Abstract
Background
The aim of this study was to evaluate the antimicrobial and antioxidant activities of the methanol extract, fractions and isolated compounds from Entada abyssinica stem bark, plant used traditionally against gastrointestinal infections.
Methods
The methanol extract of E. abyssinica stem bark was pre-dissolved in a mixture of methanol and water, and then partitioned between n-hexane, ethyl acetate and n-butanol. The ethyl acetate portion was fractionated by column chromatography and the structures of isolated compounds elucidated by analysis of spectroscopic data and comparison with literature data. Antimicrobial activity was assayed by broth microdilution techniques on bacteria and yeasts. The antioxidant activity was determined by DPPH radical scavenging method.
Results
Four known compounds [(5S,6R,8aR)-5-(carboxymethyl)-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-trimethylnaphthalenecarboxylic acid (1), methyl 3,4,5-trihydroxybenzoate (2), benzene-1,2,3-triol (3) and 2,3-dihydroxypropyltriacontanoate (4)] were isolated. Compared to the methanol extract, fractionation increased the antibacterial activities of the n-hexane and ethyl acetate fractions, while the antifungal activities increased in ethyl acetate, n-butanol and aqueous residue fractions. The isolated compounds were generally more active on bacteria (9.7 to 156.2 μg/ml) than yeasts (78.1 to 312.5 μg/ml). Apart from compound 1, the three others displayed DPPH· scavenging activity (RSa), with RSa50 values of 1.45 and 1.60 μg/ml.
Conclusion
The results obtained from this study support the ethnomedicinal use of E. abyssinica in the treatment of gastrointestinal infections and the isolated compounds could be useful in the standardisation of antimicrobial phytomedicine from this plant.
Background
Exploring the healing power of plants is an ancient concept. For many centuries people have been trying to alleviate and treat diseases with different plant extracts and formulations [1]. The interest in plants with antimicrobial properties has been revived because of current problems associated with the use of antibiotics [2]. Many microbial infections lead to the production of highly reactive molecules from the metabolism of oxygen that can cause extensive damage to cells and tissues [3]. The fact that microorganisms nowadays tend to develop resistance towards drugs, coupled to the undesirable side effects of certain antibiotics offer considerable potentials for the development of new effective antimicrobial and antioxidant agents; medicinal plants are a prolific source.
Entada abyssinica (Mimosaceae) is an understorey forest deciduous tree, 3-15 m high, with a flat spreading crown. It is widespread in central and eastern tropical Africa [4]. It has a grey stem bark, glabrous leaves and creamy white flowers [5]. A decoction of the stem bark is locally used in the treatment of coughs, rheumatic and abdominal pains, and diarrhoea while the root or leaf decoction is used in the treatment of fever and to prevent miscarriage [5]. The fresh roots are used against gonorrhoea [6].
Some biological activities of Entada abyssinica have been reported. The leaves are highly active against Semliki forest virus [7]. Weak antibacterial activities of the methanol extract of E. abyssinica stem bark has been reported by Fabry et al. [8]. A number of biologically active compounds have been isolated from E. abyssinica including a diastereoisomer of the clerodane type diterpene, kolavenol [9], flavonoids and phytosterol glycosides [10] and kolavic acid derivatives [11].
However, to the best of our knowledge, no information on its free radical scavenging activities is available. This study was therefore designed to evaluate the possible beneficial antimicrobial and antioxidant potencies of the methanol extract, fractions and compounds from this plant.
Methods
Plant material, extraction and phytochemical screening
The stem bark of E. abyssinica was collected in May 2007 in Menoua Division, West Cameroon. Botanical identification was done at the Cameroon National Herbarium in Yaounde by Mr Tadjouteu Fulbert, where a voucher specimen was kept under the reference number 44732/HNC. The stem bark was cut into pieces, air-dried under shade and ground into powder using an electric grinder. A mass of 375 g of powder was exhaustively extracted with 1 l of methanol. After filtration, the solvent was evaporated under reduced pressure in a rotary evaporator at 45°C to afford the methanol extract (47.50 g). An amount of 32.50 g of this extract was pre-dissolved in 100 ml of a mixture of methanol and water (1:9) and then 400 ml of n-hexane was added and shaken vigorously. After about 30 min, the n-hexane phase was collected and the process repeated thrice. Methanol was then evaporated from the polar phase and the aqueous residue treated sequentially with ethyl acetate and n-butanol. The n-hexane, ethyl acetate and n-butanol were evaporated under reduced pressure in rotary evaporator to afford 4.64, 15.78 and 2.63 g of fractions respectively. The aqueous residue (9.44 g) was obtained after drying the residual portion in the oven at 40°C for 48 h. The methanol extract and fractions were subjected to phytochemical screening using standard procedures [12].
