Abstract
Fibroblast growth factor (Fgf) signalling plays a crucial role in many developmental processes. Among the Fgf pathway ligands, Fgf9 (UniProt: P54130) has been demonstrated to participate in maturation of various organs and tissues including skeleton, testes, lung, heart, and eye. Here we establish a novel Fgf9 allele, discovered in a dominant N-ethyl-N-nitrosourea (ENU) screen for eye-size abnormalities using the optical low coherence interferometry technique. The underlying mouse mutant line Aca12 was originally identified because of its significantly reduced lens thickness. Linkage studies located Aca12 to chromosome 14 within a 3.6 Mb spanning interval containing the positional candidate genes Fgf9 (MGI: 104723), Gja3 (MGI: 95714), and Ift88 (MGI: 98715). While no sequence differences were found in Gja3 and Ift88, we identified an A→G missense mutation at cDNA position 770 of the Fgf9 gene leading to an Y162C amino acid exchange. In contrast to previously described Fgf9 mutants, Fgf9Y162C carriers were fully viable and did not reveal reduced body-size, male-to-female sexual reversal or skeletal malformations. The histological analysis of the retina as well as its basic functional characterization by electroretinography (ERG) did not show any abnormality. However, the analysis of head-tracking response of the Fgf9Y162C mutants in a virtual drum indicated a gene-dosage dependent vision loss of almost 50%. The smaller lenses in Fgf9Y162C suggested a role of Fgf9 during lens development. Histological investigations showed that lens growth retardation starts during embryogenesis and continues after birth. Young Fgf9Y162C lenses remained transparent but developed age-related cataracts. Taken together, Fgf9Y162C is a novel neomorphic allele that initiates microphakia and reduced vision without effects on organs and tissues outside the eye. Our data point to a role of Fgf9 signalling in primary and secondary lens fiber cell growth. The results underline the importance of allelic series to fully understand multiple functions of a gene.
Introduction
According to the World Health Organization, 315 million people are visually impaired worldwide. 43 million of them suffer from complete blindness (<3/60 presenting visual acuity), leading to a significant loss of life quality and shortened life time. Globally, the major causes of blindness are (in order of frequency) cataracts, uncorrected refractive errors, glaucoma, and macular degeneration [1]. However, vision might also be impaired by irregular eye-size parameters as anterior chamber depth or thickness of the lens. Microphakia is characterized by smaller lenses and usually occurs together with other systemic abnormalities inside and outside the eye as part of the Warburg Micro syndrome (WARBM1; MIM #600118), Marfan's syndrome (MFS; MIM #154700), or rhizomelic chondrodysplasia calcificans punctata (RCDP; MIM #215100) [2]–[4]. These syndromes are initiated by mutations in RAB3GAP (WARMB1; MIM *602536), FBN1 (MFS; MIM *134797), and PEX7 (RCDP; MIM +601757), respectively [5]–[9].
Beside these structural and receptor proteins, various members of the Fgf family play a role in lens growth by regulating developmental processes. Fgfs are widely expressed in developing and adult tissues and have diverse functions in organogenesis, tissue repair, metabolism, and physiological homeostasis. In humans and mice, 22 Fgf ligands have been described, which mediate signals through seven different isoforms of Fgf receptors (FgfRs; reviewed by [10]). At least 13 Fgf ligands are present in the eye during development and/or at maturity (reviewed by [11]). Among them, Fgf1 (UniProt: P61148) and Fgf2 (UniProt: P15655) were initially demonstrated to regulate lens development by studies in lens epithelial explants [12]–[14]. In subsequent experiments, negative effects on lens polarity could be initiated by lens-specific over-expression of Fgf1 (MGI: 95515), Fgf3 (MGI: 95517), Fgf4 (MGI: 95518), Fgf7 (MGI: 95521), Fgf8 (MGI: 99604), and Fgf9 in the mouse [11], [15], [16].
Regarding null mutants of these ligands, only Fgf9 knock-out mice displayed lens pathologies, characterized by delayed primary lens fiber cell elongation in at least a portion of Fgf9 null embryos [17]. These Fgf9 knock-out mutants further exhibited various pathologic phenotypes outside the eye including neonatal lethality caused by lung hypoplasia and male-to-female sex reversal initiated by irregular testicular embryogenesis [18]–[20]. Furthermore, irregular skeletogenesis was detected in an Fgf9N143T missense mutant (Fgf9Eks; MGI: 2182127). This is initiated by an impaired homodimerization of the altered Fgf9 protein, which increases diffusion through developing tissues due to decreased heparin binding affinity of Fgf9EKS [21], [22].
In our attempt to identify novel genes involved in determination of eye size, we established the mouse mutant line Aca12 (ACMaster abnormality #12) in a dominant ENU mutagenesis program [23] because of its significantly thinner lenses compared to data of control mice [24]. The goal of this study was the genetic and phenotypic characterization of Aca12. We identified a missense mutation in the fibroblast growth factor gene Fgf9 as causative event for the observed microphakia. We further detected reduced vision in Aca12 and demonstrate a retarded lens growth in Aca12 embryos and postnatal mice. Our study indicates that the novel Fgf9Y162C allele has functional (and clinical) consequences different from other known alleles of Fgf9. Therefore, it shows the importance of the characterization of allelic series to fully understand the function of a given gene.
Results
Establishment and genotyping of the Aca12 mouse mutant line
The mouse mutant line Aca12 was originally detected and established in a dominant ENU mutagenesis screen for eye size abnormalities because of its decreased lens thickness (polar diameter) at the age of 11 weeks (10.1% and 9.4% reduced mean lens thickness in homozygous Aca12 males and females, respectively). Further investigations revealed an increased anterior chamber depth and a reduced overall axial length. All eye-size parameters were less severely affected in heterozygotes, indicating a semidominant mode of inheritance (Figure 1A and 1B).
Figure 1. Affected eye size parameters of Fgf9Y162C mutants.
(A,B) Mean lens thickness (polar diameter) and axial length were reduced in heterozygous (A/+, hatched) and homozygous Fgf9Y162C (A/A, white) mice compared to the C57BL/6J control (WT, black). Anterior chamber depth was increased in the mutants. (A) females (n = 20); (B) males (n = 20). *p<0.02; **p<0.001.
