Abstract
For biomaterials scientists focusing on tissue engineering applications, the gold standard material is healthy, autologous tissue. Ideal material properties and construct design parameters are thus both obvious and often times unachievable; additional considerations such as construct delivery and the underlying pathology necessitating new tissue yield additional design challenges with solutions that are not evident in nature. For the past nearly two decades, our laboratory and collaborators have aimed to develop both new biomaterials and a better understanding of the complex interplay between material and host tissue to facilitate bone and cartilage regeneration. Various approaches have ranged from mimicking native tissue material properties and architecture to developing systems for bioactive molecule delivery as soluble factors or bound directly to the biomaterial substrate. Such technologies have allowed others and us to design synthetic biomaterials incorporating increasing levels of complexity found in native tissues with promising advances made towards translational success. Recent work focuses on translation of these technologies in specific clinical situations through the use of adjunctive biomaterials designed to address existing pathologies or guide host-material integration.
Keywords: biomaterials, tissue engineering, polymer scaffolds, hydrogels, biomimetics, bioreactors
Introduction
In the last 20 years, biomaterials science has expanded rapidly as a field in terms of publications, funding dollars, and recognition. Expansion in the related field of tissue engineering has similarly followed suit, with advances often closely tied to improved materials and better understanding of material-tissue interactions. While small scale yet exciting examples of clinical translation and success often reach the public consciousness through mainstream media, those directly involved in the field are often asked about more widespread translational advances, timelines, and the realization of the great potential biomaterials and tissue engineering hold.
The problems which we aim to address are often much more apparent than the progress by which we strive to reach the solution. To the non-scientist, perceptions of the field are, incorrectly perhaps, marked by exciting, promising news stories one day with little impact or follow up of note. To the invested biomaterials scientist and tissue engineer, the impact of the last 20 years lays in the building of a solid foundation of principles, understanding, and enabling technologies upon which the next 20 years will be built. In the following pages, we aim to give a broad overview of the capabilities of our laboratory followed by a discussion within this context of advances in the field to date and our views of the future of biomaterials, tissue engineering, and related fields.
Clearly Defined Goals
One of the subtleties that separate advances in tissue engineering from other fields within engineering is the existence of a clear set of goals. While buildings and bridges are ever being built higher and longer, reactors made more efficient or productive, and processors faster, tissue engineers are constantly measured against the gold standard of native tissue. Furthermore, because any viable tissue engineered product or technology will ultimately be implanted into the body to replace or restore native tissue function, these products or technologies must achieve a level of function very close to that of the known gold standard. With a very brief history upon which to build, we are being asked in a sense to build the tallest building possible with very little tolerance for any solution that falls short of the goal. The biomaterials-specific expectations are a tall task as well – provide a template for tissue growth and structure, provide functionality should there be a lag between biomaterial construct implantation and tissue maturity, and ideally, degrade at a rate equal to the rate at which the native tissues matures, infiltrates, differentiates, or functions.
Because of this goal, tissue engineering is often compared with or viewed in the context of transplantation. Recent advances in composite tissue allotransplantation (CTA), including hand and face transplants, and decreased requirements for immunosuppressive drugs have heightened awareness and expectations for this technology. Because the end goal of CTA and tissue engineering are identical, the two fields are frequently compared. Advances in tissue engineering bring in question the need for CTA and vice versa. In our opinion, the two fields are closely related. Clinically, success with CTA will likely be more rapid than tissue engineering and will have greater relative success in the immediate future in treating significant tissue defects containing multiple types of specialized tissue, for example, the loss of an entire limb. Tissue engineering ideally avoids the greatest single downside to CTA and allotransplantation in general – the need for donor tissue. Over the course of this review we will discuss in more detail certain areas where the two fields intersect.
Biomimetic Materials
Traditionally, authors and reviewers have classified a subset of the biomaterials used in tissue engineering as biomimetic based on their possessing some structural or functional characteristic found in the tissue to be engineered. Upon review, it is difficult to imagine any biomaterial used in tissue engineering that does not in some way meet the criteria to be termed biomimetic. Regardless of the approach taken or material used, the end goal of recapitulating native tissue function is a truly biomimetic goal.
Reaching that goal, however, may involve many different strategies and materials, some of which may seem to more closely mimic nature than others. In the following sections, we will examine certain native tissue properties of bone and cartilage, the two tissues upon which the majority of our laboratory’s work has focused, and explore the ways in which the biomaterials used to engineer these tissues have been biomimetic. After such an examination, we hope to identify common themes among biomaterials that may advance the field in the future.
Material Properties and Scaffold Architecture
Early work in our laboratory, as in many biomaterials laboratories, focused largely on the ex vivo interaction between materials and different cell types, measuring cell viability and proliferation when cultured in the presence of and on different types of biomaterials [1–4]. While such studies to this day remain an important step in characterizing new biomaterials [5, 6], work directed towards the ultimate goal of in vivo biomaterial applications began with a focus on porosity as a means to allow tissue ingrowth and nutrient transport within an implanted construct. Early work in this area using porous poly(L-lactic acid) (PLLA) films showed that while the rate of tissue ingrowth predictably varied directly with the pore size of the films, a balance needed to be achieved between porosity and the ingrowth of the desired tissue, nutrient delivery and waste removal, biomaterial degradation, and fibrous tissue ingrowth [7–9]. This is one of but many challenges we face in regenerating tissues and a potential breakpoint from simple biomimicry – while the body may employ a highly porous matrix as the backbone of a specific tissue, such as trabecular bone, merely copying that matrix is often not the ideal strategy due to the difference between normal tissue growth/development and the intricacies of wound healing.