Fractionation and isolation
A quantity of 10.5 g of the ethyl acetate fraction was subjected to silica gel 60 (0.20-0.500 mm) flash chromatography and eluted with mixtures of n-hexane (Hex) and ethyl acetate (EtOAc) of increasing polarity (0-100%) to yield a total of 9 fractions of 200 ml each. These fractions were combined on the basis of TLC profiles into four major fractions: F1 [4.77 g, Hex/EtOAc (100:0), (80:20), (70:30)], F2 [2.48 g, Hex/EtOAc (60:40), (50:50)], F3 [1.56 g, Hex/EtOAc (40:60)] and F4 [0.63 g, Hex/EtOAc (30:70), (20:80), (0:100)]. Fraction F1 was further dissolved in a mixture of Hex and EtOAc (60:40) and a whitish compound precipitated. It was filtered and rinsed with EtOAc to afford compound 1 (100 mg). The filtrate (4.5 g) was subjected to further silica gel column chromatography (0.063-0.200 mm) to afford compound 2 (113 mg). Fraction F3 afforded compound 3 (108 mg) as a yellowish powder from the mixture Hex/EtOAc (60:40). Fraction F4 was further subjected to column chromatography (0.063-0.200 mm) purification using Hex and EtOAc (40:60) to afford compound 4 (54 mg).
Chemical analysis
The n-hexane fraction was subjected to GC-MS using an Agilent 6890N Network GC system/5975 Inert × L Mass selective Detector at 70 eV and 20°C. The GC column was a CP-S-il 8 CB LB, fused silica capillary column (0.25 mm × 30 m, film thickness 0.25 μm). Helium was used as carrier gas at a flow rate of 1.2 ml/min. The injector port was maintained at 250°C; the oven temperature was programmed at 5°C/min from 70°C to 300°C. A solution of each fraction was prepared in chloroform at a concentration of 10% (wt/v). To 90 μl of this solution, 10 μl of trimethyl sulfonium hydroxide (TMSH) was added and 1 μl of the resulting mixture was injected into the GC -MS apparatus. The constituents were identified by comparing their mass spectra data with those stored in NIST05 and Wiley237 database libraries.
Aluminium sheet pre-coated with silica gel 60 GF254 (Merck) was used for thin layer chromatography (TLC). The spots were visualized under UV light (254 and 366 nm) with a UV lamp model 52-58 mineralight, and sprayed with 50% aqueous solution of H2SO4 followed by heating at 100°C.
IR spectra were measured with KBr disks using FT-IR-8400 S Shimadzu spectrophotometer. EI-MS were carried out on a GCT Premier CAB109 TOF mass spectrometer. 1H-, 13C-NMR and 2D-NMR (COSY 1H-1H, HMBC and HSQC) spectra were recorded in acetone-d6 (500 MHz for 1H and 125 MHz for 13C) on a Brücker-Avance-500 MHz NMR spectrometer.
(5S,6R,8aR)-5-(carboxymethyl)-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-trimethylnaphthalenecarboxylic acid (1): White powder; 13C NMR (100 MHz, CDCl3 + CD3OD): δ 14.9 (5-Me), 17.8 (C-4), 20.7 (6-Me), 21.0 (8a-Me), 25.3 (C-7), 27.0 (C-3), 29.4 (C-8), 35.2 (C-6), 37.5 (C-8a), 38.5 (C-5), 43.6 (C-1'), 44.6 (C-4a), 136.9 (C-2), 142.6 (C-1), 169.3 (1-COOH), 175.4 (C-2'); FABMS: m/z 279 ([M - H]+, 98), 153 (100), 151 (44), 46 (13); HRFABMS: m/z 279.1592 (calcd. for C16H23O4: 279.1596).
Methyl 3,4,5-trihydroxybenzoate (2): Colorless needles; 13C NMR (100 MHz, CDCl3): δ 51.7 (OCH3), 109,8 (C-2/C-6), 125.2 (C-1), 148.4 (C-3/C-5), 138.9 (C-4), EIMS: m/z 184 ([M]+, 99), 153 (100), 125 (14), 107 (2), 79 (3); HREIMS: m/z 184.0370 (calcd. for C8H8O5: 184.0372).
Benzene-1,2,3-triol (3): White powder; 13C NMR (100 MHz, CDCl3): δ 110.2 (C-4/C-6), 124.6 (C-5), 138.5 (C-2), 148.8 (C-1/C-3); EIMS: m/z 126 ([M]+, 100), 108 (21), 80 (24), 52 (27); HREIMS: m/z 126.0312 (calcd. for C6H6O3: 126.0317).
2,3-dihydroxypropyltriacontanoate (4): Whitish gum; 13C NMR (125 MHz, CDCl3): δ 14.2 (CH3), 22.7-34.2 (28 CH2), 63.4 (C-3), 65.2 (C-1), 70.3 (C-2), 172.3 (COO); EIMS: m/z 43 (100), 57 (80), 71 (44), 83 (64), 85 (28), 97 (69), 99 (7), 111 (37), 125 (19), 127 (3), 139 (8), 141 (3), 153 (5).
Microorganisms and growth conditions
The microorganisms used in this study consisted of two Gram (+) bacteria (Enterococcus faecalis ATCC 10541 and Staphylococcus aureus ATCC 25922); six Gram (-) bacteria (Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 11775, Klebsiella pneumoniae ATCC13883, Salmonella typhi ATCC 6539, Proteus mirabilis and Shigella flexneri); and 10 yeasts (Candida albicans ATCC 9002, C. albicans ATCC 2091, C. albicans ATCC 24433, C. parapsilosis ATCC 22019, C. lusitaniae ATCC 200950, C. tropicalis ATCC 750, C. krusei ATCC 6258, C. guillermondi, C. glabbrata IP 35 and Cryptococcus neoformans IP 95026). The reference strains (ATCC) were obtained from American Type Culture Collection (Rockville, USA). The two clinical bacterial isolates were collected from "Centre Pasteur" (Yaoundé, Cameroon) and the two IP fungal strains were obtained from "Institute Pasteur" (Paris, France). The bacterial and fungal strains were grown at 35°C and maintained on nutrient agar (NA, Conda, Madrid, Spain) and Sabouraud Dextrose Agar (SDA, Conda) respectively.
