Summary
Sumoylation is generally considered a repressive mark for many transcription factors. However, the in vivo importance of sumoylation for any given substrate remains unclear and is questionable because the extent of sumoylation appears exceedingly low for most substrates. Here, we permanently eliminated SF-1/NR5A1 sumoylation in mice (Sf-1K119R,K194R or 2KR) and found that Sf-12KR/2KR mice failed to phenocopy a simple gain of SF-1 function or show elevated levels of well-established SF-1 target genes. Instead, mutant mice exhibited marked endocrine abnormalities and changes in cell fate that reflected an inappropriate activation of hedgehog signaling and other potential SUMO-sensitive targets. Further, unsumoylatable SF-1 mutants activated Shh and exhibited preferential recruitment to Shh genomic elements in cells. We propose that the sumoylation cycle greatly expands the functional capacity of transcription factors such as SF-1, and is leveraged during development to achieve cell type-specific gene expression in multi-cellular organisms.
Introduction
Many proteins involved in transcription including transcription factors, histones, and chromatin remodeling proteins undergo posttranslational modification by conjugation with the small ubiquitin-like modifier (SUMO) (Gill, 2005a). The sumoylation cycle begins with activation of SUMO by the E1 enzyme, followed by transesterification to the E2 conjugating enzyme (Ubc9), and ligation of SUMO to an acceptor lysine; for most substrates this process is further stimulated by SUMO E3 ligases (Hay, 2005). Desumoylation completes this cycle and is carried out by Sentrin/SUMO-specific isopeptidases (SENPs). Genetic lesions in the general sumoylation machinery show that sumoylation is essential for life in both lower and higher eukaryotes. Deleting Ubc9 in mice or its ortholog lwr in Drosophila leads to embryonic death (Apionishev et al., 2001; Nacerddine et al., 2005), as does knocking-down Ubc9 or all three Sumo paralogs in zebrafish (Nowak and Hammerschmidt, 2006; Yuan et al., 2010). Similarly, disrupting desumoylation in mice by removing the SUMO-specific proteases Senp1 (Cheng et al., 2007) and Senp2 (Kang et al., 2010) also impairs normal development. Eliminating individual SENP proteases presumably increases the proportion of sumoylated to unmodified substrate. This was observed recently in Senp2−/− mice, which display defects in cardiac development resulting in part from the hypersumoylation of the repressor Pc2 and enhanced recruitment of the polycomb repressive complex 1 (PRC1) to Gata4 and Gata6 promoters (Kang et al., 2010). Taken together, these studies imply that a group of critical substrates must undergo sumoylation and/or desumoylation during development.
While these studies establish the essential requirement of the general SUMO cycle, it remains unclear to what extent sumoylation changes the in vivo function of a specific substrate. To date, these questions have been partially addressed in transgenic studies that ectopically delivered sumoylatable and unsumoylatable substrates. For instance, SUMO-fusions and non-sumoylatable SoxE factors function differently in Xenopus cell fate studies (Taylor and Labonne, 2005). Similarly, wild type and K107R PPARγ exhibit different phenotypes after adenoviral-mediated delivery in a rat carotid artery injury model (Lim et al., 2009). Reconstitution studies also report that non-sumoylatable variants are far less efficient at rescuing null phenotypes when compared with their sumoylatable counterparts. Indeed, rescue of uterine-vulval defects with the K17, K18 LIN-11 sumoylation mutant (Broday et al., 2004), and rescue of retinal defects with the K178R NR2E3/PNR mutant (Onishi et al., 2009) are diminished compared with wild type constructs. While these studies showed that sumoylated and unmodified substrates function differently when ectopically delivered, the in vivo consequences of sumoylation for an endogenously expressed substrate in a native setting remains to be determined.
Vertebrate members of the NR5A subfamily of nuclear receptors, including steroidogenic factor 1 (SF-1, NR5A1) and liver receptor homolog 1 (LRH-1, NR5A2), possess two conserved sumoylation sites (Chen et al., 2004; Komatsu et al., 2004; Lee et al., 2005) which are located in the large flexible hinge domain juxtaposed to either the DNA binding domain (DBD) or the ligand binding domain (LBD). SF-1 is efficiently sumoylated in vitro to nearly 100% at both Lys119 and Lys194 (Campbell et al., 2008; Ogawa et al., 2009). As found for many transcription factors, overexpression of unsumoylatable SF-1 increases cell-based reporter activity and elevates ACTH-dependent steroidogenic enzyme genes (Yang et al., 2009) without affecting protein stability (Lee et al., 2005). Similarly, unsumoylatable LRH-1 increases reporter activity when overexpressed in cultured cells (Chalkiadaki and Talianidis, 2005; Lee et al., 2005), and enhances induced pluripotency when overexpressed in mouse embryonic fibroblasts (Heng et al., 2010). Results from these cellular studies suggest that the major effect of NR5A receptor sumoylation is an overall repression of transcriptional activity. However, our previous study led us to refine this notion and hypothesize that disrupting the normal SUMO cycle of SF-1 might selectively affect a subset of SUMO-sensitive targets rather than simply altering SF-1 activity on all targets (Campbell et al., 2008).
To directly assess if sumoylation is essential for proper function of a single substrate in its native setting, we generated a knock-in mouse model harboring an unsumoylatable form of SF-1 (Sf-1K119R,K194R or 2KR). In vertebrates, SF-1 is essential for normal endocrine tissue development and regulates genes in the stress and reproductive endocrine signaling axes. Heterozygous Sf-1 mice exhibit moderate defects and are relatively normal compared to the dramatic adrenal and gonadal agenesis displayed in Sf-1−/− mice (Luo et al., 1994). Numerous heterozygous human SF-1 mutations are associated with testicular dysgenesis, premature ovarian failure and adrenal insufficiency. Interestingly, two missense mutations associated with male infertility map closely to K119 and K194 (Bashamboo et al., 2010; Lourenco et al., 2009). On the other hand, targeted overexpression of SF-1 in mouse adrenals leads to adrenal hyperplasia (Zubair et al., 2009) and results in adrenal cortical tumors (Doghman et al., 2007). Based on these Sf-1 dosage-sensitive phenotypes and the repressive effect of SF-1 sumoylation in cells, we asked if permanently eliminating SF-1 sumoylation would manifest as an increase in SF-1 dosage with a concomitant elevation of well-established target genes. Here we report that Sf-12KR/2KR mice failed to phenocopy a simple gain (or loss) of SF-1 function, and instead exhibited distinct physiological abnormalities that reflected an inappropriate activation of SF-1 targets in endocrine organ development.
Results
Endocrine tissues and their steroidogenic capacity are disrupted in Sf-12KR/2KR mice
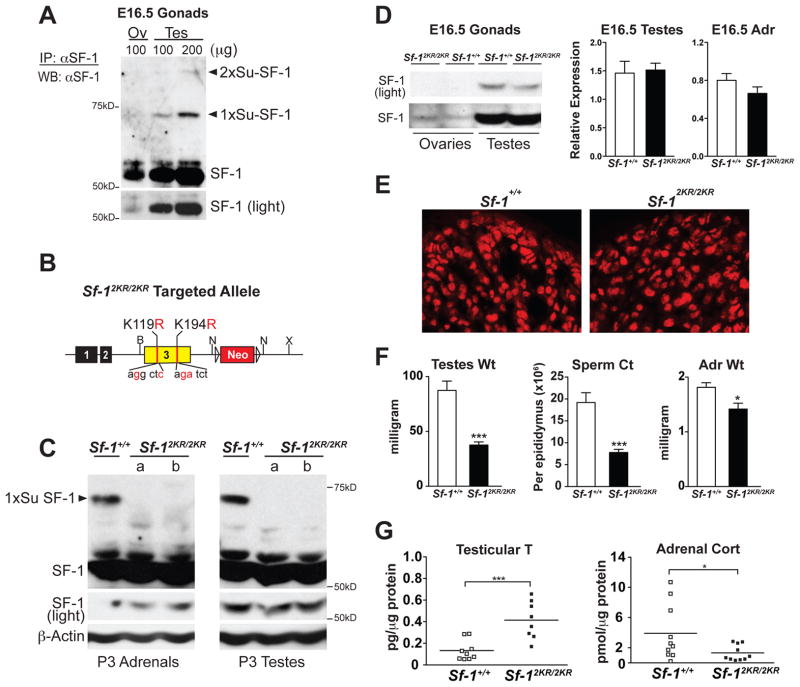
Sumoylated SF-1 is readily detected in embryonic (E) 16.5 testes and adrenals but undetectable in the embryonic ovary underscoring the marked sexually dimorphic expression of SF-1 during this early stage of gonadal development (Figures 1A and 1D). In these embryonic tissues, the mobilities of mono- and di-sumoylated SF-1 species were similar to those obtained in cellular studies, suggesting that in vivo, SF-1 is sumoylated at both the major (K194) and minor (K119) acceptor sites (Campbell et al., 2008; Chen et al., 2004; Komatsu et al., 2004; Lee et al., 2005; Luo et al., 2010; Ogawa et al., 2009)(Figures 1A and S1). Given that neither K119 nor K194 appears to be acetylated (Chen et al., 2005) or polyubiquitinated (Figure S1), and to ensure that all SF-1 sumoylation would be abolished in vivo, we chose to knock-in two point mutations, K119R/K194R (referred to as 2KR) in the endogenous Sf-1 mouse locus (Figures 1B and S1).