Rather than look to normal, healthy tissues as a biomaterial template, a more nuanced approach looks at how the body, when capable of doing so, successfully heals itself. For bone, this process first involves the formation of a callus, which at an early stage is made of woven bone that later remodels into lamellar bone eventually consisting of the typical cortical and trabecular structure of healthy bone. The dense fibrocartilage callus and woven bone prevent fibrous tissue ingrowth while providing the template used for remodeling. Particulate leaching is one method that seemingly recapitulates the process of callus formation allowing bone remodeling. By initially introducing a relatively solid construct that then begins to develop pores due to the specific leaching of some non-toxic, rapidly degradable or soluble construct component, often salt or gelatin, we have developed a method allowing temporal control of pore formation and subsequently tissue ingrowth, mechanical property changes, and scaffold degradation [10–14].
As critical as the development of porosity throughout a scaffold may be, having an ideally tailored pore network within a scaffold made of a biomaterial poorly suited for the application at hand is tantamount to failure. PLLA and poly(DL-lactic-co-glycolic acid) (PLGA) were used in much of our early work towards understanding fundamental biomaterial-cell interactions [1–4, 15–17], biomaterial processing [18, 19], and degradation [8, 9, 20]; however, early work also aimed to develop biomaterials better suited to the specific applications of bone and cartilage engineering. Again taking cues from nature, the first such material, poly(propylene fumarate) (PPF, Figure 1), is an unsaturated linear polyester based on fumaric acid, an intermediate in the citric acid cycle [21]. PPF is a well suited platform material for bone tissue engineering; when crosslinked and then degraded, it maintained structure, strength, and porosity despite significant mass losses[22] and elicited a minimal tissue response from both hard and soft tissues after 2 and 8 weeks when implanted into rabbits [23]. In addition to its utility as a bone tissue engineering scaffold, PPF has been a platform material in our laboratory for a number of studies including refinements in polymer synthesis [24, 25], fundamental chemical and photo cross-linking studies [26–32], and for the development of related materials [33–35].
Figure 1.
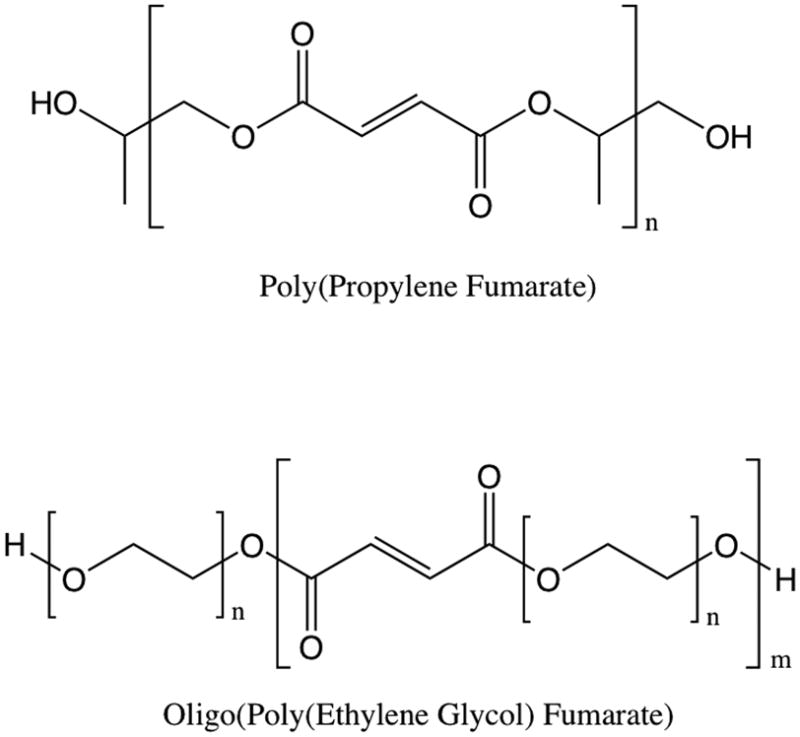
Structure of fumarate-based polymers, poly(propylene fumarate) (PPF) and oligo(poly(ethylene glycol) fumarate) (OPF).
Few, if any, tissues exist in isolation. An obvious corollary is that few, if any, tissues are injured or diseased without affecting some other surrounding, incorporated, or connected tissue. One evolving trend in tissue engineering has been towards engineering complex tissues – those with highly specific structure and/or multiple tissue types within the construct [36, 37] – and towards constructs that incorporate the interface between two tissues [38]. Our laboratory has long had an interest in cartilage tissue engineering and hydrogels, which again represent a synthetic yet biomimetic approach toward biomaterials synthesis as cartilage is itself a hydrogel. One such hydrogel developed in our laboratory, crosslinked oligo(poly(ethylene glycol) fumarate) (OPF, Figure 1), bears structural resemblance to PPF and is a useful platform for cartilage tissue engineering due to the ability to modulate the tensile mechanical properties, mesh size, swelling characteristics, and cell attachment by incorporating poly(ethylene glycol) (PEG) of varying molecular weights [39–41].
Material and Drug Delivery
Another salient consideration, although not one in which we can take cues from nature, in the development of biomaterials for tissue engineering is delivery or implantation of the material and of any cells or bioactive factors that may be delivered concurrently or at a later time. Usually, the acceptable method of delivery will be obvious based on the condition being treated, the current standards of care for that condition, and what, if any, cells and bioactive factors are to be delivered within the construct.