Antimicrobial assays
The minimum inhibitory concentration (MIC) of the crude methanol extract, fractions and isolated compounds were determined through broth microdilution method in 96-well micro-titre plates as described by Zgoda and Porter [13]. The 96-well plates were prepared by dispensing into each well 100 μl of Mueller Hinton broth for bacteria and Sabouraud Dextrose broth for yeasts. The test substances were initially prepared in 10% ethanol/tween 80 in broth medium at 3124.8 μg/ml (methanol extract and fractions), 1250 μg/ml (isolated compounds) and 50 μg/ml (reference antibiotics). A volume of 100 μl of each test sample was added into the first wells of the micro-titre plate. Serial two-fold dilutions of these test samples were made and 100 μl of inoculum standardized at 106 CFU/ml for bacteria or 2.5 × 105 CFU/ml for yeasts (at 600 nm, Jenway 6105 UV/Vis spectrophotometer- 50 Hz/60 Hz) [14] was then added into each well. The last wells (N°12) served as sterility controls (contained broth only) or negative control (broth plus inoculum). This gave final concentration ranges of 781.25-0.76 μg/ml, 312.50-0.30 μg/ml and 12.50-0.01 μg/ml for the methanol extract or fractions, isolated compounds and reference substances respectively. The plates were sealed with parafilm, then agitated with a plate shaker to mix their contents and incubated at 35°C for 24 h for bacteria and 48 h for yeast.
The MICs of each test sample was detected following addition of 50 μl (0.2 mg/ml) p-iodonitrotetrazolium chloride (INT, Sigma-Aldrich, South Africa) solution for bacteria. Viable bacteria reduced the yellow dye to a pink colour. For yeast, MICs were determined by visualising the turbidity of the wells. The MIC corresponded to the lowest well concentration where no colour or turbidity change was observed, indicating no growth of microorganism. The MBC or MFC was determined by adding 50 μl aliquots of the clear wells to 150 μl of freshly prepared broth medium and incubating at 35 °C for 48 h. The MBC or MFC was regarded as the lowest concentration of test sample which did not produce a colour or turbidity change as above. All tests were performed in triplicates.
DPPH radical scavenging activity
Radical scavenging activity of test samples (methanol extract, fractions and isolated compounds) was determined spectrophotometrically (Jenway, spectrophotometer model 1605) at 517 nm under UV/Visible light using DPPH radical [15]. The methanol extract and fractions, isolated compounds, and L-ascorbic acid were prepared in methanol and tested at concentration ranges of 200 to 6.25 μg/ml, 64 to 0.25 μg/ml and 8 to 0.25 μg/ml respectively. A volume of 900 μl of DPPH· solution (20 mg/l) was mixed with 100 μl of test sample in a curve and the absorbance (Ab) was read immediately and after 30 min incubation at room temperature (As). The experiments were carried out in triplicate. The percentages of DPPH· scavenged (RSa %) by test samples were calculated as:
The radical scavenging activity fifty (RSa50) corresponding to the amount of sample necessary to decrease by 50% the amount of free radical DPPH was determined by plotting the scavenging activity against the logarithm of sample concentration [16].
Statistical analysis
The data on antioxidant activity were subjected to the one-way analysis of variance (ANOVA) and results were expressed (where appropriate) as mean ± standard deviation. Differences between means of samples were compared using Duncan's multiple range tests at P <0.05.
Results and discussion
Chemical composition and antimicrobial activity
The qualitative analysis of the methanol extract and fractions of E. abyssinica revealed the presence of alkaloids, flavonoids, tannins, saponins and cardiac glycosides (Table 1). These results show a similarity in chemical composition between Entada abyssinica and Entada africana as well as Entada phaseoloides [17]. Lipid components identified in n-hexane fraction were mainly saturated and unsaturated fatty acids (Table 2). The structures of the isolated compounds from E. abyssinica were established by spectroscopic analysis [IR, EI-MS, H1 and C13 NMR spectra in conjunction with 2D experiments (COSY 1H-1H, HMBC and HSQC)] and direct comparison with published data. The compounds were identified as: (5S,6R,8aR)-5-(carboxymethyl)-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-trimethylnaphthalenecarboxylic acid (1) [18]; methyl 3,4,5-trihydroxybenzoate (methyl gallate) (2) [19]; benzene-1,2,3-triol (pyrogallol) (3) [20] and 2,3-dihydroxypropyltriacontanoate (4) (Figure 1).
Table 1.
Phytochemical screening of E. abyssinica methanol extract and fractions
| Groups of chemical constituents | Methanol extract | n-hexane fraction | Ethyl acetate fraction | n-butanol fraction | Aqueous residue fraction |
|---|---|---|---|---|---|
| Alkaloids | + | + | + | + | + |
| Flavonoids | + | + | + | + | + |
| Coumarins | + | - | + | + | + |
| Phenols | + | - | + | + | + |
| Tannins | + | - | + | + | + |
| Saponins | + | - | + | + | + |
| Steroids | - | - | - | - | - |
| Chalcones | + | - | + | + | + |
| Terpernoids | + | - | + | - | - |
| Cardiac glycosides | + | - | - | + | + |
+: presence; -: absence.