Figure 1. Sf-12KR/2KR mice exhibit disrupted endocrine physiology without affecting SF-1 protein levels.
A. Protein lysates from Sf-1+/+ E16.5 gonads were immunoprecipitated (IP) and western blotted (WB) using anti-SF-1 antibodies. Mono- (1xSu-SF-1) and di- (2xSu-SF-1) sumoylated SF-1 are indicated (arrowheads) with a lighter exposure of unmodified SF-1 shown in bottom panel. B. The targeted allele of unsumoylatable SF-1 mutant mouse (Sf-12KR/2KR) with individual mutations shown in Exon 3, and before removal of Neo cassette (Neo). C. Sumoylation of SF-1 is abolished in Sf-12KR/2KR mice. SF-1 levels are shown for postnatal day (P) 3 wild type and mutant tissues with βactin as loading control. D. Western blots of E16.5 ovaries and testes (left panels), and qPCR of E16.5 testes and adrenals (right panels) showed no difference in SF-1 protein and transcript levels, respectively. E. Immunostaining of SF-1 in wild type and mutant E16.5 adrenals. F. Testicular/adrenal weights and sperm counts are shown for 8 or 12 weeks old Sf-12KR/2KR males, respectively, n=7 for Sf-1+/+ and n=6 for Sf-12KR/2KR. G. Gonadal testosterone (T) levels and adrenal corticosterone (Cort) levels measured in P23 male wild type and Sf-12KR/2KR littermates. All data points in steroid assays passed the Grubbs’ outliers test. See also Figure S1, S2 and Table S1.
Sf-12KR/2KR mice were generated with both K119/K194 mutations confirmed by PCR genotyping and genomic DNA sequencing (Figure S1 and data not shown). Unlike SF-1 null mice, which die shortly after birth, Sf-12KR/2KR mice are viable and show normal Mendelian ratios (data not shown). We chose to focus initially on Sf-12KR/2KR adrenals and testes given the established roles and prominent embryonic expression of SF-1 in these two endocrine organs. If the Sf-12KR allele results in a simple gain-of-function then we would expect mutant mice to exhibit elevated Sf-1 transcripts (due to autoregulation) and adrenal hyperplasia (Doghman et al., 2007; Zubair et al., 2009). Although Sf-12KR/2KR tissues showed a pronounced loss of sumoylated SF-1 species, levels of mutant SF-1 transcripts and protein were unchanged when examined at multiple developmental stages (Figures 1C, 1D and S1). Importantly, a similar nuclear pattern of staining was observed for SF-1 in mutant and wild type adrenals (Figures 1E and S1). These data demonstrate that mutating the major and minor acceptor lysines at K119 and K194 effectively eliminated all detectable sumoylation of SF-1 in vivo, while preserving normal SF-1 protein levels and its subcellular localization.
Despite normal levels of SF-1 protein, Sf-12KR/2KR adult males showed a dramatic reduction in testicular weight, degenerated seminiferous tubules, lowered sperm count, and increased interstitial Leydig cells (Figures 1F and S2) with all mutant mice of both sexes eventually exhibiting infertility (Table S1). Sf-12KR/2KR adult adrenals revealed a significant, but subtle decrease in weight as well (Figure 1F). Morphological differences exhibited by mutant organs were accompanied by abnormal hormonal output, as evidenced by elevated testicular testosterone and progesterone levels and a slightly lower but still significant change in adrenal corticosterone (Figures 1G and S2). These altered steroid profiles together with gross changes in Sf-12KR/2KR organ morphology show that eliminating sumoylation of a specific substrate dramatically perturbs tissue organization and function.
Cell-fate specification is perturbed in Sf-12KR/2KR endocrine organs
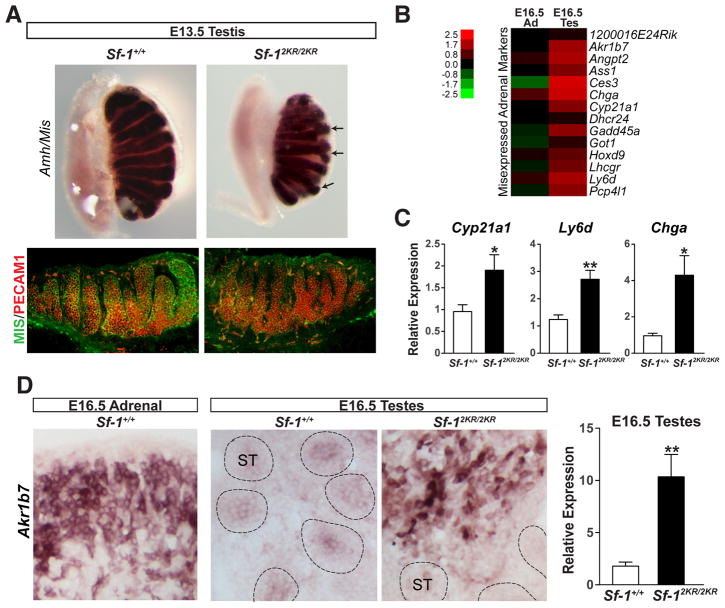
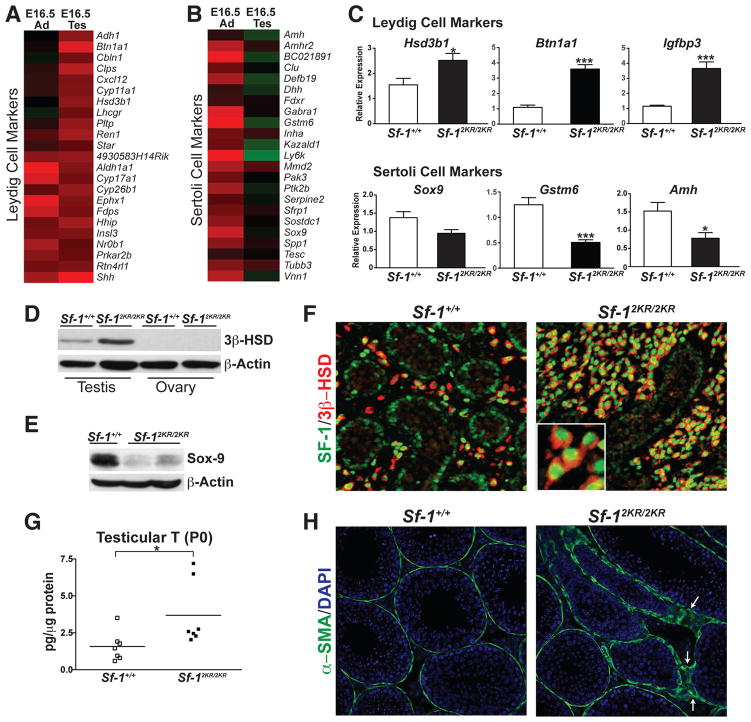
In the developing testes, SF-1 is expressed in both the Sertoli and Leydig somatic cells where it regulates genes involved in male sexual differentiation such as Mis/Amh, Sox9 and those encoding steroidogenic enzymes (Parker and Schimmer, 1997; Sekido and Lovell-Badge, 2008; Shen et al., 1994). Morphological changes in Sf-12KR/2KR testes were apparent as early as E13.5 as judged by irregularly branched, thin cords (Figure 2A). Despite the marked loss of germ cells in older testes, germ cell colonization at this early gonadal stage appeared normal with an equivalent number of germ cells found in wild type and mutant E13.5 testes (Figure 2A). To assess the molecular changes in Sf-12KR/2KR endocrine organs, RNA profiling of mutant testes and adrenals was carried out at different developmental stages (Table S2). Surprisingly, several established adrenal markers were misexpressed in Sf-12KR/2KR testes as illustrated by the prominent expression of Akr1b7 in the interstitial region of the embryonic testes (Figures 2B and 2D). Other adrenal markers found in mutant testes included Ly6d, Chga and Cyp21a1 (Figure 2C).