In order to identify the mutation underlying the Aca12 phenotype, we performed a genome-wide linkage analysis by crossing homozygous mutants on a C57BL/6J background (G1) to wild-type C3HeB/FeJ mice. Heterozygous mutants (G2) were backcrossed to C3HeB/FeJ mice. The SNP-based analysis of 55 G3 offspring revealed linkage of the Aca12 phenotype to chromosome 14 within an interval spanning 13 Mb (between rs13482161 and rs30895903; cytoband C1 to C3). A further fine mapping using 175 G3 mice located the Aca12 mutation close to the microsatellite marker D14Mit215, between rs167842243 and D14Mit234 (Figure 2A). Based on these data, the genetic order was calculated (genetic distance ± SD in parenthesis): rs167842243 (1.15±0.81 cM), Aca12/D14Mit215 (0.57±0.57 cM), D14Mit234. The critical interval of approximately 3.6 Mb includes 78 genes. 29 of them are predicted only because of the existence of ESTs, but are not yet fully annotated. Among the characterized genes, Gja3 (gap junction protein, alpha 3), Ift88 (intraflagellar transport 88 homolog [Chlamydomonas]), and Fgf9 have previously been associated with irregularities in the eye [17], [25], [26]. Sequence analysis excluded Gja3 and Ift88 as candidates for Aca12. However, heterozygous Aca12 carriers exhibited an A→G transition at cDNA-position 855 of Fgf9 (counting the starting ATG as position 1; Figure 2B). Since the transition did not generate a novel restriction site, we confirmed the presence of this particular mutation in five homozygous carriers by sequencing of the corresponding region. Moreover, sequence analysis of four different wild-type strains (C57BL/6J, C3HeB/FeJ, JF1, and CFW) excluded a general polymorphism at this site. The 855 A→G transition is predicted to cause an exchange of Tyr to Cys at amino acid position 162 of the Fgf9 protein. Consequently, we refer to the novel allele as Fgf9Y162C.
Figure 2. Haplotype analysis of the Aca12 mutation and sequence analysis of the Fgf9 coding region.
(A) The Aca12 mutation is localized between the flanking microsatellite markers D14Mit268 and D14Mit234. Black boxes illustrate the presence of two C3H marker alleles (recombination between microsatellite marker and Aca12); white boxes illustrate heterozygosity of the markers for the C3H and C57BL/6J allele (lack of recombination). The number of G3 progeny carrying the particular recombination pattern is given below the boxes. The total number of recombination (R) events between neighbouring markers is shown to the right of the boxes, including the calculated relative genetic distances (cM ± standard deviations). In total, we tested 175 G3 animals. (B) Sequence analysis of genomic DNA of a heterozygous mutant shows an A→G transition (black arrow) at cDNA position 855 of the Fgf9 gene resulting in a Y162C amino acid exchange in the Fgf9 protein.
Fgf9Y162C does not affect viability, sex determination, or skeletogenesis
In order to study whether Fgf9Y162C carriers are fully viable, heterozygous individuals were bred to produce homozygous mutant mice. Among 86 intercross offspring, the genotype distribution was at the expected Mendelian ratio (25.6%, wild-types; 47.7%, heterozygotes; and 26.7%, homozygous mutants) and consequently did not indicate a reduced viability. Regular sex development in Fgf9Y162C was proved by sex genotyping PCR. A Sry signal was generated in all tested heterozygous and homozygous mutant males. In contrast, none of the female-like samples showed this signal, excluding the presence of XY genotyped female-like individuals (Figure 3). Concerning skeletogenesis, neither heterozygous nor homozygous Fgf9Y162C newborn mice developed elbow joint fusions, knee joint dysplasia, widened sternums, thickened ribs, fused vertebrae, or fused skull sutures (Figure 4A–4E). Moreover, even after breeding of more than 10 generations, we never observed kinky tails among adult Fgf9Y162C mice further demonstrating that skeletogenesis is not affected by the novel allele Fgf9Y162C.
Figure 3. Fgf9Y162C mutants exhibit regular sexual development.
Sex genotyping PCR amplified a Sry fragment (266 bp) indicating the presence of a Y chromosome in all tested male samples, but in none of the analyzed female probes. WT, C57BL/6J control (n = 5); A/+, heterozygous Fgf9Y162C mice (n = 6–9); A/A, homozygous Fgf9Y162C mice (n = 6–12); M, marker.
Figure 4. Skeleton develops regularly in Fgf9Y162C mutants.
(A–E) Alcian blue/Alizarin red staining of (A) elbow joints (lateral view), (B) knee joints (lateral view), (C) thoraric regions (ventral view), (D) spinal columns (lateral view), and (E) skulls (dorsal view) of C57BL/6J control (WT), heterozygous Fgf9Y162C (A/+), and homozygous Fgf9Y162C (A/A) newborn mice. The mutants did not exhibit skeletal abnormalities. H, humerus; R, radius; U, ulna; Fe, femur; T, tibia; Fi, fibula; S, sagittal suture; C, coronal suture.
Fgf9Y162C mice show reduced vision that is not caused by retinal irregularities
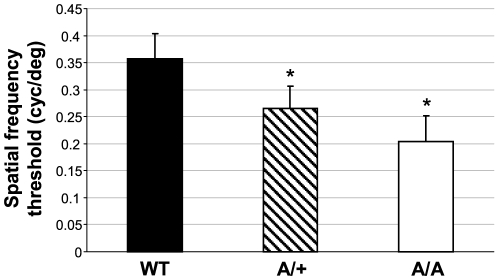
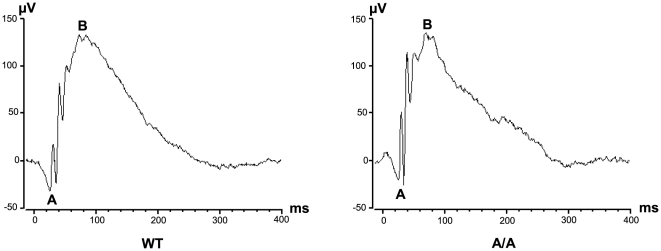
Since malformations outside the eye were excluded, we focused our studies on visual properties and development of ocular tissues. Vision of Fgf9Y162C mice was tested in a virtual optokinetic drum with three-month-old carriers. Calculation of mean spatial frequency thresholds confirmed a negative effect on vision in both homozygotes and heterozygotes (Figure 5). One possible reason for the reduced vision might be an abnormal retinal morphology and functionality, especially since Fgf9 has been suggested to play a role in retinal differentiation and maturation [27]. We therefore analyzed the retina of the more severely affected homozygous Fgf9Y162C mice in detail. An initial histological comparison between Fgf9Y162C mutants and wild-type controls did not reveal abnormalities on the cellular level (Figure 6A and 6B). These findings were confirmed by ophthalmoscopic investigations. All tested mutants exhibited a homogenously pigmented fundus with a well defined vessel pattern and a regularly developed optic disc (Figure 6C). Further investigations of retinal function by ERG measurements showed standard implicit times and regular a- and b-wave amplitudes in the electroretinogram of homozygous Fgf9Y162C retinas (Figure 7). It clearly demonstrates that the function of retinal photoreceptors, bipolar neurons, and ganglions is not affected as far as can be identified with these methods.
Figure 5. Reduced visual acuity in the Fgf9Y162C mutants.
Spatial frequency thresholds indicated a reduced vision in the Fgf9Y162C mutants. Values represent means ± standard deviation of measurements from twelve C57BL/6J controls (WT), ten heterozygous Fgf9Y162C mutants (A/+), and ten homozygous Fgf9Y162C carriers (A/A). *p<0.001.