In our laboratory and many others, significant work on injectable systems for drug delivery and tissue engineering has led to a number of different methods for construct delivery, each with application specific advantages and disadvantages [42–48]. Our work has largely focused on cell and growth factor delivery. For cellular delivery within hydrophobic scaffolds, we have used hydrophilic carriers to encapsulate cells, an approach which confers two main advantages. First, in injectable systems, it reduces exposure to any potentially cytotoxic monomers and initiators [49–51]. Second, it reduces the time needed for construct fabrication, as cell delivery is not dependent on cell adhesion to the scaffold, and the carrier properties may be tuned to provide an appropriate niche for cell proliferation and/or differentiation (Figure 2)[52]. When using a hydrophilic, injectable material alone, such as OPF hydrogels, our primary approach to cartilage tissue engineering includes directly encapsulated cells prior to OPF crosslinking [53–56].
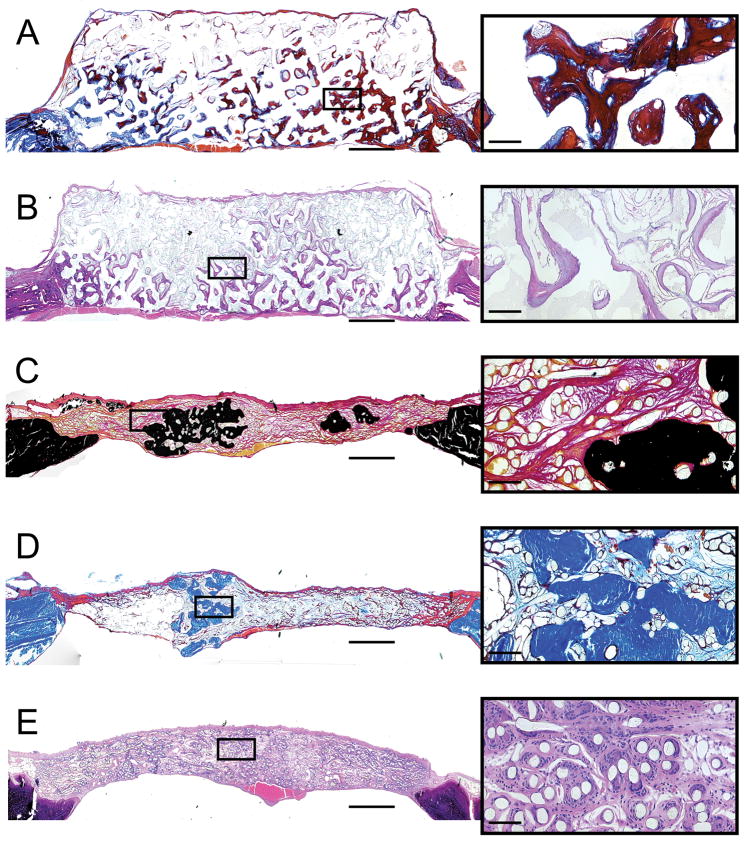
Figure 2.
Representative histological sections from rat calvarial defects 12 weeks after implantation of poly(L-lactic acid) and coral scaffolds with mesenchymal stem cells, harvested at the time of surgery and implanted without ex vivo culture, encapsulated in fibrin glue. Group and stain are as follows: A) coral scaffold, no cells, Goldner’s trichrome, B) coral scaffold with encapsulated cells and platelet rich plasma, hematoxylin and eosin, C) polymer mesh scaffold with encapsulated cells, Von Kossa/Van Gieson, D) polymer mesh scaffold with encapsulated cells and platelet rich plasma, Goldner’s trichrome, E) polymer mesh scaffold, no cells, hematoxylin and eosin. Scale bars in the full size images on the left represent 1 mm, while in the magnified micrographs to the right, the scale bars represent 100 μm. In A, mineralized tissue is stained blue or deep red due to the partial demineralization required during processing. In D, mineralized tissue is stained bright blue while osteoid is stained deep red. Reprinted with permission from [52].
Equipped with the ability to deliver cells, biomaterial scaffolds, and bioactive factors, one is then left to make the critical decision of what to deliver. Looking back at the course of our laboratory’s existence, the general trend has been towards more complex delivery schema in an attempt to mimic key extracellular cues that occur naturally. Our early efforts in this area involved the use of peptide sequences (GRGD, RGD, RGDS, and an osteopontin-derived peptide) covalently bound to OPF and poly(propylene fumarate-co-ethylene glycol) matrices to successfully guide cell adhesion and differentiation [57–63] on these hydrophilic surfaces. In cartilage, bone, and combined osteochondral tissue engineering applications we have used the controlled release of single and dual soluble growth factors to induce the differentiation of delivered mesenchymal stem cells (MSCs) and to promote local tissue healing and ingrowth [54, 55, 64–79].
Current Approaches and Future Directions
In the preceding sections we aimed to give you a broad overview of the capabilities developed and studied within our laboratory over the past nearly 20 years, highlighting general areas where engineering biomaterials and tissues has followed nature’s cues. This has resulted in a trend towards increased system complexity based on both our growing capabilities as biomaterials scientists and an increased understanding of the biological processes we aim to recapitulate. Given this context, a key question develops: where does the field, and an individual laboratory within it, go from here?
Diverging paths
We foresee two divergent paths emerging within the current fields of biomaterials and tissue engineering. First, work will continue in a similar direction to that which currently accounts for most biomaterials research - continued investigation towards understanding the principles that govern material-host interactions and the modification of current and development of new materials.