Table 2.
Percentage composition of chemical constituents in n-hexane fraction of E.abyssinica stem bark
| Chemical constituents/Molecular ion (m/z) | Chemical structure | % |
|---|---|---|
| Hexadecanoic acid (C16 H32 O2) |
CH3-CH2-[CH2]12-CH2-COOH | 17.86 |
| 9,12-Octadecadienoic acid (Z,Z) (C18 H32 O2) |
CH3-[CH2]4-CH = CH-CH2-CH = CH-[CH2]7-COOH | 21.14 |
| 9-Octadecenoic acid (Z) (C18 H34 O2) |
CH3-[CH2]6-CH2-CH = CH-[CH2]7-COOH | 27.78 |
| Octadecanoic acid (C18 H36 O2) |
CH3-CH2-[CH2]14-CH2-COOH | 2.92 |
| 298(M+ 1), 257(5), 189(26), 121(30), 107(60), 95(100), 81(54), 55(71), 41(46), 29(10) | nd | 4.27 |
| 327(M+ 1), 290(2), 274(7), 189(31), 175(16), 120(63), 107(74), 95(100), 55(48), 41(46) | nd | 8.50 |
| 347(M+ 1), 330(77), 315(30), 256(15), 203(36), 175(49), 139(100), 119(57), 107(75), 95(82), 79(64), 55(68), 41(55) | nd | 17.50 |
| Total | 99.97 |
nd: not determined.
Figure 1.
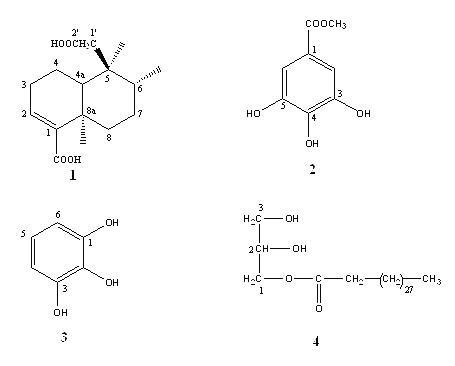
Chemical structures of compounds isolated from Entada abyssinica ethyl acetate fraction. 1:(5S,6R,8aR)-5-(carboxymethyl)-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-Trimethylnaphthalenecarboxylic acid; 2: methyl 3,4,5-trihydroxybenzoate; 3: benzene-1,2,3-triol; 4: 2,3-dihydroxypropyltriacontanoate.
The results of the antibacterial and antifungal activities of the methanol extract, fractions and isolated compounds are reported in Table 3. These substances displayed varied antibacterial and antifungal activities across the studied pathogens. The observed wide range of antimicrobial properties for the methanol extract and fractions can be explained by the presence of various groups of potentially active classes of secondary metabolites (Table 1). Indeed flavonoids [21], saponins [22], polyphenols [23], cardiac glycosides [24], coumarines [25], tannins [26], triterpenes and alkaloids [27,28] have been reported to possess antimicrobial activities. It was noted that the bacterial agents were more susceptible to the tested samples than yeasts. Yeasts like other fungi are eukaryotic organism with more complex structural organisation compared to the simple prokaryotic bacterial cells. This probably explains the difference in sensitivity of these two groups of microorganisms. According to the classification of Rios and Rico [29], the methanol extract and fractions (MIC range of 24.4 to 781.2 μg/ml) could be considered to possess significant (MIC < 100 μg/ml), moderate (100 < MIC = 625 μg/ml) or weak (MIC > 625 μg/ml) activity against the corresponding pathogens. The antibacterial activity of the methanol extract of E. abyssinica stem bark reported in this study are generally high compared to that of Fabry et al. [30]. The n-hexane fraction acted most on bacteria. Its major component, octadecenoic acid, has been reported to possess antibacterial and antifungal activities [31]. Moreover, this does not exclude the fact that the observed antibacterial activity could be a result of the combined effect of all the detected lipid components in the n-hexane fraction [32]. The antibacterial mechanism of action of fatty acids is still poorly understood, however, the prime target of these compounds is the cell membrane, where they interfere with the electron transport chain and oxidative phosphorylation [33]. The ethyl acetate and n-butanol fractions were generally more effective on both bacteria and yeasts, while the aqueous residue fraction displayed greater activity on yeasts.
Table 3.