Figure 6. Regular retinal histology and fundus morphology of Fgf9Y162C mutants.
(A,B) Morphology of four-month-old C57BL/6J control (WT) and homozygous Fgf9Y162C (A/A) retinas (A) and optic nerve heads (B) are compared. The mutants displayed a regular retinal development. (C) Funduscopy of six-month-old C57BL/6J controls (WT) and homozygous Fgf9Y162C (A/A) mice also revealed a regular pigmentation, optic disc structure, and blood vessel pattern in the eye fundi of the mutants. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS/OS, inner segment/outer segment of photoreceptor layer; RPE, retinal pigment epithelium.
Figure 7. Fgf9Y162C mice exhibit regular functionality of the retina.
Electroretinograms of six-month-old C57BL/6J control (WT) and homozygous Fgf9Y162 C (A/A) mice are shown. The mutants exhibit a regular electrical response to the given light flashes. (A), a-wave amplitude; (B), b-wave amplitude.
Fgf9Y162C retards prenatal and postnatal lens growth
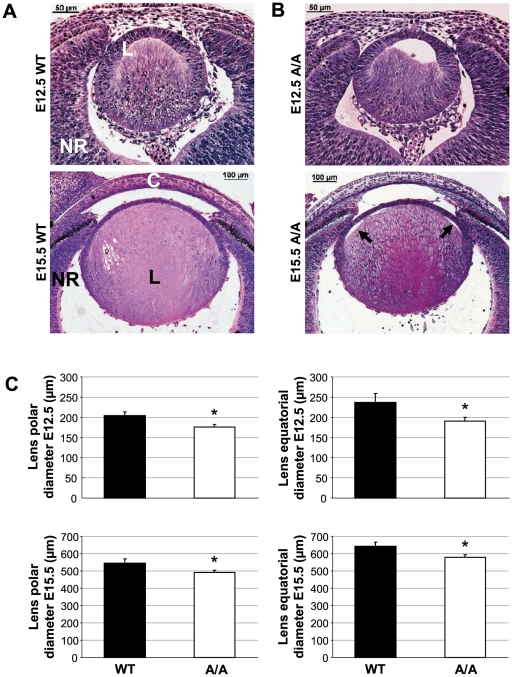
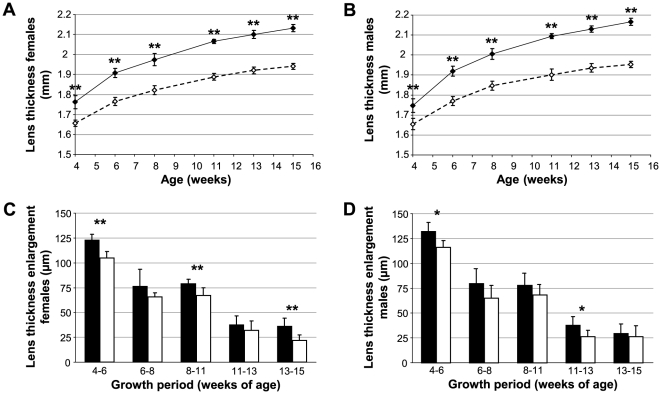
We further used the novel Fgf9Y162C missense mutation to study the role of Fgf9 signalling during lens development. Comparable to the recent findings, lens vesicles of embryonic Fgf9Y162C lenses remained partly unfilled at E12.5, pointing to retarded primary lens fiber growth (Figure 8A and 8B). At E15.5, lens fiber cells elongated completely. However, connection between fibers and lens epithelium was partly disrupted in the mutants at this particular stage (Figure 8A and 8B). Further calculations of mean lens polar and equatorial diameters revealed a significant reduction of both dimensions in prenatal Fgf9Y162C lenses (Figure 8C). Postnatal OLCI measurements confirmed decreased polar diameters (Figure 9A and 9B). Moreover, postnatal growth tracking of individual Fgf9Y162C lenses indicated a significantly reduced lens growth rate in the tested growth periods (Figure 9C and 9D).
Figure 8. Retarded primary lens fiber growth and reduced size of the Fgf9Y162C prenatal lens.
(A,B) Lens morphology of (A) C57BL/6J control (WT) and (B) homozygous Fgf9Y162C (A/A) lenses is shown at E12.5/E15.5. Growth of primary lens fibers is retarded in the mutants at E12.5. At E15.5, connection between lens fibers and lens epithelium is partly disrupted (arrows). L, lens; NR, neural retina; C, cornea. (C) Mean lens polar and equatorial diameter calculation at E12.5/E15.5 indicated a reduced size of both dimensions in the homozygous mutants. Values are means ± standard deviation (n = 5). *p<0.01.
Figure 9. Decreased Fgf9Y162C lens thickness at postnatal stages and retarded postnatal lens growth in Fgf9Y162C mutants.
(A,B) Mean lens thickness (polar diameter) of homozygous Fgf9Y162C mutants (dashed lines) obtained by OLCI at different postnatal stages are compared to the C57BL/6J control data (solid lines). Mutants exhibit significantly reduced lens sizes at all tested stages. (A) females; (B) males. (C,D) Postnatal lens growth of homozygous Fgf9Y162C mice (white bars) and C57BL/6J controls (black bars) is tracked by OLCI. In the mutants, lens growth is most strikingly reduced at early postnatal stages. (C) females; (D) males. Values are means ± standard deviation (n = 10–12). *p<0.01; **p<0.001.
Reduced lens size did not affect transparency, at least at younger stages. Histological analysis of four-month-old lenses confirmed normal nuclear degradation as well as regular lens fiber cell arrangement and size (Figure 10A–10C). However, 11 out of 18 twelve-month-old mutant lenses exhibited faint opacities of fiber cells at the anterior pole (Figure 11), indicating a tendency towards age related cataract formation in Fgf9Y162C mice.
Figure 10. Regular equatorial lens histology and fiber cell size of Fgf9Y162C mutants.
(A,B) Morphology of (A) four-month-old C57BL/6J control (WT) and (B) homozygous Fgf9Y162C (A/A) lenses is shown. The mutants display a regularly developed lens bow. L, lens; R, retina. (C) Mean lens fiber cell thickness at the age of four months calculated by averaging the breadth of ten fiber cell layers in the equatorial outer cortex. Lens fiber cell size did not differ significantly in the homozygous Fgf9Y162C mutants. Values are means ± standard deviation (n = 5).
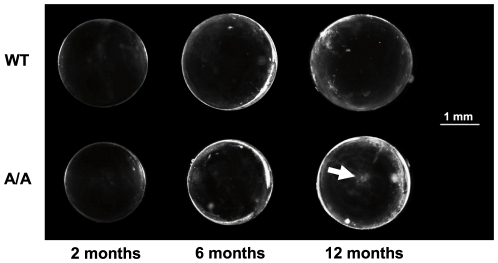
Figure 11. Age-related cataract formation in Fgf9Y162C lenses.