The second path, driven by the promise of recent and highly publicized clinical translations in tissue engineering along with increasing translational focus among funding agencies, will involve rapidly translatable technologies incorporating existing regulated biomaterials in combination or towards new applications. Recently in the United States, a conglomerate of funding sources combined to form the Armed Forces Institute of Regenerative Medicine (AFIRM), marking a large investment in tissue engineering and biomaterials research with the goal of fostering rapid clinical translation of new technology for the treatment of wounded warriors [80, 81]. Within our laboratory, a recent focus towards the goal of increasing applications in which scaffold-based tissue engineering approaches can be employed of has resulted in the development of porous poly(methylmethacrylate) (PMMA) space maintainers to better prepare hostile wound environments for eventual scaffold or construct implantation [82–85]. Although these two paths differ in approach and timeline, greater clinical success will only reinforce the potential of biomaterials and tissue engineering, expanding interest and hopefully funding for the development of novel biomaterials that improve upon those in use.
Intersecting fields
Expansion in the field of biomaterials will continue to be driven by our ability to collaborate. The continued development of new materials and advancement of our basic understanding of biomaterials will undoubtedly be the result of advances in related fields. For example, in the last decade, we and others have worked at the intersection of biomaterials and nanotechnology, resulting in materials with improved mechanical properties [47, 86–88] without significantly affecting biocompatibility [89, 90]. These scaffolds, composed of PPF and single-walled carbon nanotubes, exhibited favorable hard and soft tissue responses after 12 weeks when implanted in a bone defect.
The intersection of matrix biology and biomaterials has led to further advances in tissue engineering. Well deserved attention has been given to work using decellularized tissues and organs that are then recellularized with autologous cells, using biological cues preserved throughout the decellularization process to drive cell and tissue function [91–94]. These approaches to tissue engineering seem to straddle the line between biomaterials and transplant biology. Clinical products based on this same principle, chiefly demineralized bone matrix and acellular dermal matrices, are in widespread use. Limitations with regard to donor tissue availability and suitability, potential disease transmission, and patient acceptance of xenografts somewhat tempers the enthusiasm for such approaches, particularly as adjuncts to solid organ transplantation. Decellularized matrices and organs remain an exciting and promising development within the field.
Addressing complexity
Returning to the focus on biomimicry, a semantic argument may exist over whether decellularizing and recellularizing an existing organ or tissue represents a biomimetic or strictly biological approach. An alternative, perhaps more biomimetic or bioinspired approach has been taken with matrix analogues and combinations of synthetic biomaterials and matrix components. These approaches are hardly new – fibronectin has long been used to mediate cell attachment for in vitro applications [95, 96].
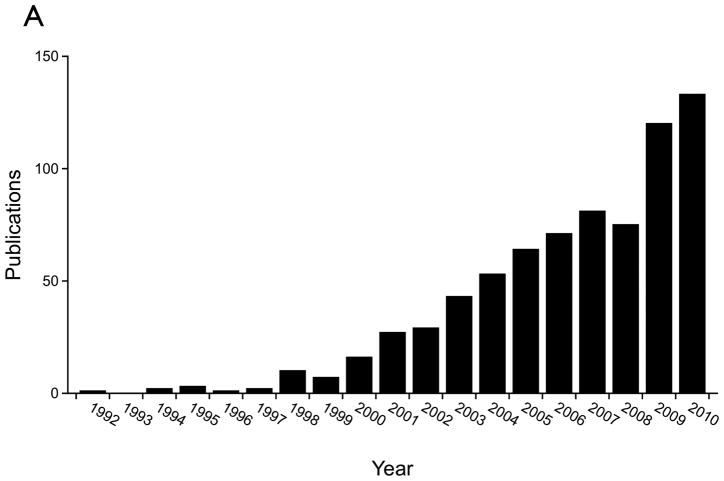
As biomaterials-science has advanced, so has our ability to increase the complexity with which we mimic the native extracellular matrix and cell signaling processes. Early studies in our lab and others focused on harnessing the bioactive capabilities of short peptide sequences, such as RGD, derived from extracellular matrix proteins [57, 58, 60–63, 97–99]. More recently, fusion proteins have been used to bind larger peptide growth factors to matrices, resulting in increased bone regeneration when compared to a released soluble growth factor [100]. With the capability to include more peptide sequences, proteins, soluble growth factors and other extracellular matrix (ECM) components to direct cellular behavior and tissue regeneration, research is necessary to determine which components of the native ECM and signaling cascades are optimal for tissue engineering applications (Figure 3) [101–105].
Figure 3.
Publication trends based on the MEDLINE database demonstrating the very recent increase in tissue engineering related publications which consider the extracellular matrix. A) Publications by year containing the term “extracellular matrix” in the title or abstract and the term “tissue engineering” or “tissue engineered” in the title. B) Publications by year containing the terms “tissue engineering” or “tissue engineered” in the title.