Minimal inhibitory concentration (MIC)/minimum bactericidal or fungicidal concentration (MBC or MFC) of test substances (μg/ml)
| Microorganisms | Parameters | Test samplesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | HF | EF | nB | AR | 1 | 2 | 3 | 4 | Ref | ||
| Gram (-) bacteria | |||||||||||
| Pseudomonas | MIC | 195.3 | 24.4 | 781.2 | 390.6 | 781.2 | 39.0 | 9.7 | 9.7 | 312.5 | 1.5 |
| aeruginosa | MBC | 781.2 | 390.6 | nab | na | na | 156.2 | 19.5 | 9.7 | > 312.5 | 12.5 |
| Proteus | MIC | 195.3 | 390.6 | 390.6 | 781.2 | na | 156.2 | 19.5 | 39.0 | na | 0.3 |
| mirabilis | MBC | 781.2 | na | 390.6 | na | na | 156.2 | 39.0 | 156.2 | na | 1.5 |
| Shigella flexneri | MIC | 781.2 | 48.8 | 390.6 | 24.4 | 390.6 | 78.1 | 39.0 | 39.0 | na | 0.2 |
| MBC | na | 390.6 | 390.6 | 195.3 | na | 156.2 | 156.2 | 78.1 | na | 6.2 | |
| Klebsiella | MIC | 781.2 | 24.4 | 97.6 | 781.2 | na | 78.1 | 19.5 | 78.1 | na | 0.2 |
| pneumoniae | MBC | na | 195.3 | 390.6 | na | na | 156.2 | 39.0 | 156.2 | na | 3.1 |
| Salmonella typhi | MIC | 781.2 | 390.6 | 97.6 | 781.2 | na | 156.2 | 39.0 | 78.1 | na | 0.3 |
| MBC | na | na | 390.6 | na | na | 312.5 | 156.2 | 156.2 | na | 1.5 | |
| Escherichia coli | MIC | 781.2 | 24.4 | 97.6 | 24.4 | na | 78.1 | 39.0 | 156.2 | 312.5 | 0.2 |
| MBC | na | 390.6 | 390.6 | 390.6 | na | 312.5 | 312.5 | 156.2 | > 312.5 | 1.5 | |
| Gram (+) bacteria | |||||||||||
| Enterococcus | MIC | 195.3 | 390.6 | 390.6 | 781.2 | na | 78.1 | 39.0 | 78.1 | na | 0.2 |
| faecalis | MBC | na | 781.2 | 781.2 | na | na | 312.5 | 312.5 | 156.2 | na | 1.5 |
| Staphylococcus | MIC | 390.6 | 195.3 | 97.6 | 781.2 | 390.6 | 78.1 | 39.0 | 78.1 | na | 1.5 |
| aureus | MBC | na | na | 390.6 | na | na | 39.0 | 156.2 | 156.2 | na | 12.0 |
| Yeast | |||||||||||
| C. albicans | MIC | na | na | 195.2 | 390.6 | 195.3 | 312.5 | 312.5 | 78.1 | na | 0.01 |
| ATCC 2091 | MFC | na | na | 195.3 | 390.6 | 1953 | 312.5 | 312.5 | 156.2 | na | 0.08 |
| C. albicans | MIC | 781.2 | 781.2 | 195.3 | 195.3 | 195.3 | 312.5 | 156.2 | 156.2 | 312.2 | 0.01 |
| ATCC9002 | MFC | na | na | 781.2 | 195.3 | 390.6 | 312.5 | 156.2 | 156.2 | > 312.5 | 0.08 |
| C. albicans | MIC | 195.3 | 390.6 | 195.3 | 390.6 | 195.3 | 156.2 | 312.5 | 78.1 | na | 0.02 |
| ATCC 24433 | MFC | 781.2 | na | 195.3 | na | 195.3 | 312.5 | 312.5 | 156.2 | na | 0.02 |
| Candida | MIC | 781.2 | 781.2 | 195.3 | 390.6 | 390.6 | 312.5 | 156.2 | 78.1 | na | 0.01 |
| parapsilosis | MFC | na | 390.6 | 781.2 | 390.6 | 781.2 | 312.5 | 156.2 | 156.2 | na | 0.08 |
| Candida | MIC | 195.3 | na | 48.8 | 390.6 | 195.3 | 312.5 | 312.5 | 78.1 | na | 0.04 |
| tropicalis | MFC | 781.2 | na | 390.6 | 781.2 | 195.3 | 312.5 | 312.5 | 156.2 | na | 0.08 |
| Candida krusei | MIC | na | 781.2 | 781.2 | 390.6 | 195.3 | 312.5 | 312.5 | 78.1 | na | 0.04 |
| MFC | na | na | na | 390.6 | 195.3 | 312.5 | 312.5 | 156.2 | na | 0.04 | |
| Candida glabrata | MIC | na | 781.2 | 195.3 | 390.6 | 390.6 | 78.1 | 312.5 | 78.1 | na | 0.02 |
| MFC | na | na | 195.3 | na | 781.2 | 78.1 | 312.5 | 156.2 | na | 0.02 | |
| Candida | MIC | 781.2 | 390.6 | 195.3 | 195.3 | 195.3 | 156.2 | 312.5 | 78.1 | na | 0.01 |
| lusitaniae | MFC | na | 781.2 | 390.6 | 195.3 | 195.3 | 156.2 | 312.5 | 78.1 | na | 0.08 |
| Cryptococcus | MIC | 390.6 | 781.2 | 97.6 | 390.6 | 195.3 | 195.3 | 78.1 | 78.1 | na | 1.00 |
| neoformans | MFC | na | na | 781.2 | 390.6 | 195.3 | 312.5 | 156.2 | 78.1 | na | 1.00 |
| Candida | MIC | 781.2 | 781.2 | 195.3 | 390.6 | 390.6 | 312.5 | 156.2 | 78.1 | na | 0.01 |
| guillermondi | MFC | na | na | na | na | 781.2 | 312.5 | 156.2 | 156.2 | na | 0.08 |
aME: methanol extract; HF: n-hexane fraction; EF: Ethyl acetate fraction; nB: n-butanol fraction; AR: Aqueous residue; 1: (5S,6R,8aR)-5-(carboxymethyl)-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-Trimethylnaphthalenecarboxylic acid; 2: methyl 3,4,5-trihydroxybenzoate; 3: benzene-1,2,3-triol; 4: 2,3-dihydroxypropyltriacontanoate and Ref (reference drugs): ciprofloxacin for bacteria and nystatin for yeasts.
bna: not active at concentrations upto 781.2 μg/ml.