Figure 2. Misexpression of adrenal markers in Sf-12KR/2KR testes.
A. Wholemount in situ hybridization of Amh/Mis and immunofluorescent staining of MIS (green) and PECAM1 (red) in Sf-1+/+ and Sf-12KR/2KR E13.5 testes showing aberrant testes cords branching without an apparent loss of germ cells. B. Heat map showing upregulated adrenal markers in E16.5 mutant testes compared to wild type, with color scale bar shown. C. Increased expression levels of selected adrenal markers in E16.5 mutant testes. D. In situ hybridization and transcript levels (right panel) of the adrenal marker Akr1b7 in E16.5 mutant and wild type testes. ST, seminiferous tubules. See also Table S2.
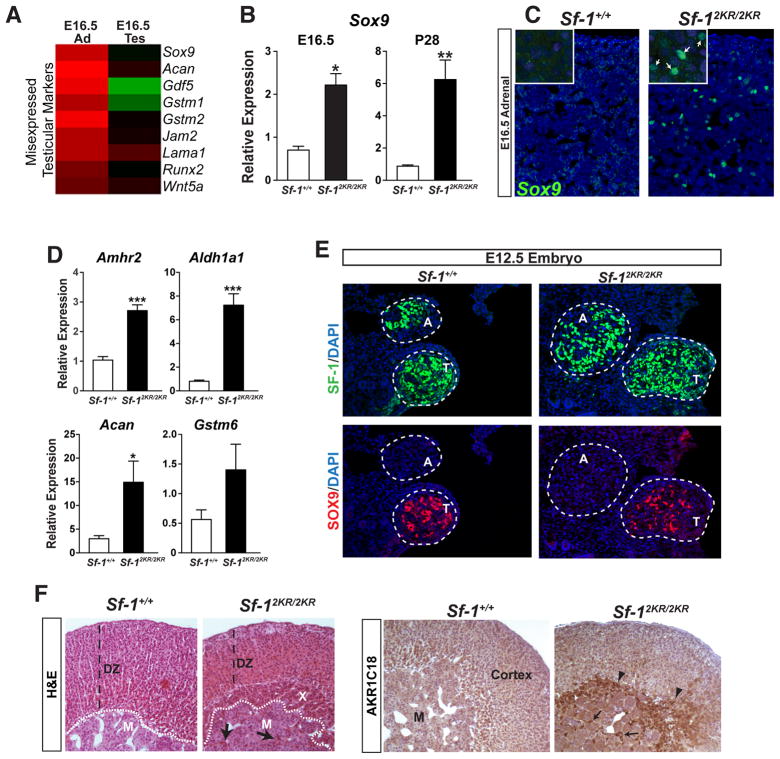
During development SF-1 is essential for adrenal maturation and is known to regulate multiple genes in the adrenal cortex. Similar to mutant embryonic testes, E16.5 Sf-12KR/2KR adrenals misexpress a number of testicular markers (Figure 3A). Perhaps the most unexpected embryonic marker to be misexpressed in mutant adrenals is the well-characterized Sertoli cell marker Sox9, which is never found in developing or adult adrenals (Figures 3A and B, and (Reiprich et al., 2008)). Sox9 misexpression is observed at E16.5 and continues into adulthood with SOX9-positive cells found scattered throughout the adrenal cortex of mutant mice (Figures 3B and 3C). Interestingly, while both male and female mutant adrenals misexpressed Sox9, no Sox9 could be detected in embryonic ovaries (data not shown). Other misexpressed markers included Amhr2, Aldh1a1, and two known targets of SOX9, Acan and Gstm6 (Figure 3D). No ectopic SOX9 expression was observed at an earlier stage of adrenal development (E12.5), just after separation of adrenals from the common adrenogonadal primordia (Figure 3E). Thus, misexpression of Sox9 at E16.5 appears to be ectopic in nature and is unlikely to result from a migratory defect of testicular SOX9-positive cells into the developing adrenal.
Figure 3. Misexpression of testicular markers such as SOX9, and retention of the fetal X-zone in Sf-12KR/2KR adrenals.
A. Heat map showing upregulated testicular markers in E16.5 mutant adrenals compared to wild type. B. Elevated expression of the testis-specific marker Sox9 in E16.5 and P28 mutant male adrenals. C. SOX9 staining in E16.5 male adrenals (green) in wild type or mutant adrenals (higher magnification shown in inset). D. Expression of testicular markers and Sox9 target genes in E16.5 wild type and mutant adrenals. E. Transverse sections of E12.5 embryos were stained for SF-1 (green) to locate adrenals (A) and testes (T), as indicated by dashed circles. SOX9 (red) signal is absent in adrenals in both wild type and Sf-12KR/2KR embryos. F. Representative photomicrographs of H&E and immunohistochemical staining of 20α-HSD (AKR1C18) in 8-week old male adrenals show cortical hypoplasia and persistence of the fetal X-zone as indicated by AKR1C18 staining (right panel, arrowheads). X-zone infiltration into adrenal medulla is indicated with arrows. DZ, definitive zone; M, adrenal medulla; X, X-zone. See Table S2.
Mutant adrenals also exhibited a persistent fetal X-zone (Figure 3F). Aldo-keto reductase 1c18 (Akr1c18), which encodes the enzyme 20α-hydroxysteroid dehydrogenase (20α-HSD), marks the fetal adrenal zone (Hershkovitz et al., 2007) and is elevated in adult mutant postnatal males or virgin females (Figure 3F and data not shown). While the origin and exact function of the murine adrenal X-zone are still mysterious, this zone is considered a remnant of the fetal adrenal and regresses at puberty in males and during the first pregnancy in females (Beuschlein et al., 2002). That Sf-12KR/2KR mice retain this fetal tissue implies that eliminating SF-1 sumoylation interferes with the normal maturation of this endocrine tissue. Collectively, these data show that SF-1 must be sumoylated for proper adrenal and testicular cell-specification.
Increased SHH signaling and expansion of steroidogenic progenitors in mutant mice
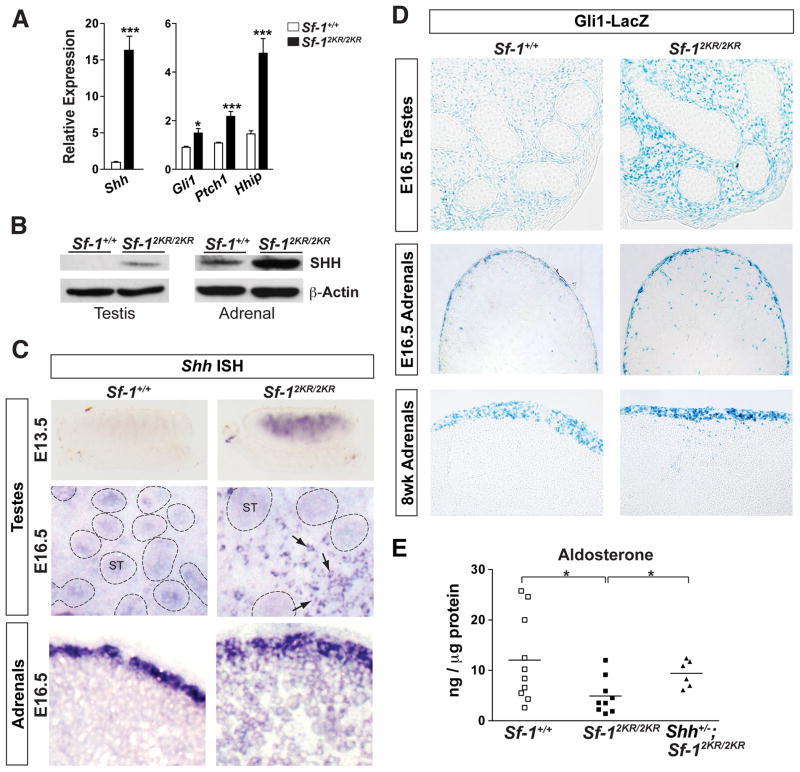
Hedgehog signaling is known to play a role in the recruitment of steroidogenic progenitors in both testes and adrenals. Testicular Sertoli cells secrete desert hedgehog (Dhh) and promote steroid-producing Leydig cell differentiation (Bitgood et al., 1996; Yao et al., 2002). In the adrenal cortex, expression of sonic hedgehog (Shh) rather than Dhh is needed to maintain steroidogenic progenitors and initiate steroidogenic cell differentiation (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). Unexpectedly, Shh, which is normally undetectable in testes, is switched on in embryonic Sf-12KR/2KR testes (Figures 4A, 4B and S3). Beginning at E13.5, strong Shh expression is observed in the interstitial compartment of Sf-12KR/2KR testes but not in wild type littermates (Figure 4C). In contrast, Dhh expression is decreased in mutant testes (Figure S3). Further analyses confirmed that Shh- and GLI1-positive cells were markedly elevated in E16.5 mutant testes (Figures 4C and 4D) along with downstream effectors of hedgehog signaling such as Gli1, Ptch1 and Hhip (Figure 4A).