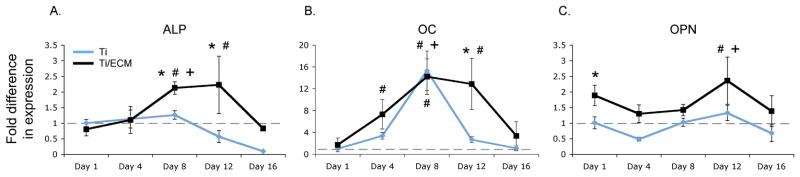
Rather than optimize or simplify native ECM, our laboratory has taken the approach of generating ex situ a native ECM homologue using a flow perfusion bioreactor. In this process, a cell culture media flowing through a porous scaffold generates a shear stress on seeded osteoblasts or osteoblast precursor cells which, likely in response to the mechanical stimulus, deposit an ECM which contains many bioactive signaling molecules that, following decellularization and recellularization can effectively direct gene expression and differentiation of reseeded precursor cells (Figure 4) [106–113]. Advantages of this process are the potential for scale up and potential “off the shelf” usage, ability to generate a matrix using autologous cells for later implantation, and the lack of need for donor tissues. While many components of the bone ECM have been recapitulated through this process, it is unclear how the generated ECM differs from native ECM and how this process can be applied to other tissue types.
Figure 4.
Gene expression profiles of bone-specific markers of osteoblastic differentiation alkaline phosphatase (ALP), osteocalcin (OC) and osteopontin (OP) as determined by RT-PCR. The data demonstrate significantly increased expression in mesenchymal stem cells cultured on titanium fiber meshes with ex vivo generated ECM (blue line) compared to those cells cultured on control titanium fiber meshes without ECM (black line). A * symbol indicates a significant difference in expression level at the specified timepoint (p <0.05); a # symbol indicates a significant difference in expression level with respect to the baseline (p <0.05); a + symbol indicates a significant increase in expression level from the previous timepoint. The dashed grey line indicates the baseline expression level. Reprinted in part and with permission from [111].
The future
Biomaterials and tissue engineering face a number of challenges in the future, and the solutions for addressing these challenges are not obvious. In the United States alone, the federal government has spent over $4 billion on tissue engineering in the last 20 years, much of which has focused on biomaterials [114]. Over the same period, cell-based products have been used to treat over 300,000 patients worldwide [115]. Depending on the observer, such numbers can be seen as a sign that biomaterials-based tissue engineering strategies are becoming a viable therapeutic option or that clinical translation and product development still lag significantly behind research.
If the viewpoint that product development lags behind research is adopted, then certainly one major component in this disparity is the time and cost associated with gaining regulatory approval for clinical translation. Regulatory considerations have long been a topic for publication [116]; however, little progress has been made in addressing many concerns regarding the development, evaluation, and regulation of these often complex products.
Indeed, as alluded to in the discussion of the divergent pathways of biomaterials and tissue engineering research, as we develop more complicated strategies for tissue regeneration, which better approximate the complexity of nature and perhaps yield superior results or publications, we may actually be developing technologies for which no clear pathway or perhaps chance of gaining regulatory approval exists [117]. Cell-based technologies in particular have a much shorter history with regulatory agencies and thus may be particularly cumbersome to bring to market [118–120]. It should also be noted that even after potentially being translated into clinical use, unanswered questions remain about the financial viability of these products [121].
Conclusions
Over the last 20 years, rapidly advancing biomaterial technologies and our increased understanding of biomaterial-host interactions have allowed us to create systems for tissue engineering that mimic native tissues and processes with increasing complexity. These approaches hold great potential for future clinical applications and have significantly increased our fundamental understanding of biomaterials.
In the field at large and the microcosm of our laboratory, we have observed a recent divergence within our materials-based tissue engineering research. While continued exploration and development of new technologies will continue to drive most research, particularly in academic settings, recent funding trends and clinical successes have reinforced the principle that simpler is better, particularly when dealing with regulatory issues and the pressure of rapid clinical translation. It is our hope that by embracing the simple technologies, the field can establish greater confidence among healthcare providers and regulatory agencies so that later, as more promising and complex technologies emerge, the road to clinical success is faster and more straightforward.
Acknowledgments
The authors acknowledge financial support by the United States National Institutes of Health, National Science Foundation, NASA, Whitaker Foundation, Armed Forces Institute of Regenerative Medicine, and other agencies and companies for biomaterials – related research over the past 20 years (AGM). Additionally, thanks and recognition must go out to all of the collaborators, fellows, students, and staff both at Rice University and elsewhere without whom this work would not have been possible.
Abbreviations
- AFIRM
Armed Forces Institute of Regenerative Medicine
- CTA
composite tissue allotransplantation
- ECM
extracellular matrix
- OPF
oligo(poly(ethylene glycol) fumarate)
- PEG
poly(ethylene glycol)
- PLGA
poly(DL-lactic-co-glycolic acid)
- PLLA
poly(L-lactic acid)
- PMMA
poly(methylmethacrylate)
- PPF
poly(propylene fumarate)
Footnotes
This invited contribution presents a 20-year perspective of the biomaterials research conducted in the laboratory of Professor Antonios G. Mikos of Rice University. Professor Mikos was the recipient of the 2011 Founders Award of the Society For Biomaterials.