C57BL/6J control (WT) and homozygous Fgf9Y162C (A/A) lenses are compared at three different stages (two, six, and twelve months of age). All lenses remained transparent at least up to six months of age. Some twelve-month-old mutant lenses developed an age-related polar cataract (arrow).
Fgf9Y162C does not alter binding affinity for heparin
In order to get first insights into pathologic effects at the protein level, we performed modeling analysis of Fgf9Y162C. Structural changes were not predicted by the 3D-JIGSAW comparative modeling server (Figure 12A–12C). To test if the affinity towards heparin is influenced by the mutation Y162C, N-terminally truncated mFgf9WT and mFgf9Y162C were recombinantly expressed in bacteria and purified to approximately 95% purity. The proteins were subsequently applied to analytical heparin affinity chromatography. The major portions of both protein samples eluted exactly at the same ionic strength (Figure 13A). SDS PAGE (Figure 13B and 13C) as well as Western Blots (not shown) confirmed this observation. Thus, the mutation seems to have no effect on the heparin binding affinity.
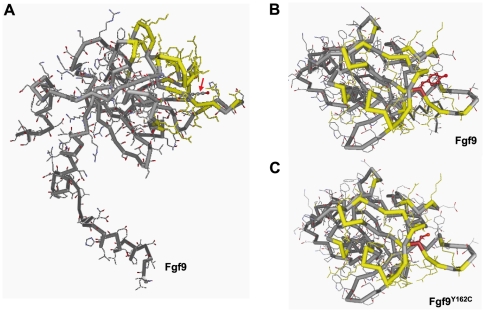
Figure 12. Predictive modeling of the Fgf9Y162C protein.
(A) The lateral view on Fgf9WT structure shows that the exchanged amino acid residue (red arrow) lies close to essential residues of the heparin binding region (marked in yellow). (B,C) Dorsal view on the wild-type protein (B) and Fgf9Y162C (C) indicates that structural changes within the heparin binding region (yellow marked residues) are not predicted. The amino acid residues at position 162 are marked in red.
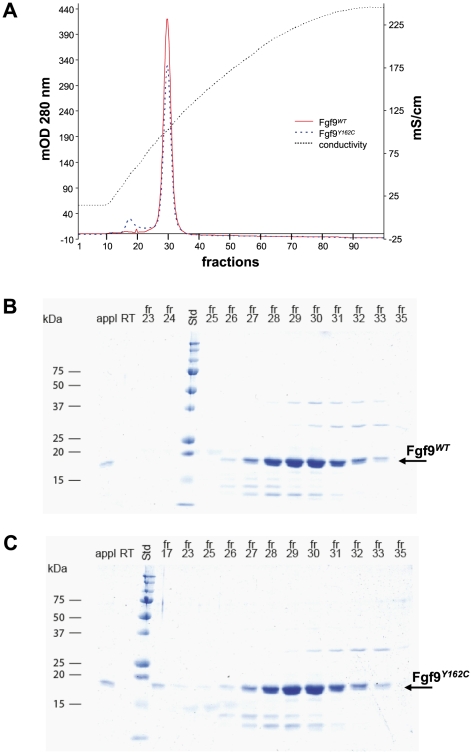
Figure 13. Affinity for Fgf9WT and Fgf9Y162C to heparin.
(A) Chromatographic analysis of the affinities of mFgf9WT and mFgf9Y162C for heparin is shown. mFgf9WT and mFgf9Y162C were loaded onto a HiTrap heparin column (1.5 mg each) and eluted with a linear gradient of NaCl from 120 mM to 2.0 M (conductivity line). Elution profiles of mFgf9WT (red) and mFgf9Y162C (blue) were determined by monitoring absorbance at 280 nm. (B,C) Coomassie stained SDS-PAGE gels monitoring the chromatographic analysis for mFgf9WT (B) and mFgf9Y162C (C). Std, standard; appl, sample load; RT, run through; fr, fraction.
Discussion
We have established the novel mouse mutant Aca12 that is primarily characterized by changes of ocular structures, e.g. larger anterior chambers, thinner lenses, and shorter axes. A genome wide linkage analysis and fine mapping placed the mutation on chromosome 14 within a 3.6 Mb interval. Sequence studies of positional candidate genes identified a base pair exchange within the Fgf9 gene (chromosome position 58.69–58.73 Mb); this mutation co-segregates with the phenotype among our breeding colony and was not found within other wild-type strains of mice. The facts that (i) a recombination between the Aca12 mutation and D14Mit215 (chromosome position 58.99 Mb) did not occur among 175 G3 carriers of the linkage study, (ii) the 855 A→G transition co-segregates with the phenotype and does not occur as a polymorphic site in other mouse strains, and (iii) Fgf9 has previously been associated with ocular malformations [17] further support the conclusion that the 855 A→G mutation represents the causative event for the observed pathologic phenotypes.
The replaced tyrosine residue 162 in Fgf9 is conserved between mice and humans and is an essential component of one of the 12 β strands defining the FGF trefoil fold; it is present also in FGF16 (UniProt: O43320) and FGF20 (UniProt: Q9NP95; they form together with FGF9 [UniProt: P31371] one of the FGF subfamilies [28]). Pathologic missense mutations in FGF9 (MIM #600921)/Fgf9 have been identified in humans affecting amino acid position 99 (S99N [29]) and position 143 in mice (N143T [22]). Among other features, in both cases, skeletal malformations have been described; however, effects on the skeleton have not been identified in the new Fgf9Y162C allele. Nevertheless, we demonstrate for the first time an essential role of this conserved residue Tyr-162 for the functional integrity of the Fgf protein. Position 162 is localized close to essential residues of the heparin binding site [30]. However, experimental data with recombinant proteins suggest no influence of the mutation on the heparin binding affinity.
Because of the unique phenotype, the novel Fgf9Y162C allele represents most likely a dominant-negative or neomorphic mutation, since the pathological phenotype is present in the heterozygotes. This feature is different from the recessive mode of inheritance in the Fgf9 knockouts; these heterozygotes are phenotypically normal [18]. However, similar to our new Fgf9Y162C allele, the Fgf9Eks allele (characterized by a N143T substitution [22]) is also characterized by a dominant mode of inheritance [21]. In contrast to our new Fgf9Y162C allele, the Fgf9Eks allele leads to several skeletal defects. The molecular explanation includes the prevention of homodimerization of Fgf9Eks, a reduced binding affinity to heparin [22], and increased diffusion properties. Therefore, Fgf9Eks is present in regions, where it usually does not occur; this ectopic Fgf9 signalling represses skeletal joint and suture development [21]. In contrast to Fgf9Eks, the regular skeletogenesis, the viability of the homozygous mutants and the regular sex determination in Fgf9Y162C mutants strongly points to a different pathologic mechanism in these mutants.