Figure 4. SHH signaling is amplified in Sf-12KR/2KR adrenals and ectopically activated in Sf-12KR/2KR testes.
A. Expression of Shh and signaling targets in E16.5 testes. B. Increased SHH protein levels in E16.5 mutant endocrine tissues. C. Ectopic expression of Shh in embryonic mutant testes at E13.5 by wholemount ISH and at E16.5 by section ISH. Embryonic mutant adrenals also showed increased cortical Shh expression at E16.5 by section ISH. Arrows indicate Shh-positive interstitial cells with seminiferous tubules indicated (ST, dotted circles). D. GLI1-positive cells in Sf-12KR/2KR tissues were visualized by crossing Sf-12KR/2KR mice to the Gli1LacZ (Gli1tm2Alj) reporter line. X-gal staining revealed increased GLI1-positive cells in Sf-12KR/2KR E16.5 testes and an altered, more intense pattern of GLI1-positive cells in mutant E16.5 or 8 wks adrenals. E. Adrenal aldosterone measured from Sf-1+/+, Sf-12KR/2KR, and Shh+/−; Sf-12KR/2KR P23 males showed partial rescue of hormone deficits by lowering Shh gene dosage. See Figure S3.
Shh expression in embryonic adrenals is always restricted to the immediate subcapsular layer. However, in Sf-12KR/2KR adrenals this restricted pattern is expanded, penetrating deep into cortical layers (Figure 4C). More important, and consistent with an increase in SHH protein, hedgehog signaling is greatly enhanced in mutant adrenals as demonstrated by the notable expansion of GLI1-positive cells in the subcapsular layer and definitive zone (Figure 4D). In older adrenals, these scattered Gli1-positive cells persist and are distributed throughout the cortex (Figure 4D). Thus, in both mutant adrenals and testes, loss of SF-1 sumoylation either elevates or ectopically activates Shh expression to amplify hedgehog signaling. In this regard, the altered steroid profiles observed in mutant adrenals was partially restored by reducing Shh gene dosage in Sf-12KR/2KR animals (Figure 4E).
Eliminating SF-1 sumoylation in the embryonic testes resulted in gross morphological and transcriptional changes that reflected increased Leydig cell function (Figures 5A and 5C). This change was met with a corresponding decrease in Sertoli cell function, as judged by the loss of Sertoli cell markers, such as SOX9 (Figures 5B, 5C, 5E and S3 and (Abel et al., 2009)). Expansion of fully differentiated Leydig cells begins early in E16.5 mutant testes, as judged by the increased 3β-HSD expression and staining, as well as the significantly elevated levels of testosterone (Figures 5D, 5F and 5G). The presence of scattered SOX9-positive cells in the interstitial region and the aberrant staining pattern of the peritubular myoid cell marker, alpha smooth muscle actin suggested strongly that the integrity of the seminiferous tubules was severely compromised in mutant testes (Figures 5H, S3, and (Clark et al., 2000)). These data show that disrupting the normal SUMO cycle of SF-1 not only increased SHH signaling, but also impaired normal endocrine organ development by expanding or contracting specialized cell types.
Figure 5. Steroidogenic Leydig cells are expanded in Sf-12KR/2KR mutant testes.
Heat maps showing relative levels (fold over wild type) of Leydig cell markers (A) and Sertoli cell markers (B) in Sf-12KR/2KR mutant testes and adrenals. C. QPCR showed increased expression of Leydig cell markers with a corresponding decrease in Sertoli cell markers in E16.5 Sf-12KR/2KR mutant testes. Western blots of 3β-HSD (D), and SOX9 (E) in E16.5 gonads or testes with β– actin as loading control. F. SF-1 (green) and 3β-HSD (red, marking Leydig cells) staining in E16.5 testes with higher magnification showing nuclear SF-1 and cytoplasmic 3β-HSD staining in mutant interstitial Leydig cells (lower left panel). G. Testosterone levels in mutant and wild type P0 testes. H. Immunofluorescent staining of the peritubular myoid cell marker alpha smooth muscle actin (α-SMA) exhibited an abnormal staining pattern in 4-week old mutant testes (arrows). See Figure S3.
SF-1 unsumoylatable mutant protein directly regulates Shh expression
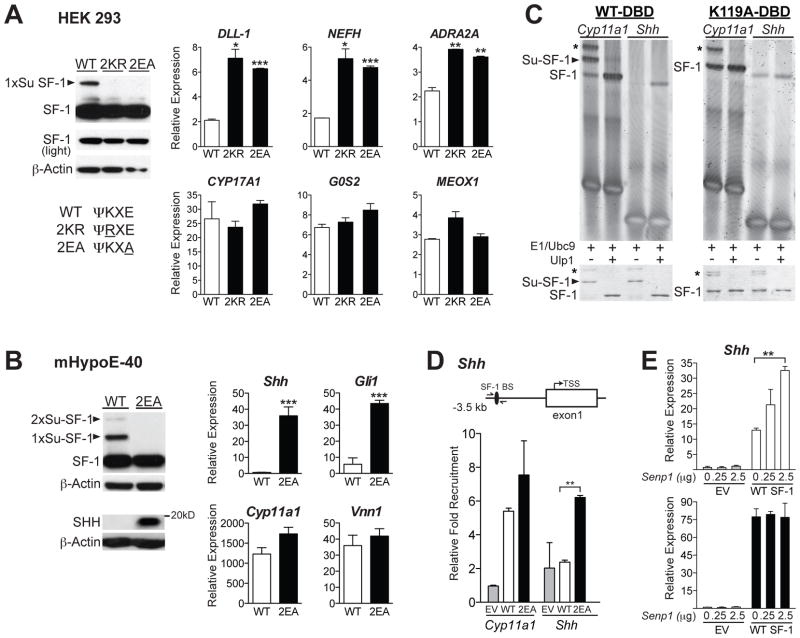
We next asked whether misexpression of SUMO-sensitive genes such as Shh observed in mutant mice is a direct consequence of loss of SF-1 sumoylation. Initially, inducible cell lines that express a panel of unsumoylatable SF-1 variants were generated in HEK 293 cells (Figures 6A and S4). In addition to the 2KR mutant, individual lysine mutants (K119R and K194R), as well as an SF-1 SUMO consensus site mutant E121A/E196A (2EA) were also included in this panel. By disrupting the UBC9 substrate recognition sequence, the E to A mutation prevents sumoylation but leaves the acceptor lysines available for alternative modifications, including acetylation and ubiquitination (Sampson et al., 2001). As expected, neither the 2KR nor 2EA SF-1 proteins are sumoylated (Figure 6A). In HEK 293 cells, we were able to directly compare the transcriptional effects of all SF-1 variants when expressed at equivalent protein levels. Profiling of these stable cell lines showed that direct SF-1 targets such as CYP17A1, G0S2 and MEOX1 (Whitby et al., 2011) are induced equally by wild type and all SF-1 SUMO mutants (Figures 6A and S4), with some well-established targets such as StAR and CYP11A1 trended slightly higher (data not shown) consistent with results from Hammer and colleagues (Yang et al., 2009). However, similar to what was found in Sf-12KR/2KR tissues, several distinct targets including DLL-1, NEFH and ADRA2A were induced by both the 2KR and 2EA variants, but not by wild type SF-1 (Figures 6A and S4). Interestingly, full activation of these SUMO-sensitive targets varied among different SUMO-mutants. For example, activation of some genes (DLL-1 and ADRA2A) was nearly equivalent for the single K194R and 2KR and 2EA double mutants. For other genes (NEFH), full activation was only observed with the double mutants (Figure S4). Although it remains to be determined how each mutant activates this subset of SUMO-sensitive targets, our data confirm that for most targets, the 2KR and 2EA SF-1 variants are functionally similar.