References
- 1.Ishaug SL, Yaszemski MJ, Bizios R, Mikos AG. Osteoblast function on synthetic biodegradable polymers. J Biomed Mater Res. 1994;28:1445–1453. doi: 10.1002/jbm.820281210. [DOI] [PubMed] [Google Scholar]
- 2.Thomson RC, Giordano GG, Collier JH, Ishaug SL, Mikos AG, Lahiri-Munir D, et al. Manufacture and characterization of poly(alpha-hydroxy ester) thin films as temporary substrates for retinal pigment epithelium cells. Biomaterials. 1996;17:321–327. doi: 10.1016/0142-9612(96)85570-0. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Garcia CA, Mikos AG. Retinal pigment epithelium cell culture on thin biodegradable poly(DL-lactic-co-glycolic acid) films. J Biomater Sci Polym Ed. 1998;9:1187–1205. doi: 10.1163/156856298x00721. [DOI] [PubMed] [Google Scholar]
- 4.Wake MC, Gerecht PD, Lu L, Mikos AG. Effects of biodegradable polymer particles on rat marrow-derived stromal osteoblasts in vitro. Biomaterials. 1998;19:1255–1268. doi: 10.1016/s0142-9612(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 5.Klouda L, Hacker MC, Kretlow JD, Mikos AG. Cytocompatibility evaluation of amphiphilic, thermally responsive and chemically crosslinkable macromers for in situ forming hydrogels. Biomaterials. 2009;30:4558–4566. doi: 10.1016/j.biomaterials.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew SA, Hacker MC, Saraf A, Raphael RM, Kasper FK, Mikos AG. Biodegradable branched polycationic polymers with varying hydrophilic spacers for nonviral gene delivery. Biomacromolecules. 2009;10:2436–2445. doi: 10.1021/bm9003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wake MC, Patrick CW, Jr, Mikos AG. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994;3:339–343. doi: 10.1177/096368979400300411. [DOI] [PubMed] [Google Scholar]
- 8.Lu L, Peter SJ, Lyman MD, Lai H-L, Leite SM, Tamada JA, et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21:1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Peter SJ, Lyman MD, Lai H-L, Leite SM, Tamada JA, et al. In vitro degradation of porous poly(L-lactic acid) foams. Biomaterials. 2000;21:1595–1605. doi: 10.1016/s0142-9612(00)00048-x. [DOI] [PubMed] [Google Scholar]
- 10.Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci Polym Ed. 1995;7:23–38. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 11.Wake MC, Gupta PK, Mikos AG. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 1996;5:465–473. doi: 10.1177/096368979600500405. [DOI] [PubMed] [Google Scholar]
- 12.Habraken WJEM, Wolfe JGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17:1057–1074. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 13.Habraken WJ, Zhang Z, Wolke JG, Grijpma DW, Mikos AG, Feijen J, et al. Introduction of enzymatically degradable poly(trimethylene carbonate) microspheres into an injectable calcium phosphate cement. Biomaterials. 2008;29:2464–2476. doi: 10.1016/j.biomaterials.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Habraken WJ, de Jonge LT, Wolke JG, Yubao L, Mikos AG, Jansen JA. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J Biomed Mater Res A. 2008;87:643–655. doi: 10.1002/jbm.a.31703. [DOI] [PubMed] [Google Scholar]
- 15.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27:183–189. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 16.Evans GR, Brandt K, Widmer MS, Lu L, Meszlenyi RK, Gupta PK, et al. In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials. 1999;20:1109–1115. doi: 10.1016/s0142-9612(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 17.Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, et al. Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23:841–848. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- 18.Mikos AG, Lyman MD, Freed LE, Langer R. Wetting of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams for tissue culture. Biomaterials. 1994;15:55–58. doi: 10.1016/0142-9612(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 19.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, et al. Preparation and characterization of poly(L-lactic acid) foams. Polymer. 1994;35:1068–1077. [Google Scholar]
- 20.von Recum HA, Cleek RL, Eskin SG, Mikos AG. Degradation of polydispersed poly(L-lactic acid) to modulate lactic acid release. Biomaterials. 1995;16:441–447. doi: 10.1016/0142-9612(95)98816-w. [DOI] [PubMed] [Google Scholar]
- 21.Yaszemski MJ, Payne RG, Hayes WC, Langer RS, Aufdemorte TB, Mikos AG. The Ingrowth of New Bone Tissue and Initial Mechanical Properties of a Degrading Polymeric Composite Scaffold. Tissue Eng. 1995;1:41–52. doi: 10.1089/ten.1995.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Fisher JP, Holland TA, Dean D, Mikos AG. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part II. In vitro degradation. Biomacromolecules. 2003;4:1335–1342. doi: 10.1021/bm0300296. [DOI] [PubMed] [Google Scholar]
- 23.Fisher JP, Vehof JW, Dean D, van der Waerden JP, Holland TA, Mikos AG, et al. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res. 2002;59:547–556. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 24.Peter SJ, Suggs LJ, Yaszemski MJ, Engel PS, Mikos AG. Synthesis of poly(propylene fumarate) by acylation of propylene glycol in the presence of a proton scavenger. J Biomater Sci Polym Ed. 1999;10:363–373. doi: 10.1163/156856299x00423. [DOI] [PubMed] [Google Scholar]
- 25.Shung AK, Timmer MD, Jo S, Engel PS, Mikos AG. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. J Biomater Sci Polym Ed. 2002;13:95–108. doi: 10.1163/156856202753525963. [DOI] [PubMed] [Google Scholar]
- 26.Timmer MD, Jo S, Wang C, Ambrose CG, Mikos AG. Characterization of the cross-linked structure of fumarate-based degradable polymer networks. Macromolecules. 2002;35:4373–4379. [Google Scholar]
- 27.Timmer MD, Horch RA, Ambrose CG, Mikos AG. Effect of physiological temperature on the mechanical properties and network structure of biodegradable poly(propylene fumarate)-based networks. J Biomater Sci Polym Ed. 2003;14:369–382. doi: 10.1163/156856203321478874. [DOI] [PubMed] [Google Scholar]