To understand the role of homodimerization, Kalinina et al. [28] introduced structure-based mutations into the dimer interfaces of FGF9 (D195A, L200A, I204A, L205A). The corresponding mutant proteins occur more frequently as a monomer, exhibit enhanced proliferative activities, and bind heparin more weakly than wild-type Fgf. It would be of great interest to see whether the novel Fgf9Y162C allele leads to similar biochemical consequences.
Since the resulting pathologic symptoms are restricted to decreased eye sizes and reduced visual properties, we focused our detailed analysis on the ocular function. Since ERG and retinal histology were normal in the mutants, reduced vision is obviously not caused by irregular development of retinal layers or optic nerve head. Consequently, the altered ocular structures of the Fgf9Y162C mutants characterized by deeper anterior chambers (about 4%), thinner lenses (about 10%), and shorter axes (about 5%) might be responsible for the reduced vision. Moreover, reduced vision might be due also to irregular development of visual system in the brain, since Fgf9 also is expressed in the visual cortex [31].
Our histological studies of embryonic Fgf9Y162C lenses indicated a retarded growth of primary lens fibers, since the lens vesicle of the mutants remained only partly filled at E12.5. This is in accordance with previous findings in Fgf9 null embryos [17]. We additionally identified a dominant negative effect of Fgf9Y162C on postnatal lens growth. Since secondary lens fiber cells are of regular shape and thickness, equatorial diameter of Fgf9Y162C lenses is most likely reduced because of a decreased total number of fiber cell layers. This would point to an essential role of Fgf9 signalling in proliferation of lens epithelial cells and fiber cell differentiation, respectively. Moreover, the reduced lens polar diameter might further indicate effects of Fgf9Y162C on lens fiber elongation. The decreased number of fiber cell layers does not affect transparency of the young lens, but about half of the tested lenses developed age-dependent polar opacifications.
Considering the confirmed role of Fgf9 signalling in lens development, it seems surprising that ocular Fgf9 expression is restricted to retinal layers [17], [32], [33]. It therefore seems very likely that Fgf9 is secreted by retinal cells and diffuses through the vitreous to the lens. This would further explain the requirement of normal Fgf9 mobility for regular lens development. Taking into account that an increased diffusion of Fgf9Y162C can be excluded as discussed above, retarded lens growth might consequently be the result of reduced Fgf9Y162C concentrations in the lens tissue due to decreased diffusion abilities. This hypothesis is further supported by dose dependent effects of Fgf9 on lens development. In particular, transgenic overexpression of Fgf9 resulted in severe lens defects including irregular polarity and postnatal cataract formation [16]. Loss of Fgf9 function in homozygous knockout mutants initiated mild lens pathologies [17] which can be compared to the situation in heterozygous Fgf9Y162C mutants. However, completely different pathologic mechanisms (like altered receptor binding affinity) underlying the irregular lens development in Fgf9Y162C can currently not be excluded; polarity of lenses might develop normally because of an Fgf9Y162C concentration gradient. This hypothesis would fit to previous results that indicate an essential role of FGF gradients on determining anterior-posterior patterns of the lens [34], [35].
The target genes of Fgf9 signalling during lens development remain to be identified. Effects on lens fiber maturation have previously been described in various gap junction protein mutants including connexin23 (UniProt: Q9CX92), connexin43 (UniProt: P23242), connexin46 (UniProt: Q64448), and connexin50 (UniProt: P28236) [36]–[39]. Similar to the situation in homozygous Fgf9Y162C mice, irregular cell-to-cell appositions between lens epithelial cells and fiber cells were found in the connexin23- and connexin43 mutants [36], [37]. It seems very likely that Fgf9 signalling interacts with gap junction protein expression in the lens. Since Fgf9 binds specifically to the Fgf receptors 2 and 3 [40], studies using conditional knockout mice lacking Fgf receptor expression further provided hints for putative Fgf9 signalling targets. Triple knockout mice (Fgfr1–3; MGI: 95522–95524) exhibited irregular lens fiber elongation and proliferation together with aberrant or reduced expression of p27kip1 (UniProt: P46414), p57kip2 (UniProt: P49919), Prox1 (UniProt: P48437), Pax6 (UniProt: P63015), c-Maf (UniProt: P54843), E-cadherin (UniProt: P09803), and crystallins (UniProt: P24622, P02525, and P04345) [41].
In conlcusion, the Fgf9Y162 mouse line described here provides a novel Fgf9 allele with yet unknown properties. It will be useful for getting more insights into the role of Fgf9 signalling in lens development; moreover, our data suggest FGF9 as a candidate for human age-related cataracts. Since the new Fgf9 allele leads to a novel phenotype not observed in other of FGF9 mutants, it is of great interest to uncover the underlying biochemical differences.
Materials and Methods
Ethics statement
The use of animals was in accordance with the German Law of Animal Protection, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the tenets of the Declaration of Helsinki. Animal experiments of this study were approved by the ethics committee of the Upper Bavarian government (TVA #55.2-1-54-2531-78-06).
Mice
Mice were kept under specific pathogen-free conditions at the Helmholtz Center Munich. Male C57BL/6J mice were treated with ENU (80 mg/kg body weight applied by intraperitoneal injection in three weekly intervals) at the age of 10–12 weeks as previously described [42] and mated to untreated female C57BL/6J mice [23]. The offspring of the ENU-treated mice were screened at the age of 11 weeks for abnormalities of the eye size [24]. Mice with phenotypic deviations were tested for a dominant mode of inheritance.
Linkage analysis
Heterozygous carriers (first generation) were mated to wild-type C3HeB/FeJ mice, and the offspring (second generation) were backcrossed to wild-type C3HeB/FeJ mice. DNA was prepared from tail tips of affected offspring of the third generation (G3). For genome-wide linkage analysis, genotyping of a genome-wide mapping panel consisting of 149 single nucleotide polymorphisms (SNP) was performed using MassExtend, a MALDI-TOF (matrix-assisted laser/desorption ionization, time of flight analyzer) mass spectrometry high-throughput genotyping system supplied by Sequenom (San Diego, CA, USA; [43]). Fine mapping was performed with the microsatellite markers D14Mit141, D14Mit101, D14Mit142, D14Mit268, D14Mit215, and D14Mit234. Furthermore, recombination frequency between Aca12 and the SNP rs167842243 (chromosome 14, genome coordinates 56,682,854) was determined using the primer pair SNP56.68-1 and SNP56.68-2 (Table 1) and subsequent sequence analysis.