Figure 6. Unsumoylatable SF-1 directly regulates a subset of SUMO-sensitive target genes.
A. Levels of unmodified and sumoylated (arrowheads) 3x-FLAG tagged WT and unsumoylatable 2EA and 2KR SF-1 variants in HEK293 cells after 24 hrs Tetracycline (Tet) induction. SUMO consensus sequence and mutations (underlined) are listed. Graphs represent relative expression of the SUMO-sensitive targets (DLL-1, NEFH and ADRA2A, top), and SUMO-insensitive targets (CYP17A1, G0S2 and MEOX1, bottom) following 24 hrs Tet-induced SF-1 expression. Bars indicate fold over vehicle control (+ SD). B. Protein in mHypoE-40 stable cells was detected with anti-FLAG, anti-SHH or anti-β-Actin antibodies. Relative expression of Shh and SHH target Gli1 (top) was increased after induction of 2EA SF-1 proteins compared to equivalent induction of Cyp11A1 and Vnn1. C. EMSAs of unsumoylated SF-1 binding to either a non-canonical site found in the Shh promoter or to the canonical Cyp11a1 site (left panel). Input of in vitro sumoylated SF-1 DBD protein for each reaction is shown below EMSAs. In the absence of the major K119 acceptor lysine, one cryptic sumoylation of SF-1 is observed at either K100 or K106, as indicated by the *. D. ChIP-qPCR results showing relative fold recruitment with all values normalized to empty vector (EV) control in mHypoE-40 stable cell lines; forward and reverse primers are provided in Supplemental Information. E. Relative levels of Shh expression after overexpressing Senp1 plasmid in mHypoE-40 cells stably expressing WT SF-1 (upper panel) or 2EA (lower panel), with each compared to the EV control. Experiments have been performed at least three times. See also Figure S4.
To determine if the 2KR and 2EA variants directly activate SHH signaling, as observed in Sf-12KR/2KR mice, we used an embryonic cell line (mHypoE-40) that is highly responsive to exogenous SF-1. Stable mHypoE-40 cell lines were generated with equal WT or 2EA protein levels (Figure 6B); 2KR lines were also created but consistently expressed far less protein (Figure S4). As observed with Sf-12KR/2KR, eliminating sumoylation in the 2EA (and K119R) variant increased Shh and activated SHH signaling as judged by elevated SHH protein and an increase in the hedgehog target gene Gli1 (Figures 6B and S4). Shh was also induced in the 2KR SF-1 mHypoE-40 cells despite reduced SF-1 protein levels (Figure S4). In contrast, well-characterized SF-1 targets Vnn1 and Cyp11a1 were largely unaffected by the sumoylation status of SF-1 (Figures 6B and S4). These cellular data support our hypothesis that loss of SF-1 sumoylation in Sf-12KR/2KR tissues leads to inappropriate activation of SUMO-sensitive genes in select cell types.
Previously we reported that when the minor site at K119 is sumoylated, recognition by SF-1-DBD of nonconsensus binding sites is diminished (Campbell et al., 2008). Given that Shh is also induced in K119R stable mHypoE-40 cells (Figure S4), we asked if SF-1 binding to Shh upstream genomic regions correlated with the sumoylation status of SF-1. In vitro sumoylated SF-1 protein containing the DBD and hinge region that includes K119 but not K194 (SF-1-DBD), binds poorly to a putative SF-1 nonconsensus binding site in the Shh regulatory region, but binds well to the high affinity Cyp11a1 canonical site (Figure 6C and (Campbell et al., 2008)). When sumoylation is eliminated at K119, binding to the Shh site is restored (Figure 6C, K119A DBD, far right lanes). Consistent with the gel shift data, only the SF-1 2EA mutant protein is recruited to the non-canonical site upstream of the Shh gene in a ChIP-qPCR assay (Figures 6D and S4). Unfortunately, the much lower expression of the SF-1 2KR mutant in stable mHypoE-40 cells precluded a similar ChIP analysis (Figure S4). Overexpressing the SUMO-isopeptidase Senp1 in mHypoE-40 cells provides further proof that unsumoylatable SF-1 selectively activates Shh. Indeed, a Senp1 dose-dependent increase in endogenous Shh was observed with wild type SF-1 but not with SF-1 SUMO mutant cells including 2KR, 2EA and K119R (Figures 6E and S4). We infer from these data that sumoylation modulates DNA binding specificity of SF-1 and that SUMO-sensitive targets such as Shh, are activated once SF-1 is desumoylated.
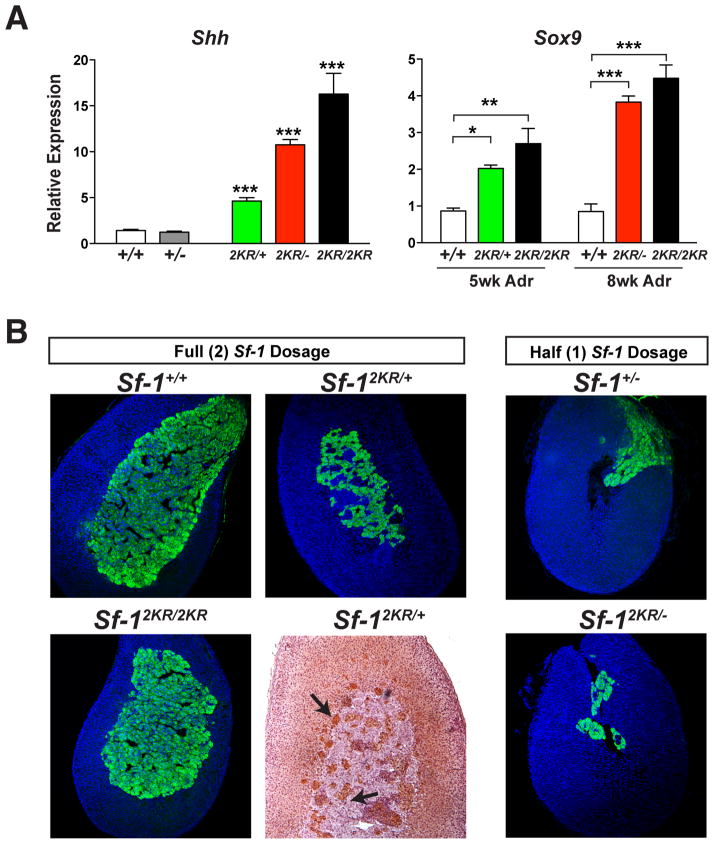
The Sf-12KR unsumoylatable allele is dominant
Ectopic expression of Shh, Sox9 and AKR1C18 in mutant endocrine organs was used to determine if the Sf-12KR allele is dominant or recessive. In all cases, we found a clear and marked increase in the expression of these SUMO-sensitive markers in Sf-12KR/+ heterozygous tissues. Both Shh and Sox9 are ectopically activated in Sf-12KR/+ heterozygous and Sf-12KR/− hemizygous testes or adrenals, respectively (Figure 7A). For Shh, the presence of one wild type allele in Sf-12KR/+ testes resulted in less ectopic activation compared to hemizygous Sf-12KR/− testes suggesting that the sumoylatable allele can partially oppose the dominant effects of the Sf-12KR mutant allele. The presence of 20α-HSD (AKR1C18)-positive cells infiltrating the adrenal medulla and the persistent fetal X-zone in Sf-12KR/+ heterozygous adrenals also illustrates the dominance of the Sf-12KR allele (Figure 7B). Pronounced differences in adrenal morphology were also observed between Sf-1+/− and Sf-12KR/− genotypes, underscoring the functional differences between the wild type and unsumoylatable alleles (Figure 7B). These genetic data provide further proof that the Sf-12KR allele is functionally distinct from the wild type allele and dominates to potently activate SUMO-sensitive genes such as Shh.