The isolated compounds were equally more active on bacteria than yeasts. Compounds 2 and 3 showed greater activity (MIC = 9.7-312.5 μg/ml) than compounds 1 (MIC = 39.0-312.5 μg/ml) and 4 (78.12-312.5 μg/ml). To the best of our knowledge, the antimicrobial and antioxidant activities of compound 1 are reported herein for the first time. The presence of methyl groups on the carbon rings of this compound could be critical for its activity [34]. Compound 2 is a known medicinally important substance that was previously isolated from Entada africana [35], Acer ginnala [36] and Galla rhois, and has been reported to possess antimicrobial activities [37] and anti-asthmatic effects [38]. The activities of this compound herein reported corroborates those of Jang-Gi et al. [37] with MICs of 250 and 500 μg/ml on resistant bacterial strains. Compound 3 (pyrogallol) was as well active on both bacteria (MIC of 9.7 to 78.1 μg/ml) and yeasts (MIC of 78.1 to 156.2 μg/ml), this antimicrobial activity was found to be greater in comparison to that reported by Jin et al. [20]. Pyrogallol contains both phenolic hydroxyl groups and a system of delocalized electrons, conditions critical for its antimicrobial activity [20]. The presence of hydroxyl groups on compounds 2 and 3 could be responsible for the observed antimicrobial activity. Compound 4 recorded weak and selective antimicrobial activities against the tested microorganisms and these activities could be due to the 2,3-dihydroxypropyl moiety [39].
Antioxidant activity
The free radical scavenging activities of samples (RSa50) are reported in Table 4. The ethyl acetate fraction (RSa50 of 3.07 μg/ml) showed the greatest activity while the n-hexane fraction had the least (RSa50 of 35.87 μg/ml). Phenols, flavonoids, saponins and tannins identified in the extract and fractions may be responsible for the observed antioxidant activities [40]. Compounds 2, 3 (phenolic compounds) and 4 (fatty ester) demonstrated interesting radical scavenging activities (RSa50 of 1.45, 1.45 and 1.60 μg/ml respectively. The antioxidative effects of phenolic compounds (2 and 3) are mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides [40]. It is known that the antioxidant activity of an aromatic compound is proportional to the number of hydroxyl groups it contains [41]. This probably explains the high radical scavenging activities of compounds 2 and 3. The activity of 2 in this assay was higher than that (RSa50 = 2.8 μg/ml) reported by Seong et al. [37] against DPPH free radical.
Table 4.
Test sample's concentration reducing 50% of free radical DPPH (RSa50)
| Test samples | RSa50 (μg/ml) |
|---|---|
| Plant extract and fractions | |
| Methanol extract | 7.12 ± 0.21* |
| n-hexane fraction | 35.87 ± 0.07* |
| Ethyl acetate fraction | 3.07 ± 0.04* |
| n-butanol fraction | 3.10 ± 0.10* |
| Aqueous residue fraction | 7.15 ± 0.08* |
| Isolated compoundsa | |
| 1 | 298 ± 0.57* |
| 2 | 1.45 ± 0.00* |
| 3 | 1.45 ± 0.00* |
| 4 | 1.60 ± 0.01* |
| Reference substance | |
| L-ascorbic acid | 0.68 ± 0.00 |
a1:(5S,6R,8aR)-5-(carboxymethyl)-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-Trimethylnaphthalenecarboxylic acid; 2: methyl 3,4,5-trihydroxybenzoate; 3: benzene-1,2,3-triol and 4: 2,3-dihydroxypropyltriacontanoate.
*Scavenging activity significantly lower than the reference, Duncan (p < 0.05).
Conclusion
The results obtained from this study reveal that the stem bark of E. abyssinica may be useful in the development of an antimicrobial phytomedicine which can be standardised using the isolated compounds.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GNT and PKL carried out the assays in this study; HKP participated to extract fractionation and isolation of compounds; HK and YO participated to structural elucidation of compounds 1 and 2; GV and GG YO participated to structural elucidation of compounds 3 and 4; JRK designed and supervised the work along with the manuscript writing. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Gerald N Teke, Email: ngobob@yahoo.com.
Paul K Lunga, Email: lungapaul@yahoo.ca.
Hippolyte K Wabo, Email: hkamdemw@yahoo.ca.
Jules-Roger Kuiate, Email: jrkuiate@yahoo.com.
Gerard Vilarem, Email: gerard.vilarem@ensiacet.fr.
Geraldine Giacinti, Email: Geraldine.Giacinti@ensiacet.fr.
Haruhisa Kikuchi, Email: hal@mail.pharm.tohoku.ac.jp.
Yoshiteru Oshima, Email: oshima@mail.pharm.tohoku.ac.jp.
