) and alleviated Aβ-induced neurotoxicity (Fig. 4) was impaired by the anti-Wnt5a antibody and Box5, Aβ likely induces Wnt5a secretion, although the kinetics of the secretion is currently unknown. In peripheral non-neuronal systems, Wnt5a is implicated in inflammation of multiple chronic disorders, including rheumatoid arthritis [64], sepsis [56], atherosclerosis [65], melanoma [66], and psoriasis [67]. Our results provide the initial evidence for a critical role of Wnt5a signaling in the regulation of inflammatory responses in CNS disorders. Because the primary cortical cultures used in this study contain neurons and glia (including microglia and astrocytes), we currently do not know the specific type of glial cells through which Wnt5a evokes the observed inflammatory responses. In a recent study, Halleskog et al. reported that Wnt3a stimulated the expression of proinflammatory cytokines in microglia [68]. It would be interesting to know if Wnt5a regulates neuroinflammation by stimulating the same or different types of glia. Nonetheless, the findings on Wnt5a and Wnt3a indicate that proteins in the Wnt family may orchestrate neuroinflammatory response during AD pathogenesis.
Supporting Information
Cell survival rates revealed by trypan blue staining. Cortical cultures at 24 hrs after indicated treatments were used. Dying cells were stained due to the increase of membrane permeability to the dye.
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the start-up funds from the University of Texas Medical Branch to S-JT and by grant No. 30873457 from the National Natural Science Foundation of China and No. 2008A060202010 and 2010B050700019 from the Scientific Technology Project of Guangdong Province of China to LZ. BL is a Ph.D. student fellow supported by China Scholarship Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer's Disease: Progress and Problems on the Road to Therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-[beta] in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004;295:245–257. doi: 10.1016/j.yexcr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Gorlovoy P, Larionov S, Pham TT, Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. Faseb J. 2009;23:2502–2513. doi: 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- 8.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 9.Carter MD, Simms GA, Weaver DF. The development of new therapeutics for Alzheimer's disease. Clin Pharmacol Ther. 2010;88:475–486. doi: 10.1038/clpt.2010.165. [DOI] [PubMed] [Google Scholar]
- 10.Scali C, Giovannini MG, Prosperi C, Bellucci A, Pepeu G, et al. The selective cyclooxygenase-2 inhibitor rofecoxib suppresses brain inflammation and protects cholinergic neurons from excitotoxic degeneration in vivo. Neuroscience. 2003;117:909. doi: 10.1016/s0306-4522(02)00839-4. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, et al. [beta]-Amyloid-Induced Inflammation and Cholinergic Hypofunction in the Rat Brain in Vivo: Involvement of the p38MAPK Pathway. Neurobiology of Disease. 2002;11:257. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- 12.Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer's disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maezawa I, Zimin PI, Wulff H, Jin L-W. Amyloid-β Protein Oligomer at Low Nanomolar Concentrations Activates Microglia and Induces Microglial Neurotoxicity. Journal of Biological Chemistry. 2011;286:3693–3706. doi: 10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parachikova A, Vasilevko V, Cribbs DH, LaFerla FM, Green KN. Reductions in Amyloid-β-Derived Neuroinflammation, with Minocycline, Restore Cognition but do not Significantly Affect Tau Hyperphosphorylation. Journal of Alzheimer's Disease. 2010;21:527. doi: 10.3233/JAD-2010-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gitter BD, Cox LM, Rydel RE, May PC. Amyloid beta peptide potentiates cytokine secretion by interleukin-1 beta-activated human astrocytoma cells. Proc Natl Acad Sci U S A. 1995;92:10738–10741. doi: 10.1073/pnas.92.23.10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong Y. Effect of a carboxy-terminal fragment of the Alzheimer's amyloid precursor protein on expression of proinflammatory cytokines in rat glial cells. Life Sci. 1997;61:2323–2333. doi: 10.1016/s0024-3205(97)00936-3. [DOI] [PubMed] [Google Scholar]
- 17.Sondag CM, Dhawan G, Combs CK. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J Neuroinflammation. 2009;6:1. doi: 10.1186/1742-2094-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meda L, Baron P, Prat E, Scarpini E, Scarlato G, et al. Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid[25–35]. J Neuroimmunol. 1999;93:45–52. doi: 10.1016/s0165-5728(98)00188-x. [DOI] [PubMed] [Google Scholar]
- 19.Salinas PC, Zou Y. Wnt Signaling in Neural Circuit Assembly. Annual Review of Neuroscience. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Park CS, Tang SJ. Activity-dependent synaptic WNT release regulates hippocampal long-term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 21.Tang SJ. The synaptic Wnt signaling hypothesis. Synapse. 2007;61:866–868. doi: 10.1002/syn.20434. [DOI] [PubMed] [Google Scholar]