Figure 7. Unsumoylatable SF-1 prevails over the wild type SF-1 and ectopically activates SUMO-sensitive genes in vivo.
A. Shh and Sox9 transcripts levels are shown for E16.5 testes and adult male adrenals, respectively. Statistical significance was determined by one-way ANOVA. B. Comparison of adult adrenals obtained from different allelic combinations as indicated, by either tyrosine hydroxylase staining of adrenal medulla (green), or AKR1C18/20α-HSD staining, which marks cells of the fetal X-zone (brown, black arrows). The eccentric smaller medulla observed in heterozygous Sf-1+/− adrenal is further exaggerated in Sf-12KR/− adrenals.
Discussion
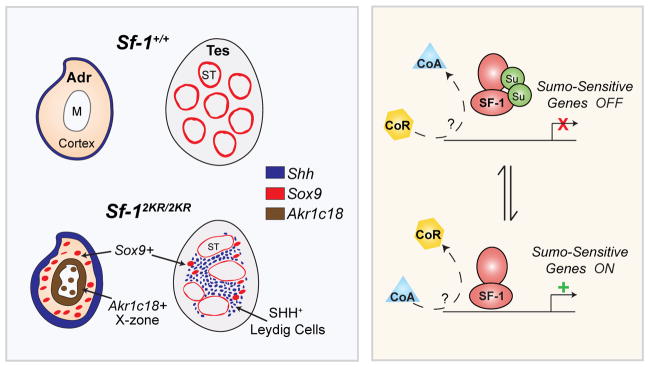
Here, we report that loss of substrate-specific sumoylation in a multi-cellular organism causes profound changes in cell fate specification, organogenesis, and normal physiology. The ability to permanently alter the endogenous ratio of sumoylated to unmodified SF-1 protein by knocking-in the mutant Sf-12KR allele allowed us to clarify the full in vivo function of substrate-specific sumoylation. Unlike other studies aimed at disrupting the sumoylation cycle (Cheng et al., 2007; Kang et al., 2010; Nacerddine et al., 2005), this report shows that establishing and maintaining SUMO modification on a specific substrate is critical for orchestrating complex gene expression patterns. Specifically, we find that mice harboring only the non-sumoylatable variant of the nuclear receptor SF-1 exhibit marked changes in endocrine tissue development and function. While most cellular data suggest that sumoylation simply represses or shuts off transcriptional activity, our in vivo data show that the sumoylation cycle of a substrate functions instead to ensure appropriate gene expression during development. Thus, rather than simply activating all SF-1 targets, a distinct subset of SF-1 targets was activated in mutant Sf-12KR/2KR mice, which correlated well with the cellular and morphological changes observed in mutant endocrine organs, as depicted in Figure 8.
Figure 8. Sumoylation of SF-1 is critical for normal endocrine tissue development.
A schematic summarizing our results showing that shifting the normal ratio of sumoylated to unsumoylated SF-1 to only unmodified SF-1 (left bottom panel) in Sf-12KR/2KR mice interferes with normal endocrine tissue development and results in the ectopic expression of SUMO-sensitive SF-1 target genes, such as Shh (dark blue), Sox9 (red), and Akr1c18, which marks the fetal X-zone (brown ring in adrenal). We hypothesize that the correct ratio of sumoylated to unmodified substrate is needed to selectively turn on/off developmental gene programs (right panel). Transcriptional co-activators (CoA) and/or co-repressors (CoR) might also be involved in this process.
Our data suggest that disrupting SF-1 sumoylation affects expression of endocrine organ-specific markers as evidenced by expression of classic adrenal markers in mutant testes (Akr1b7 and Cyp21a1) and the surprising expression of the testicular marker Sox9 and its downstream targets (Acan, Gstm6, Gdf5, and Aldh1a1) in mutant adrenals. The appearance of inappropriate markers in mutant tissues might reflect an under-differentiated cell population possessing intermediate steroidogenic cell fates as shown by others (Bernichtein et al., 2008; O’Shaughnessy et al., 2003; Val et al., 2006). However, we find no evidence for subpopulations of interstitial cells in mutant testes and have yet to identify a marker that colocalizes with SOX9 in mutant adrenals. The dramatic changes in cell fate markers observed in Sf-12KR/2KR mice are similar to transcriptional changes observed after overexpressing either unmodified NR2E3 in embryonic retinal tissue or a Sox9-SUMO fusion in Xenopus embryos (Onishi et al., 2009; Taylor and Labonne, 2005). Together with our knock-in study, we conclude that sumoylation provides additional regulatory capacity and greatly enhances the functional diversity of transcriptional factors to orchestrate proper gene expression (Gill, 2005b).
We find that ectopic or expanded SHH signaling correlates well with the increase in interstitial Leydig-like cells, elevated steroid hormone production, and disturbed adrenal zonation in mutant mice. These data are consistent with known roles of hedgehog signaling in promoting gonadal steroidogenesis (Barsoum et al., 2009; Bitgood et al., 1996) and in maintaining steroidogenic lineages in mouse adrenals (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). In Sf-12KR/2KR embryonic adrenals, Shh- and GLI1-positive cells increase and expand well beyond the subcapsular region. Similar changes are observed for Cyp17a1-positive cells (data not shown), suggesting that SF-1 sumoylation normally restricts expression of both Shh and Cyp17a1 to the immediate subcapsular zone. Paradoxically, older mutant adrenals eventually shrink with age and exhibit reduced Shh (data not shown) and a noticeably thinner subcapsular layer of GLI1-positive cells. This apparent reduction in adrenal stem cells is consistent with increased cortical thinning and reduced adrenal weights in older mice. We propose that the early expansion of Shh-positive adrenal progenitors in Sf-12KR/2KR mice might ultimately disrupt steroidogenic cell differentiation by recruiting progenitors prematurely or interfering with the stem cell niche. In support of this hypothesis, reducing Shh gene dosage in Sf-12KR/2KR animals partially restored the hormone deficit observed in mutant adrenals. Still unknown is how changes in pituitary and/or hypothalamic endocrine signaling impinge on adrenal physiology in Sf-12KR/2KR mice, and how other targets activated by unsumoylatable SF-1 contribute to mutant phenotypes.
Our study provides compelling evidence that unmodified and sumoylated substrates function differently, with both variants presumably needed to regulate the full spectrum of SF-1 dosage-sensitive and SUMO-sensitive targets. The pronounced differences observed between Sf-1+/− and Sf-12KR/− genotypes in adrenal morphology and testicular Shh expression establish that the wild type and 2KR alleles are distinct. Moreover, the Sf-12KR allele is clearly dominant over the wild type allele as evidenced by elevated Shh and Sox9 in Sf-12KR/+ tissues. We therefore infer that the correct ratio of sumoylated to unmodified SF-1 must be maintained for proper development. Additionally, disturbing this ratio for SF-1 or other receptors sharing similar DNA recognition, such as LRH-1 could inappropriately activate SHH signaling; this scenario might be particularly relevant to certain types of cancer such as pancreatic adenocarcinomas (Morton et al., 2007; Petersen et al., 2010).
The unexpected ectopic induction of SHH signaling in Sf-12KR/2KR embryonic endocrine tissues and in a cellular model system strongly supports the idea that Shh is extremely sensitive to the sumoylation status of SF-1. While sumoylation appears to be the predominant modification on K119 and K194, it is possible that mutating these lysines disrupts other modifications and contributes to phenotypes observed in Sf-12KR/2KR mice. In this regard, although mHypoE-40 cellular data suggest that 2KR behaves slightly differently than the 2EA variant (which blocks UBC9 recruitment), both variants also activated Shh in these cells and appeared to be virtually identical in HEK 293 cells. Further examination of these two unsumoylatable variants in different cellular contexts should help resolve their subtle functional differences noted in our cellular studies, and might also reveal sumoylation-independent roles for UBC9 as recently described (Suda et al., 2011).