References
- Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Reviews. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Bansal D, Bhasin P, Anjali. Antimicrobial activity and phytochemical screening of five wild plants against Escherichia coli, Bacillus subtilis and Staphylococcus aureus. Journal of Pharmaceutical Research. 2010;3:1260–1262. [Google Scholar]
- Kamlesh D, Yogesh S, Ajit P. Evaluation of in vitro antioxidant activity of Sida rhombifolia (L.) Ssp. retusa (L.) Journal of Medicinal Food. 2007;10:683–688. doi: 10.1089/jmf.2006.129. [DOI] [PubMed] [Google Scholar]
- Katende AB. Useful trees and shrubs for Uganda. Identification, Propagation and Management for Agricultural and Pastoral Communities. Regional Soil Conservation Unit (RSCU), Swedish International Development Authority (SIDA) 1995.
- Bekele-Tesemma A, Birnie A, Tengnas B. Useful trees and shrubs for Ethiopia. Regional Soil Conservation Unit (RSCU), Swedish International Development Authority (SIDA) 1993.
- Haile Y, Delenasaw Y. Traditional medicinal plant knowledge and use by local healers in Sekoru District, Jimma Zone, Southwestern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2007;3:24. doi: 10.1186/1746-4269-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos P, Hermans N, De Bruyne T, Apers S, Sindambiwe JB, Witvrouw M, De Clercq E, Vanden Berghe D, Pieters L, Vlietinck AJ. Antiviral activity of Rwandan medicinal plants against human immunodeficiency virus type-1 (HIV-1) Phytomedicine. 2002;9:62–68. doi: 10.1078/0944-7113-00083. [DOI] [PubMed] [Google Scholar]
- Fabry W, Paul O, Rainer A. Antibacterial activity of East African medicinal. Journal of Ethnopharmacology. 1998;60:79–84. doi: 10.1016/S0378-8741(97)00128-1. [DOI] [PubMed] [Google Scholar]
- Freiburghaus AF, Steck HP, Brun R. Bioassay-guided isolation of a diastereoisomer of kolavenol from Entada abyssinica active on Trypanosoma brucei rhodesiense. Journal of Ethnopharmacology. 1998;61:179–183. doi: 10.1016/S0378-8741(98)00035-X. [DOI] [PubMed] [Google Scholar]
- Asfaw D, Olaf K, Martin GS, Günter M, Franz B, Dawit A, Ernst H. A diterpene, a flavonoid glycoside and a phytosterol from Entada abyssinica stem bark. Monatshefte Chemie. 2000;131:401–408. doi: 10.1007/s007060050321. [DOI] [Google Scholar]
- Nyasse B, Ngantchou I, Tchana EM, Sonké B, Denier C, Fontaine C. Inhibition of both Trypanosoma brucei bloodstream form and related glycolytic enzymes by a new kolavic acid derivative isolated from Entada abyssinica. Pharmazie. 2004;59:873–875. [PubMed] [Google Scholar]
- Sofowara A. Medicinal plants and traditional medicine in Africa. Ibadan, Nigeria, Spectrum books Ltd; 1993. [Google Scholar]
- Zgoda JR, Porter JR. A convenient microdilution method for screening natural products against bacteria and fungi. Pharmaceutical Biology. 2001;39:221–225. doi: 10.1076/phbi.39.3.221.5934. [DOI] [Google Scholar]
- Tereschuk ML, Riera MVQ, Castro GR, Abdala LR. Antimicrobial activity of flavonoid from leaves of Tagetes minuta. Journal of Ethnopharmacology. 1997;56:227–232. doi: 10.1016/S0378-8741(97)00038-X. [DOI] [PubMed] [Google Scholar]
- El-Ghorab A, Mahgoub M, Bekheta M. Effect of some bioregulators on the chemical composition of essential oil and its antioxidant activity of Egyptian carnation (Dianthus caryophyllus) Journal of Essential Oil Bearing Plants. 2006;9:214–222. [Google Scholar]
- Yassa N, Razavi BH, Hadjiakhoondi A. Free radical scavenging and lipid peroxidation activity of the shahani black grape. Pakistan Journal of Biological Science. 2008;11:2513–2516. doi: 10.3923/pjbs.2008.2513.2516. [DOI] [PubMed] [Google Scholar]
- Tibiri A, Richard WS, Noufou O. Evaluation of antioxidant activity, total phenolic and flavonoid contents of Entada africana Guill. et Perr. (Mimosaceae) organ extracts. Research Journal of Medical Sciences. 2010;4:81–87. [Google Scholar]
- Aquino R, Ciavatta ML, De Tommasi N, Gacs-Baitz E. Tetranorditerpenes from Detarium microcarpum. Phytochemistry. 1992;31:1823–1825. doi: 10.1016/0031-9422(92)83158-U. [DOI] [Google Scholar]
- Seon WH, Jun L, Hyun SK, Kyung DL, Sang HN, Ki HP, Min SY. Comparison of tyrosinase inhibitory effect of the natural antioxidants from Cedrela sinensis. Agricultural and Chemical Biotechnologies. 2005;48:144–147. [Google Scholar]
- Jin AL, In KO, Il SY, Seung HB. Antimicrobial activity of phenolic compounds against skin inflammation microorganisms. Journal of Cosmetics and Public Health. 2005;1:1–5. [Google Scholar]