- 28.He S, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials. 2000;21:2389–2394. doi: 10.1016/s0142-9612(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 29.He S, Timmer MD, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)-diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer. 2001;42:1251–1260. [Google Scholar]
- 30.Fisher JP, Holland TA, Dean D, Engel PS, Mikos AG. Synthesis and properties of photocross-linked poly(propylene fumarate) scaffolds. J Biomater Sci Polym Ed. 2001;12:673–687. doi: 10.1163/156856201316883476. [DOI] [PubMed] [Google Scholar]
- 31.Fisher JP, Dean D, Mikos AG. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials. 2002;23:4333–4343. doi: 10.1016/s0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 32.Fisher JP, Timmer MD, Holland TA, Dean D, Engel PS, Mikos AG. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part I. Determination of network structure. Biomacromolecules. 2003;4:1327–1334. doi: 10.1021/bm030028d. [DOI] [PubMed] [Google Scholar]
- 33.Suggs LJ, Kao EY, Palombo LL, Krishnan RS, Widmer MS, Mikos AG. Preparation and characterization of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomater Sci Polym Ed. 1998;9:653–666. doi: 10.1163/156856298x00073. [DOI] [PubMed] [Google Scholar]
- 34.Suggs LJ, Krishnan RS, Garcia CA, Peter SJ, Anderson JM, Mikos AG. In vitro and in vivo degradation of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res. 1998;42:312–320. doi: 10.1002/(sici)1097-4636(199811)42:2<312::aid-jbm17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Suggs LJ, Shive MS, Garcia CA, Anderson JM, Mikos AG. In vitro cytotoxicity and in vivo biocompatibility of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res. 1999;46:22–32. doi: 10.1002/(sici)1097-4636(199907)46:1<22::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 36.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable Biomaterials for Regenerating Complex Craniofacial Tissues. Adv Mater. 2009;21:3368–3393. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15:127–141. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo S, Shin H, Shung AK, Fisher JP, Mikos AG. Synthesis and characterization of oligo(poly(ethylene glycol) fumarate) macromer. Macromolecules. 2001;34:2839–2844. [Google Scholar]
- 40.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59:429–437. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 41.Temenoff JS, Steinbis ES, Mikos AG. Effect of drying history on swelling properties and cell attachment to oligo(poly(ethylene glycol) fumarate) hydrogels for guided tissue regeneration applications. J Biomater Sci Polym Ed. 2003;14:989–1004. doi: 10.1163/156856203322381465. [DOI] [PubMed] [Google Scholar]
- 42.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Behravesh E, Jo S, Zygourakis K, Mikos AG. Synthesis of in situ cross-linkable macroporous biodegradable poly(propylene fumarate-co-ethylene glycol) hydrogels. Biomacromolecules. 2002;3:374–381. doi: 10.1021/bm010158r. [DOI] [PubMed] [Google Scholar]
- 44.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and Characterization of Injectable, Thermally and Chemically Gelable, Amphiphilic Poly(N-isopropylacrylamide)-Based Macromers. Biomacromolecules. 2008;9:1558–1570. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 45.Habraken WJ, Wolke JG, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17:1057–1074. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 46.Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos A. Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J Biomed Mater Res. 1999;44:314–321. doi: 10.1002/(sici)1097-4636(19990305)44:3<314::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Injectable nanocomposites of single-walled carbon nanotubes and biodegradable polymers for bone tissue engineering. Biomacromolecules. 2006;7:2237–2242. doi: 10.1021/bm060391v. [DOI] [PubMed] [Google Scholar]
- 48.Suggs LJ, Mikos AG. Development of poly(propylene fumarate-co-ethylene glycol) as an injectable carrier for endothelial cells. Cell Transplant. 1999;8:345–350. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- 49.Payne RG, McGonigle JS, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 3. Proliferation and differentiation of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002;23:4381–4387. doi: 10.1016/s0142-9612(02)00186-2. [DOI] [PubMed] [Google Scholar]
- 50.Payne RG, McGonigle JS, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 2. Viability of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002;23:4373–4380. doi: 10.1016/s0142-9612(02)00185-0. [DOI] [PubMed] [Google Scholar]
- 51.Payne RG, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 1. Encapsulation of marrow stromal osteoblasts in surface crosslinked gelatin microparticles. Biomaterials. 2002;23:4359–4371. doi: 10.1016/s0142-9612(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 52.Kretlow JD, Spicer PP, Jansen JA, Vacanti CA, Kasper FK, Mikos AG. Uncultured marrow mononuclear cells delivered within fibrin glue hydrogels to porous scaffolds enhance bone regeneration within critical-sized rat cranial defects. Tissue Eng Part A. 2010;16:3555–3568. doi: 10.1089/ten.tea.2010.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Temenoff JS, Shin H, Conway DE, Engel PS, Mikos AG. In vitro cytotoxicity of redox radical initiators for cross-linking of oligo(poly(ethylene glycol) fumarate) macromers. Biomacromolecules. 2003;4:1605–1613. doi: 10.1021/bm030056w. [DOI] [PubMed] [Google Scholar]
- 54.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta 1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 55.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jo S, Shin H, Mikos AG. Modification of oligo(poly(ethylene glycol) fumarate) macromer with a GRGD peptide for the preparation of functionalized polymer networks. Biomacromolecules. 2001;2:255–261. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 58.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Modulation of differentiation and mineralization of marrow stromal cells cultured on biomimetic hydrogels modified with Arg-Gly-Asp containing peptides. J Biomed Mater Res A. 2004;69:535–543. doi: 10.1002/jbm.a.30027. [DOI] [PubMed] [Google Scholar]