Profiling endocrine tissues and cells expressing only unsumoylatable SF-1 identified scores of SUMO-sensitive targets. The precise mechanisms underlying the transcriptional and phenotypic changes in mutant mice and cells remain to be determined. Unsumoylatable SF-1 might displace transcriptional repressors sharing the same DNA binding specificity, or instead alter recruitment of corepressors such as ARIP4 (Ogawa et al., 2009), or transcriptional activators. In this regard, no changes in Co-REST or NCoR recruitment on SUMO-sensitive promoters were identified by ChIP analyses (data not shown). Ligand-dependent sumoylation of other nuclear receptors such as PPARγ, LXR and LRH-1 is proposed to maintain a repressor complex and inhibit gene expression in the inflammatory and hepatic acute phase responses (Ghisletti et al., 2007; Pascual et al., 2005; Venteclef et al., 2010). This transrepression model explains nicely how an exceedingly small amount of sumoylated receptor exerts a potent dominant effect. However, for most SUMO-sensitive targets identified here, the unsumoylatable Sf-12KR allele is dominant. In other words, the unsumoylatable SF-1 allele prevails even in the presence of wild type SF-1. Moreover, in mHypoE-40 cells, Shh is induced strongly only after expressing the mutant SF-1 variants or with wild type SF-1 in combination with SENP1. Thus, in addition to SUMO-dependent transrepression, or derepression as recently reported in Sp3 sumoylation deficient MEFs (Stielow et al., 2010), our data suggest that during developmental stages substrate desumoylation is also used to activate SUMO-sensitive genes. Future comprehensive genomic analysis of these highly SUMO-sensitive genes may reveal a common motif or cofactor that functions similar to the proposed synergy control model (Iniguez-Lluhi and Pearce, 2000).
The fact that SF-1 is efficiently sumoylated in vitro, but appears to be inefficiently sumoylated in endocrine tissues or cells (this study and (Lee et al., 2005)) underscores the perplexing discrepancy between the ability to detect sumoylated substrates and the functional importance of general sumoylation. This so-called “SUMO enigma” (Hay, 2005) might simply reflect a highly dynamic cycle of modification coupled with suboptimal methods for detecting sumoylation. In this regard we find that the slightest manipulation of endocrine tissues completely abrogates detection of sumoylated-SF-1. On the other hand, and as previously proposed, sumoylation might be transient but impose a permanent imprinted functional mark on a substrate, such as SF-1, or on chromatin components (Shiio and Eisenman, 2003) that regulate expression of SUMO-sensitive genes. Despite this lingering dilemma, we demonstrate that SUMO modification of transcription factors such as SF-1 is important in vivo and provides an essential, added layer of regulation for fine-tuning temporal and spatial gene expression in mammalian organ development.
Experimental Procedures
Generation of 2KR Knock-In Mice
The mouse Sf-1 (Nr5a1) BAC clone 21761 (Genome Systems, Inc.) was used to create the targeting construct, as shown in Figure S1. Site-directed mutagenesis was carried out to create the K119R/K194R mutations and introduce XhoI and BglII sites into the PCR amplified fragment corresponding to Chr2: 38,566,194-38,560,555 (based on NCBI m37 mouse assembly) containing exon 3. The full targeting construct was sequenced and contained a floxed IRES-neo cassette inserted into intron 3 and the diphtheria toxin A (DT-A) cassette inserted into the 3′ targeting vector arm. ES cell targeting and generation of chimera mice were performed by the Transgenic Animal Model Core at U. of Michigan. Southern blotting confirmed the presence of the XhoI and BglII sites in one clone out of 500 screened. Routine genotyping of mutant animals was carried out using primers IE3 Fwd: 5′ CACAGCTGTGCGTGCTGATC 3′ and IE3 Rev: 5′ GGTACCCACCTCCAGCTCCTT 3′ to amplify exon 3 followed by restriction digestion using either XhoI or BglII. Mice were kept on a 12 h light/dark cycle. Heterozygous SHH mutant mice (Shhtm1Amc) were bred with Sf-12KR/+ heterozygous mice to generate Sf-12KR/2KR; Shh+/− mice. All research with animals was performed according to guidelines approved by the UCSF IACUC.
Cell Lines and Transfections
Murine immortalized hypothalamic mHypoE-40 cells were generated from E17 mouse hypothalami obtained from BALB/c females and DC1 males (Charles River Laboratories) and dissected individually, dispersed through trituration, and plated as primary culture. Cultures were immortalized with recombinant murine retrovirus harboring simian virus (SV40) T-antigen and the neomycin resistance gene from the pZIP-Neo SV(X)1 vector, as previously described (Mayer et al., 2009). Immortalized neurons were selected by geneticin (G418). The clonal mHypoE-40 cell line was analyzed for the expression of specific markers by semi-quantitative reverse transcriptase PCR (see http://www.cellutionsbiosystems.com/). The Flp-In T-REx system was introduced into mHypoE-40 cells cultured in DMEM (Invitrogen), 10% Tetracycline-screened FBS (Hyclone), 1% pen-strep and selected with 250 ug/mL Zeocin and 5ug/mL Blasticidin (Invitrogen). Stable cell lines expressing 3xFLAG tagged WT, 2EA, 2KR, K119R, K194R, and empty vector (pcDNA5-frt/TO) were created in the Flp-In T-REx HEK 293 and mHypoE-40 cell lines and selected with 100 or 150ug/mL Hygromycin, respectively (Campbell et al., 2008). For the 2EA mutant Glu121 and Glu196 were changed to Ala by PCR mutagenesis (Stratagene). mHypoE-40 empty vector or 3xFLAG-WT SF-1 stable cells were transfected with pCI-Neo or pCI-Neo-mSENP1 using TransIT-2020 reagent (Mirus). Six hours after transfection cells were treated with tetracycline (Teknova), and total RNAs were harvested 72 hours post-transfection.
Microarrays
Mouse Exonic Evidence Based Open-source (MEEBO) arrays were printed at the Center for Advanced Technology (CAT) at University of California, San Francisco (UCSF). Hybridizations were carried out as described (Kurrasch et al., 2007) using total RNA isolated from E16.5 adrenal (8.5 μg), E16.5 testes (10 μg) and P28 adrenal or testes (10 μg). All hybridizations were performed at 65°C for 16 hr using MAUI® hybridization chamber mixers with the MAUI® hybridization systems (Biomicro Systems, Inc.). Arrays were scanned using an Axon Scanner 4000B and data were analyzed by Genepix 6.0 software (Molecular Devices). Heat maps were generated using Cluster 3 and Treeview (http://bonsai.hgc.jp/~mdehoon/software/cluster). The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE30078 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30078).
SF-1 In Vitro Sumoylation and Electrophoretic Mobility Shift Assays
In vitro sumoylation of SF-1 DBD fragment were described previously (Campbell et al., 2008) and carried out in 50 μL reactions with 1 μM SF-1-DBD, 0.12 μM E1, 4 μM UBC9, and 10 μM SUMO-1 in buffer containing 50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 10 mM MgCl2, 2 mM ATP, and 2 μM DTT at 37° for 1.5 hours. Electrophoretic mobility shift assays (EMSAs) were carried out with sumoylated SF-1 DBD, 2 mM DTT, 10 μM double-stranded oligonucleotides, with or without 3 units of Ulp1 protease (Lifesensors) and incubated at room temperature for 30 min. Each reaction was then resolved by 7.5% SDS-PAGE and by 6% native PAGE in 1× TBE buffer at 4°C. DNA-protein complexes were detected using the fluorescence-based EMSA kit (Molecular Probes) and a Typhoon scanner. EMSA primers are as follows: hCYP11A1 FWD 5′-ACATTTTATCAGCTTCTGGTATGGCCTTGAGCTGGTAGTTATAATCTTGGC-3′, hCYP11A1REV 5′-GCCAAGATTATAACTACCAGCTCAAGGCCATACCAGAAGCT-GATAAAATGT-3′, mShh BS1 FWD, 5′-ACGCCATCTAGCGGCAGGGCCAGAA-GGGGGAAG-3′, mShh BS1 REV, 5′-CTTCCCCCTTCTGGCCCTGCCGCTAGATGGCGT-3′.
Statistical Analysis
Data are represented as mean + SEM (or SD, as indicated), * = p < 0.05; ** = p < 0.005; *** = p < 0.001. Statistical analyses were performed using Prism 5 (Graphpad) software. Unless otherwise indicated, statistical significance was determined by Student’s t-test.