- Fairouz M, Amar Z, Narimane S, Ahmed T, Salah R. Flavonoid constituents from Algerian launaea resedifolia (O.K.) and their antimicrobial activity. Records of Natural Products. 2010;4:91–95. [Google Scholar]
- Pavithra PS, Janani VS, Charumathi KH, Indumathy R, Potala S, Verma RS. Antibacterial activity of plants used in Indian herbal medicine. International Journal of Green Pharmacy. 2010;4:22–28. doi: 10.4103/0973-8258.62161. [DOI] [Google Scholar]
- Okoro IO, Auguster O, Edith OA. Antioxidant and antimicrobial activities of polyphenols from ethnomedicinal plants of Nigeria. African Journal of Biotechnology. 2010;9:2989–2993. [Google Scholar]
- Ebana RUB, Madunagu BE, Ekpe ED, Otung IN. Microbiological exploitation of cardiac glycosides and alkaloids from Garcinia kola, Boreria ocymoides, Kola nitida and Citrus aurantifolia. Journal of Applied Bacteriology. 1991;71:398–401. doi: 10.1111/j.1365-2672.1991.tb03807.x. [DOI] [PubMed] [Google Scholar]
- Aziz B Kozeta V Islam K Antibacterial activity of coumarin derivatives synthesized from hydroxy-4-2H-[1]-Benzopyran-2-one. The comparison with standard drug Journal of Internet Environmental Application & Science 20105247–252.21742412 [Google Scholar]
- Selime MÇ, Binnur MY, Ali NY. Determination of antimicrobial activity of tannic acid in pickling process. Romanian Biotechnological Letters. 2010;15:5325–5330. [Google Scholar]
- Faizi S, Khan RA, Azher S, Khan SA, Taussef S, Ahmad A. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta Medica. 2003;69:350–355. doi: 10.1055/s-2003-38883. [DOI] [PubMed] [Google Scholar]
- Gonzaga WA, Weber AD, Glacomeli SR, Dalcol II, Hoelzel SC, Morel AF. Antibacterial alkaloids from Zanthoxylum rhoifolium. Planta Medica. pp. 371–374. [DOI] [PubMed]
- Rios JL, Recio MC. Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Ching TH. New bioactive fatty acids. Asia Pacific Journal of Clinical Nutrition. 2008;17:192–195. [PubMed] [Google Scholar]
- Zhao J, Shan T, Huang Y, Liu X, Gao X, Wang M, Jiang W, Zhou L. Chemical composition and in vitro antimicrobial activity of the volatile oils from Gliomastix murorum and Pichia guilliermondii, two endophytic fungi in Paris polyphylla var. yunnanensis. Natural Products Communication. 2009;4:1491–1496. [PubMed] [Google Scholar]
- Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Applied Microbiology and Biotechnology. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- Subbanwad GR, Baseer MA, Vibhute YB. Synthesis and antimicrobial activity of some new isoxazolines and 1,5-benzothiazepines. Indian Journal of Pharmaceutical Sciences. 2002;64:264–266. [Google Scholar]
- Cioffi G, D'auria M, Braca A, Mendez J, Castillo A, Morelli I, De Simone F, De Tommasi N. Antioxidant and free-radical scavenging activity of constituents of the leaves of Tachigalia paniculata. Journal of Natural Products. 2002;65:1526–1529. doi: 10.1021/np0200764. [DOI] [PubMed] [Google Scholar]
- Seong SH, Seog CL, Yong WC, Jin HK, Seung HB. Antioxidant activity of crude extract and pure compounds of Acer ginnala Max. Bulletin of Korean Chemical Society. 2004;25:389–3891. [Google Scholar]
- Jang-Gi C, Ok-Hwa K, Young-Seob L, You-Chang O, Hee-Sung C, Hye-Jin J, Dong-Won S, Dong-Yeul K. Antibacterial activity of methyl gallate isolated from Galla rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules. 2009;14:1773–1780. doi: 10.3390/molecules14051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohini C, Deshpande VH, Deshpande NR. Methyl gallate, the medicinally important compound: A review. Electronic Journal of Environmental, Agricultural Food and Chemistry. 2005;4:956–962. [Google Scholar]
- Weiguang Z, Emily E, Brendon C, Lara G, David CJ. Structural requirement for the agonist activity of the TLR2 ligand Pam2Cys. Biomedical and Life Sciences. 2010;39:471–480. doi: 10.1007/s00726-009-0463-0. [DOI] [PubMed] [Google Scholar]
- Yasukazu O, Takuma S. Antioxidant and photo-antioxidant activities of chalcone derivatives. Journal of the Japan Petroleum Institute. 2008;51:298–308. doi: 10.1627/jpi.51.298. [DOI] [Google Scholar]
- Osawa T. In: Post Harvest Biochemistry of Plant Food-materials in the Tropics. Garcia I, Mendoza, V, editor. Uritani. Scientific Societies Press, Japan; 1994. Novel natural antioxidants for utilization in food and biological systems; pp. 241–251. [Google Scholar]
- Simić A, MManojlović D, Šegan D, Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007;12:2327–2340. doi: 10.3390/12102327. [DOI] [PMC free article] [PubMed] [Google Scholar]