Table 1. PCR primers used in this study.
| Short name | Sequence | Product size | Tm |
| SNP56.68-1 | 5′-GCAAATAAAGTTTGCATGACCA-3′ | 63.5°C | |
| SNP56.68-2 | 5′-TTTGGAGCTGAGACGAAAGG-3′ | 398 bp | 64.3°C |
| Fgf9-IV-1 | 5′-TTGAAGACTATTCTGGTTCAAAGA-3′ | 60.6°C | |
| Fgf9-IV-2 | 5′-CAAAGTTTGGCAACAGTGGA-3′ | 471 bp | 63.7°C |
| Sry-1 | 5′-GAGAGCATGGAGGGCCAT-3′ | 65.3°C | |
| Sry-2 | 5′-CCACTCCTCTGTGACACT-3′ | 266 bp | 55.8°C |
| Fgf9-I-1 | 5′-TCGCCTAGTGTCTCCTGGTT-3′ | 63.7°C | |
| Fgf9-I-2 | 5′-GACCAGGCCCACTGCTATAC-3′ | 398 bp | 63.4°C |
| Fgf9-II-1 | 5′-CGGTACTATCCAGGGAACCA-3′ | 63.6°C | |
| Fgf9-II-2 | 5′-CAACAGTGGAGCTGAGGTGA-3′ | 497 bp | 64.2°C |
| Gja3-I-1 | 5′-GGAATCCAGCACTGTCAGGT-3′ | 54.0°C | |
| Gja3-I-2 | 5′-GCATGAAGATGACAAAGATGG-3′ | 700 bp | 50.0°C |
| Gja3-II-1 | 5′-AGGCCCACAGAGAAGACCAT-3′ | 54.0°C | |
| Gja3-II-2 | 5′-GGAATCCAGCACTGTCAGGT-3′ | 791 bp | 54.0°C |
| Ift88-I-1 | 5′-ATCAGGCGTCGCTTCTTC-3′ | 63.1°C | |
| Ift88-I-2 | 5′-TGATGTCAGGGATGTCTTGG-3′ | 297 bp | 63.6°C |
| Ift88-II-1 | 5′-ATCAGGCGTCGCTTCTTC-3′ | 63.1°C | |
| Ift88-II-2 | 5′-CTTTCTTCTCCAACTGTCTAATTTTT-3′ | 499 bp | 60.8°C |
| Ift88-III-1 | 5′-ATTTGACCCCCTTGGTCAGT-3′ | 64.4°C | |
| Ift88-III-2 | 5′-TTTATGGACACTTGGGATCTGA-3′ | 469 bp | 63.1°C |
| Ift88-IV-1 | 5′-GCAATGCAGGAAGACTGAAA-3′ | 62.9°C | |
| Ift88-IV-2 | 5′-CAAAAGACGCTTCGATCACA-3′ | 498 bp | 63.9°C |
| Ift88-V-1 | 5′-AAGGAAAGCCATGGCAGAA-3′ | 64.4°C | |
| Ift88-V-2 | 5′-TCCAGACGGTTCAGCTTCTT-3′ | 495 bp | 63.9°C |
| Ift88-VI-1 | 5′-AGAGGCCCTGAGAAACGACT-3′ | 64.2°C | |
| Ift88-VI-2 | 5′-AGCTACCATCAGCTGCCACT-3′ | 460 bp | 63.9°C |
| Ift88-VII-1 | 5′-TGCGAGAAAGCCATTCAGTA-3′ | 63.4°C | |
| Ift88-VII-2 | 5′-TTCTATCTGAGGGCCCAGTG-3′ | 486 bp | 64.1°C |
| Ift88-VIII-1 | 5′-CAAAGAAATAGATGCCTCCTACG-3′ | 62.6°C | |
| Ift88-VIII-2 | 5′-AAGTTCCAGGCCAGAGGAA-3′ | 250 bp | 63.7°C |
| mFgf9for-33aa_NcoI | 5′-TTTCCATGGATGGACCACCTGGGTCAG-3′ | 47.4°C | |
| mFgf9rev_HindIII | 5′-TTTAAGCTTTCAGCTTTGGCTTAGAATATC-3′ | 474 bp | 48.5°C |
Genotyping, sex determination, and sequencing
Genomic DNA was isolated from tail tips of C57BL/6J, C3HeB/FeJ, JF1, and CFW wild-type mice or homozygous/heterozygous mutants according to standard procedures.
PCR of the mutated site was performed with the primers Fgf9-IV-1 and Fgf9-IV-2, sex genotyping PCR was carried out with the primers Sry-1 and Sry-2 amplifying the sex determining region of chromosome Y (Table 1). For sequencing of promising candidate genes, the primer pairs Fgf9-I/Fgf9-II, Gja3-I/Gja3-II, and Ift88-I to Ift88-VIII were used (Table 1).
PCR was performed with a PTC-225 thermocycler (MJ Research, Waltham, USA). Products were analyzed by electrophoresis on a 1.5% agarose gel. Sequencing was performed commercially (GATC Biotech, Konstanz, Germany) after direct purification of the PCR products (Nucleospin Extract II, Macherey-Nagel, Düren, Germany).
The coding sequence of the novel Fgf9Y162C allele is available under GenBank accession HM988990.
Protein modeling
The PDB files used to model Fgf9WT and Fgf9Y162C was generated from the 3D-JIGSAW comparative modeling server [44]. The proteins were modeled using the 3D Mol-Viewer component of the Vector NTI Suite software 9.0.0 (Invitrogen, Darmstadt, Germany).
Fgf9 bacterial expression, purification and heparin binding
Fgf9WT and Fgf9Y162C were expressed and purified essentially as previously described [22], [45]. Residues 34 to 208 each of mFgf9WT and mFgf9Y162C were cloned into the vector pET28a(+) (Novagen, Darmstadt, Germany) between NcoI and HindIII sites. Forward primer mFgf9for-33a_NcoI and reverse primer mFgf9rev_HindIII were used (Table 1). The resulting open reading frames coded for proteins of ∼20 kDa. Sequence integrity was verified by Sanger sequencing. Plasmid constructs were transformed into E.coli BL21 DE3 (Stratagene, Waldbronn, Germany) and protein expression was induced by 0.5 mM IPTG in LB/Kan medium for four hours at 37°C or overnight at 20°C. Bacteria were harvested, suspended in lysis buffer (25 mM HEPES pH 7.5, 150 mM NaCl, 10% glycerol, 1 mM EDTA, protease inhibitor Complete [Roche, Mannheim, Germany]) and subsequently disrupted by lysozyme treatment and sonication. DNA was digested by DNaseI (New England Biolabs, Frankfurt, Germany). After removal of cell debris by centrifugation, ammonium sulfate was added to the supernatants to reach 25% saturation. After centrifugation Fgf9 proteins were precipitated over night from the supernatant at 50% saturation of ammonium sulfate. Pellets were dissolved in 40 volumes 25 mM HEPES pH 7.5, 120 mM NaCl, centrifuged, and the supernatants applied to a 1 ml HiTrap heparin affinity column (GE Healthcare, Little Chalfont, United Kingdom). Proteins were eluted with a linear gradient (120 mM to 2 M NaCl) in HEPES pH 7.5. Fractions containing enriched mFgf9 proteins were applied to a second round of heparin affinity chromatography. Identity of mFgf9 proteins was verified by SDS-PAGE and Western blotting (polyclonal antibodies from Antibodies-Online, Aachen, Germany). Protein concentration of purified proteins was determined by Bradford assay (Bio-Rad, München, Germany) using BSA as standard.