Supplementary Material
Acknowledgments
We thank members of the Ingraham lab and Dr. D. Julius for helpful suggestions and discussion and are also grateful for the initial contributions by Drs. K. Hata (Osaka University) and M. Show. We thank Drs. J. Reiter, G. Martin and S. Pleasure for experimental reagents and mouse strains and Drs. J. Weinstein, M. Wegner, W. Miller, T. Tachibana, and L. Reichardt for their generous gift of antibodies. This research was funded by T32 DK07418 to F.Y.L., Ruth L. Kirschstein NRSA-NIDDK fellowships to F.Y.L. and E.J.F., and by an NIH-NIDDK-RO1 DK070024 to H.A.I.
Footnotes
See Supplemental Information for additional experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel MH, Baban D, Lee S, Charlton HM, O’Shaughnessy PJ. Effects of FSH on testicular mRNA transcript levels in the hypogonadal mouse. J Mol Endocrinol. 2009;42:291–303. doi: 10.1677/JME-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apionishev S, Malhotra D, Raghavachari S, Tanda S, Rasooly RS. The Drosophila UBC9 homologue lesswright mediates the disjunction of homologues in meiosis I. Genes Cells. 2001;6:215–224. doi: 10.1046/j.1365-2443.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol. 2009;329:96–103. doi: 10.1016/j.ydbio.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashamboo A, Ferraz-de-Souza B, Lourenco D, Lin L, Sebire NJ, Montjean D, Bignon-Topalovic J, Mandelbaum J, Siffroi JP, Christin-Maitre S, et al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010;87:505–512. doi: 10.1016/j.ajhg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernichtein S, Alevizaki M, Huhtaniemi I. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol Metab. 2008;19:231–238. doi: 10.1016/j.tem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Beuschlein F, Keegan CE, Bavers DL, Mutch C, Hutz JE, Shah S, Ulrich-Lai YM, Engeland WC, Jeffs B, Jameson JL, et al. SF-1, DAX-1, and acd: molecular determinants of adrenocortical growth and steroidogenesis. Endocr Res. 2002;28:597–607. doi: 10.1081/erc-120016972. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Broday L, Kolotuev I, Didier C, Bhoumik A, Gupta BP, Sternberg PW, Podbilewicz B, Ronai Z. The small ubiquitin-like modifier (SUMO) is required for gonadal and uterine-vulval morphogenesis in Caenorhabditis elegans. Genes Dev. 2004;18:2380–2391. doi: 10.1101/gad.1227104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Faivre EJ, Show MD, Ingraham JG, Flinders J, Gross JD, Ingraham HA. Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1) Mol Cell Biol. 2008;28:7476–7486. doi: 10.1128/MCB.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A, Talianidis I. SUMO-dependent compartmentalization in promyelocytic leukemia protein nuclear bodies prevents the access of LRH-1 to chromatin. Mol Cell Biol. 2005;25:5095–5105. doi: 10.1128/MCB.25.12.5095-5105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Juan LJ, Chung BC. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol Cell Biol. 2005;25:10442–10453. doi: 10.1128/MCB.25.23.10442-10453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1) J Biol Chem. 2004;279:38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47:628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. Something about SUMO inhibits transcription. Curr Opin Genet Dev. 2005a;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO changes Sox for developmental diversity. Mol Cell. 2005b;20:495–496. doi: 10.1016/j.molcel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6:167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151:1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez-Lluhi JA, Pearce D. A common motif within the negative regulatory regions of multiple factors inhibits their transcriptional synergy. Mol Cell Biol. 2000;20:6040–6050. doi: 10.1128/mcb.20.16.6040-6050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Qi Y, Zuo Y, Wang Q, Zou Y, Schwartz RJ, Cheng J, Yeh ET. SUMO-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol Cell. 2010;38:191–201. doi: 10.1016/j.molcel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, et al. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol. 2004;18:2451–2462. doi: 10.1210/me.2004-0173. [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol. 2005;25:1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Ahn BY, Chung SS, Park HS, Cho BJ, Kim M, Choi SH, Lee IK, Lee SW, Choi SJ, et al. Effect of a peroxisome proliferator-activated receptor gamma sumoylation mutant on neointimal formation after balloon injury in rats. Atherosclerosis. 2009;206:411–417. doi: 10.1016/j.atherosclerosis.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Lourenco D, Brauner R, Lin L, De Perdigo A, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, Maciel-Guerra AT, Achermann JC, et al. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360:1200–1210. doi: 10.1056/NEJMoa0806228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Luo Z, Wijeweera A, Oh Y, Liou YC, Melamed P. Pin1 facilitates the phosphorylation-dependent ubiquitination of SF-1 to regulate gonadotropin beta-subunit gene transcription. Mol Cell Biol. 2010;30:745–763. doi: 10.1128/MCB.00807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CM, Fick LJ, Gingerich S, Belsham DD. Hypothalamic cell lines to investigate neuroendocrine control mechanisms. Front Neuroendocrinol. 2009;30:405–423. doi: 10.1016/j.yfrne.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M, Lewis BC. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Nowak M, Hammerschmidt M. Ubc9 regulates mitosis and cell survival during zebrafish development. Mol Biol Cell. 2006;17:5324–5336. doi: 10.1091/mbc.E06-05-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Fleming LM, Jackson G, Hochgeschwender U, Reed P, Baker PJ. Adrenocorticotropic hormone directly stimulates testosterone production by the fetal and neonatal mouse testis. Endocrinology. 2003;144:3279–3284. doi: 10.1210/en.2003-0277. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Komatsu T, Hiraoka Y, Morohashi K. Transcriptional Suppression by Transient Recruitment of ARIP4 to Sumoylated nuclear receptor Ad4BP/SF-1. Mol Biol Cell. 2009;20:4235–4245. doi: 10.1091/mbc.E08-12-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi A, Peng GH, Hsu C, Alexis U, Chen S, Blackshaw S. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron. 2009;61:234–246. doi: 10.1016/j.neuron.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18:361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen GM, Amundadottir L, Fuchs CS, Kraft P, Stolzenberg-Solomon RZ, Jacobs KB, Arslan AA, Bueno-de-Mesquita HB, Gallinger S, Gross M, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiprich S, Stolt CC, Schreiner S, Parlato R, Wegner M. SoxE proteins are differentially required in mouse adrenal gland development. Mol Biol Cell. 2008;19:1575–1586. doi: 10.1091/mbc.E07-08-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson DA, Wang M, Matunis MJ. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem. 2001;276:21664–21669. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stielow B, Kruger I, Diezko R, Finkernagel F, Gillemans N, Kong-a-San J, Philipsen S, Suske G. Epigenetic silencing of spermatocyte-specific and neuronal genes by SUMO modification of the transcription factor Sp3. PLoS Genet. 2010;6:e1001203. doi: 10.1371/journal.pgen.1001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda N, Shibata H, Kurihara I, Ikeda Y, Kobayashi S, Yokota K, Murai-Takeda A, Nakagawa K, Oya M, Murai M, et al. Coactivation of SF-1-Mediated Transcription of Steroidogenic Enzymes by Ubc9 and PIAS1. Endocrinology. 2011 doi: 10.1210/en.2010-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Labonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell. 2005;9:593–603. doi: 10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299:250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, Nilsson LM, Parini P, Janne OA, Gustafsson JA, et al. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010;24:381–395. doi: 10.1101/gad.545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby RJ, Stec J, Blind RD, Dixon S, Leesnitzer LM, Orband-Miller LA, Williams SP, Willson TM, Xu R, Zuercher WJ, et al. Small Molecule Agonists of the Orphan Nuclear Receptors Steroidogenic Factor-1 (SF-1, NR5A1) and Liver Receptor Homologue-1 (LRH-1, NR5A2) J Med Chem. 2011 doi: 10.1021/jm1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Heaton JH, Brevig H, Mukherjee S, Iniguez-Lluhi JA, Hammer GD. SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol Cell Biol. 2009;29:613–625. doi: 10.1128/MCB.00295-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zhou J, Deng M, Liu X, Le Bras M, de The H, Chen SJ, Chen Z, Liu TX, Zhu J. Small ubiquitin-related modifier paralogs are indispensable but functionally redundant during early development of zebrafish. Cell Res. 2010;20:185–196. doi: 10.1038/cr.2009.101. [DOI] [PubMed] [Google Scholar]
- Zubair M, Oka S, Parker KL, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009;23:1657–1667. doi: 10.1210/me.2009-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.