- 22.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, et al. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 24.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, et al. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, et al. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 27.Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry. 2010;15:272–285, 228. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- 28.Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 29.De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, et al. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- 31.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosi MC, Luccarini I, Grossi C, Fiorentini A, Spillantini MG, et al. Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. Journal of Neurochemistry. 2010;112:1539–1551. doi: 10.1111/j.1471-4159.2009.06566.x. [DOI] [PubMed] [Google Scholar]
- 33.Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, et al. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J Biol Chem. 2008;283:9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, et al. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 35.Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res. 2005;304:91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Cerpa W, Farias GG, Godoy JA, Fuenzalida M, Bonansco C, et al. Wnt-5a occludes Abeta oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol Neurodegener. 2010;5:3. doi: 10.1186/1750-1326-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, et al. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 38.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 39.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- 40.Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma F, Wang H, Chen B, Wang F, Xu H. Metallothionein 3 attenuated the apoptosis of neurons in the CA1 region of the hippocampus in the senescence-accelerated mouse/PRONE8 (SAMP8). Arq Neuropsiquiatr. 2011;69:105–111. doi: 10.1590/s0004-282x2011000100020. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 43.Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, et al. Short Protocols in Neuroscience: Systems and Behavioral Methods. Hoboken: Wiley; 2007. pp. 2–30. [Google Scholar]
- 44.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, et al. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 45.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 46.Parodi J, Sepulveda FJ, Roa J, Opazo C, Inestrosa NC, et al. Beta-amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J Biol Chem. 2010;285:2506–2514. doi: 10.1074/jbc.M109.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 48.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 49.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 50.Qin L, Liu Y, Cooper C, Liu B, Wilson B, et al. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 51.Tabner BJ, El-Agnaf OM, Turnbull S, German MJ, Paleologou KE, et al. Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J Biol Chem. 2005;280:35789–35792. doi: 10.1074/jbc.C500238200. [DOI] [PubMed] [Google Scholar]
- 52.Schubert D, Behl C, Lesley R, Brack A, Dargusch R, et al. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci U S A. 1995;92:1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenei V, Sherwood V, Howlin J, Linnskog R, Safholm A, et al. A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc Natl Acad Sci U S A. 2009;106:19473–19478. doi: 10.1073/pnas.0909409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, et al. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 55.Safholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, et al. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006;281:2740–2749. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- 56.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunology. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 58.Sen M, Ghosh S. Transcriptional outcome of Wnt-Frizzled signal transduction in inflammation: evolving concepts. J Immunology. 2008;181:4441–4445. doi: 10.4049/jimmunol.181.7.4441. [DOI] [PubMed] [Google Scholar]
- 59.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 60.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, et al. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, et al. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer's disease. Bipolar Disord. 2002;4:153–165. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 63.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, et al. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 66.Camilli TC, Weeraratna AT. Striking the target in Wnt-y conditions: intervening in Wnt signaling during cancer progression. Biochem Pharmacol. 2010;80:702–711. doi: 10.1016/j.bcp.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011;131:391–400. doi: 10.1038/jid.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halleskog C, Mulder J, Dahlström J, Mackie K, Hortobágyi T, et al. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell survival rates revealed by trypan blue staining. Cortical cultures at 24 hrs after indicated treatments were used. Dying cells were stained due to the increase of membrane permeability to the dye.
(TIF)