Analytical heparin affinity chromatography
1.5 mg each of ∼95% pure mFgf9WT and mFgf9Y162C were loaded onto a 1 ml HiTrap heparin HP column (GE Healthcare, Little Chalfont, United Kingdom) equilibrated with 25 mM HEPES pH 7.5, containing 120 mM NaCl. The bound proteins were eluted with a linear gradient of NaCl (120 mM to 2.0 M) in the same buffer.
Skeleton preparation
Newborn mice were eviscerated and the skin was removed. After fixation in 100% ethanol (four days) and incubation in acetone (three days), mice were washed and stained in Alcian blue/Alizarin red solution (150 mg Alcian blue solved in 50 ml 70% ethanol, 50 mg Alizarin red solved in 50 ml 95% ethanol, 50 ml acetic acid, 850 ml 100% ethanol) for ten days. After staining, mice were washed, cleared in 1% KOH/20% glycerol (two weeks), and stored in 100% glycerol.
Histological preparation, embryonic lens area determination, and mean lens fiber cell thickness calculation
Eyes of embryos and adult mice were histologically analyzed for retinal irregularities, lens area, and lens fiber cell pathologies. Embryo heads or separated eyes were fixed for seven days in Davidson solution and embedded in JB-4 plastic medium (Polyscience Inc. Eppelheim, Germany) according to the manufacturer's protocol. Sectioning was performed with an ultramicrotome (OMU3; Reichert-Jung, Walldorf, Germany). Serial transverse 3-µm sections were cut with a glass knife and stained with methylene blue and basic fuchsin. The sections were evaluated with a light microsocope (Axioplan, Carl Zeiss, Jena, Germany). Images were acquired by means of a scanning camera (AxioCam; Jenoptik, Jena, Germany) and imported into an image-processing program (Photoshop 10.0, Adobe, Unterschleissheim, Germany). Embryonic lens diameters and lens fiber cell sizes were analyzed using the size determination tools provided by the AxioVision 4.6.3.0 software (Carl Zeiss, Jena, Germany). Mean lens fiber cell thickness was calculated by averaging the breadth of ten fiber cell layers in the equatorial outer cortex.
Lens growth determination and eye size measurement
The sizes of ocular parameters were examined between four and 15 weeks of age using optical low coherence interferometry (OLCI; “ACMaster”, Meditec, Carl Zeiss, Jena, Germany). Briefly, mice were anesthetized with an intraperitoneal injection of 137 mg ketamine and 6.6 mg xylazine per kilogram body weight. The anesthetized mouse was placed on a platform and oriented in an appropriate position using light signals from six infrared LEDs arranged in a circle that must be placed in the center of the pupil. Central measurements of lens thickness (polar diameter), axial length, corneal thickness, and anterior chamber depth as well as data evaluation were performed essentially as described [24], [46].
Virtual vision test
Vision tests were performed between 9 am and 4 pm using a virtual optomotor system (Cerebral Mechanics, Lethbridge, Canada) as described previously [47]. Briefly, a rotating cylinder covered with a vertical sine wave grating was calculated and drawn in virtual three-dimensional space on four computer monitors facing to form a square. Visually unimpaired mice track the grating with reflexive head and neck movements (head-tracking). Vision threshold of the tested mice was quantified by a simple staircase test. Rotation speed and contrast were set to 12.0 d/s and 100%, respectively. Since no significant threshold differences were observed between males and females (P>0.05; calculated by Mann-Whitney U-test), data of both sexes were combined. Thresholds of wild-type C57BL/6J and homozygous Fgf9Y162C mice were compared using the Mann-Whitney U-test.
Funduscopy
Nine-month-old mice with clear lenses were administered 1% atropine to each eye to dilate their pupils. The fundus examination was performed with a Heine Sigma 150K indirect ophthalmosocope (Haag-Streit GmbH, Wedel, Germany) and a Volk 90D superfield lens (Haag-Streit GmbH). Digital fundus images were taken with a Heine Video Omega 2C indirect ophthalmoscope connected to a VRmAVC Video Grabber (Dieter Mann GmbH, Mainaschaff, Germany) and a Volk 40D or 60D lens (Fronhäuser GmbH, Unterhaching, Germany). The images were imported in an image-processing program (Photoshop 10.0; Adobe).
Elektroretinography (ERG)
Ganzfeld ERGs were recorded simultaneously from both eyes of six-month-old mice to examine the retinal function as described [48]. In brief, mice were dark-adapted for at least 12 hours and anaesthetized. After pupil dilation (1 drop Atropine 1%), individual mice were fixed on a sled and gold wires (as active electrodes) were placed on the cornea. The ground electrode was a subcutaneous needle in the tail; a reference electrode was placed subcutaneously between the eyes. The mice were introduced into an ESPION ColorBurst Handheld Ganzfeld LED stimulator (Diagnosys LLC, Littleton, MA, USA) on a rail to guide the sled (High-Throughput Mouse-ERG, STZ for Biomedical Optics and Function Testing, Tübingen, Germany). 10 ms light pulses were delivered at a frequency of 0.48 Hz and a light intensity of 12,500 cd/m2. Responses were recorded with an ESPION Console (Diagnosys LLC, Littleton, MA, USA).
General
Chemicals were from Merck (Darmstadt, Germany) or Sigma Chemicals (Deisenhofen, Germany).
Acknowledgments
The authors thank Erika Bürkle, Monika Stadler, Maria Kugler, Jan Enke, and Tommy Fuchs for expert technical assistance and Megan Fisher for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work has been supported by the European Community (European Mouse Disease Clinic) and by National Genome Network Grants (National Genome Research Network, National Genome Research Network plus). Grant Identifications: LSHG-2006-037188, BMBF 01KW9923, and BMBF 01GS0850. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. The Global Burdon of Disease: 2004 update. WHO-Press, Geneva, Switzerland; 2008. [Google Scholar]
- 2.Warburg M, Sjö O, Fledelius HC, Pedersen SA. Autosomal recessive microcephaly, microcornea, congenital cataract, mental retardation, optic atrophy, and hypogenitalism. Micro syndrome. Am J Dis Child. 1993;147:1309–1312. doi: 10.1001/archpedi.1993.02160360051017. [DOI] [PubMed] [Google Scholar]
- 3.Menez B. A case of Marfan's syndrome in a black African. Med Trop (Mars) 1981;41:569–571. [PubMed] [Google Scholar]
- 4.Sanchez E, Munier F, Evéquoz B, Marcoz JP, Balmer A. Ocular phenotype in a child with chondrodysplasia punctata, rhizomelic form. Klin Monatsbl Augenheilkd. 1997;210:329–331. doi: 10.1055/s-2008-1035067. [DOI] [PubMed] [Google Scholar]
- 5.Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, et al. Mutations of the catalytic subunit of RABGAP cause Warburg Micro syndrome. Nat Genet. 2005;37:221–223. doi: 10.1038/ng1517. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, et al. Marfan syndrome caused by a recurrent de novo missence mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 7.Braverman N, Steel G, Obie C, Moser A, Moser H, et al. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 8.Motley AM, Hettema EH, Hogenhout EM, Brites P, ten Asbroek AL, et al. Rhizomelic chondrodysplasia punctata is a peroximal protein targeting disease caused by a non-functional PTS2 receptor. Nat Genet. 1997;15:377–380. doi: 10.1038/ng0497-377. [DOI] [PubMed] [Google Scholar]
- 9.Purdue PE, Zhang JW, Skoneczny M, Lazarow PB. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat Genet. 1997;15:381–384. doi: 10.1038/ng0497-381. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an à la carte menu of heparin sulphate motifs. Curr Opin Struc Biol. 2005;15:506–516. doi: 10.1016/j.sbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain CG, McAvoy JW. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res. 1987;6:1165–1169. doi: 10.3109/02713688709034890. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain CG, McAvoy JW. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth factors. 1989;1:125–134. doi: 10.3109/08977198909029122. [DOI] [PubMed] [Google Scholar]
- 14.McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- 15.Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, et al. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol. 1998;198:13–31. doi: 10.1006/dbio.1998.8879. [DOI] [PubMed] [Google Scholar]
- 16.Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S, Hung FC, Colvin JS, White A, Dai W, et al. Patterning the optic neuroepithelium by FGF signalling and Ras activation. Development. 2001;128:5051–5060. doi: 10.1242/dev.128.24.5051. [DOI] [PubMed] [Google Scholar]
- 18.Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- 19.Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 20.Cotton LM, O'Bryan MK, Hinton BT. Cellular signalling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev. 2008;29:193–216. doi: 10.1210/er.2007-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami H, Okawa A, Yoshida H, Nishikawa S, Moriya H, et al. Elbow knee synostosis (Eks): a new mutation on mouse chromosome 14. Mamm Gen. 2002;13:341–344. doi: 10.1007/s00335-001-2143-6. [DOI] [PubMed] [Google Scholar]
- 22.Harada M, Murakami H, Okawa A, Okimoto N, Hiraoka S, et al. FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nat Genet. 2009;41:289–298. doi: 10.1038/ng.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hrabé de Angelis M, Flaswinkel H, Fuchs H, Rathkolb B, Soewarto D, et al. Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat Genet. 2000;25:444–447. doi: 10.1038/78146. [DOI] [PubMed] [Google Scholar]
- 24.Puk O, Dalke C, Favor J, Hrabé de Angelis M, Graw J. Variations of eye size parameters among different strains of mice. Mamm Gen. 2006;17:851–857. doi: 10.1007/s00335-006-0019-5. [DOI] [PubMed] [Google Scholar]
- 25.Gong X, Li E, Klier G, Huang Q, Wu Y, et al. Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 26.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinaroglu A, Ozmen Y, Ozdemir A, Ozcan F, Ergorul C, et al. Expression and possible function of fibroblast growth factor 9 (FGF9) and its cognate receptors FGFR2 and FGFR3 in postnatal and adult retina. J Neurosci Res. 2005;79:329–339. doi: 10.1002/jnr.20363. [DOI] [PubMed] [Google Scholar]
- 28.Kalinina J, Byron SA, Makarenkova HP, Olsen SK, Eliseenkova AV, et al. Homodimerization controls the fibroblast growth factor 9 subfamily's receptor binding and heparan sulfate-dependent diffusion in the extracellular matrix. Mol Cell Biol. 2009;29:4663–4678. doi: 10.1128/MCB.01780-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu XL, Gu MM, Huang L, Liu XS, Zhang HX, et al. Multiple synostoses syndrome is due to a missense mutation in exon 2 of FGF9 gene. Am J Hum Genet. 2009;85:53–63. doi: 10.1016/j.ajhg.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plotnikov AN, Eliseenkova AV, Ibrahimi OA, Shriver Z, Sasisekharan R, et al. Crystal structure of fibroblast growth factor 9 reveals regions implicated in dimerization and autoinhibition. J Biol Chem. 2001;276:4322–4329. doi: 10.1074/jbc.M006502200. [DOI] [PubMed] [Google Scholar]
- 31.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 32.Colvin JS, Feldman B, Nadeau JH, Goldfarb M, Ornitz DM. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev Dyn. 1999;216:72–88. doi: 10.1002/(SICI)1097-0177(199909)216:1<72::AID-DVDY9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, et al. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn. 2005;234:371–386. doi: 10.1002/dvdy.20441. [DOI] [PubMed] [Google Scholar]
- 34.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Robinson ML. An essential role for FGF receptor signalling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puk O, Löster J, Dalke C, Soewarto D, Fuchs H, et al. Mutation in a novel connexin-like gene (Gjf1) in the mouse affects early lens development and causes a variable small-eye phenotype. Invest Ophthalmol Vis Sci. 2008;49:1525–1532. doi: 10.1167/iovs.07-1033. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Spray DC. Structural changes in lenses of mice lacking the gap junction protein connexin 43. Invest Ophthalmol Vis Sci. 1998;39:1198–1209. [PubMed] [Google Scholar]
- 38.Dunia I, Cibert C, Gong X, Xia C, Recouvreur M, et al. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur J Cell Biol. 2006;85:729–752. doi: 10.1016/j.ejcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, et al. Disruption of Gja8 (α8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–174. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 40.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Res. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehling UH, Charles DJ, Favor J, Graw J, Kratochvilova J, et al. Induction of gene mutations in mice: the multiple endpoint approach. Mutat Res. 1985;150:393–401. doi: 10.1016/0027-5107(85)90136-8. [DOI] [PubMed] [Google Scholar]
- 43.Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, et al. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe b-cell loss in Munich Ins2 C95S mutant mice. Diabetes. 2007;56:1268–1276. doi: 10.2337/db06-0658. [DOI] [PubMed] [Google Scholar]
- 44.Bates PA, Kelley LA, MacCallum RM, Sternberg MJE. Enhancement of protein modelling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins. 2001;Suppl 5:39–46. doi: 10.1002/prot.1168. [DOI] [PubMed] [Google Scholar]
- 45.Koyama N, Ohmae H, Tsuji S, Tanaka Y, Kurokawa T, et al. Improved preparation and crystallization of 25 kDa human fibroblast growth factor – 9. Biotechnol Appl Biochem. 2001;33:117–121. doi: 10.1042/ba20000075. [DOI] [PubMed] [Google Scholar]
- 46.Schmucker C, Schäffel F. In vivo biometry in the mouse eye with low coherence interferometry. Vis Res. 2004;44:2445–2456. doi: 10.1016/j.visres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 48.Dalke C, Löster J, Fuchs H, Gailus-Durner V, Soewarto D, et al. Electroretinography as a screening method for mutations causing retinal dysfunction in mice. Invest Ophthalmol Vis Sci. 2004;45:601–609. doi: 10.1167/iovs.03-0561. [DOI] [PubMed] [Google Scholar]