- 59.Behravesh E, Mikos AG. Three-dimensional culture of differentiating marrow stromal osteoblasts in biomimetic poly(propylene fumarate-co-ethylene glycol)-based macroporous hydrogels. J Biomed Mater Res A. 2003;66:698–706. doi: 10.1002/jbm.a.10003. [DOI] [PubMed] [Google Scholar]
- 60.Behravesh E, Zygourakis K, Mikos AG. Adhesion and migration of marrow-derived osteoblasts on injectable in situ crosslinkable poly(propylene fumarate-co-ethylene glycol)-based hydrogels with a covalently linked RGDS peptide. J Biomed Mater Res A. 2003;65:260–270. doi: 10.1002/jbm.a.10461. [DOI] [PubMed] [Google Scholar]
- 61.Shin H, Jo S, Mikos AG. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethyleneglycol) spacer. J Biomed Mater Res. 2002;61:169–179. doi: 10.1002/jbm.10193. [DOI] [PubMed] [Google Scholar]
- 62.Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, et al. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26:3645–3654. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 63.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25:895–906. doi: 10.1016/s0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 64.Lu L, Stamatas GN, Mikos AG. Controlled release of transforming growth factor beta1 from biodegradable polymer microparticles. J Biomed Mater Res. 2000;50:440–451. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 65.Peter SJ, Lu L, Kim DJ, Stamatas GN, Miller MJ, Yaszemski MJ, et al. Effects of transforming growth factor beta1 released from biodegradable polymer microparticles on marrow stromal osteoblasts cultured on poly(propylene fumarate) substrates. J Biomed Mater Res. 2000;50:452–462. doi: 10.1002/(sici)1097-4636(20000605)50:3<452::aid-jbm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 66.Lu L, Yaszemski MJ, Mikos AG. TGF-beta1 release from biodegradable polymer microparticles: its effects on marrow stromal osteoblast function. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S82–91. [PubMed] [Google Scholar]
- 67.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 68.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94:101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75:156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 71.Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata Y, Mikos AG, et al. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr Cartil. 2007;15:187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, et al. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. Journal of biomedical materials research Part A. 2009;88:889–897. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- 73.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008 doi: 10.1016/j.actbio.2008.04.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008 doi: 10.1007/s11095-008-9685-1. In press. [DOI] [PubMed] [Google Scholar]
- 75.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A(Suppl 3):75–81. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 76.Ruhe PQ, Boerman OC, Russel FG, Spauwen PH, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 77.Ruhe PQ, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J Mater Sci Mater Med. 2006;17:919–927. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 78.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, et al. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72:343–353. doi: 10.1002/jbm.a.30265. [DOI] [PubMed] [Google Scholar]
- 79.Hedberg EL, Tang A, Crowther RS, Carney DH, Mikos AG. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J Control Release. 2002;84:137–150. doi: 10.1016/s0168-3659(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 80.Atala A. AFIRM: moving regenerative medicine onto the battlefield. Regenerative Med. 2008;3:463–464. doi: 10.2217/17460751.3.4.463. [DOI] [PubMed] [Google Scholar]
- 81.Holden C. Regenerative medicine. Rebuilding the injured warrior. Science. 2008;320:437. doi: 10.1126/science.320.5875.437a. [DOI] [PubMed] [Google Scholar]
- 82.Shi M, Kretlow JD, Spicer PP, Tabata Y, Demian N, Wong ME, et al. Antibiotic-releasing porous polymethylmethacrylate/gelatin/antibiotic constructs for craniofacial tissue engineering. Journal of controlled release: official journal of the Controlled Release Society. 2011 doi: 10.1016/j.jconrel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kretlow JD, Shi M, Young S, Spicer PP, Demian N, Jansen JA, et al. Evaluation of soft tissue coverage over porous polymethylmethacrylate space maintainers within nonhealing alveolar bone defects. Tissue Eng Part C Methods. 2010;16:1427–1438. doi: 10.1089/ten.tec.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi M, Kretlow JD, Nguyen A, Young S, Scott Baggett L, Wong ME, et al. Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials. 2010;31:4146–4156. doi: 10.1016/j.biomaterials.2010.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen C, Young S, Kretlow JD, Mikos AG, Wong M. Surface characteristics of biomaterials used for space maintenance in a mandibular defect: a pilot animal study. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2011;69:11–18. doi: 10.1016/j.joms.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 86.Mistry AS, Cheng SH, Yeh T, Christenson EM, Jansen JA, Mikos AG. Fabrication and in vitro degradation of porous fumarate-based polymer/alumoxane nanocomposite scaffolds for bone tissue engineering. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32010. In press. [DOI] [PubMed] [Google Scholar]
- 87.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Rheological behaviour and mechanical characterization of injectable poly(propylene fumarate)/single-walled carbon nanotube composites for bone tissue engineering. Nanotechnology. 2005;16:531–538. doi: 10.1088/0957-4484/16/7/030. [DOI] [PubMed] [Google Scholar]
- 88.Shi X, Sitharaman B, Pham QP, Liang F, Wu K, Edward Billups W, et al. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28:4078–4090. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mistry AS, Mikos AG, Jansen JA. Degradation and biocompatibility of a poly(propylene fumarate)-based/alumoxane nanocomposite for bone tissue engineering. J Biomed Mater Res A. 2007;83:940–953. doi: 10.1002/jbm.a.31280. [DOI] [PubMed] [Google Scholar]
- 90.Shi X, Sitharaman B, Pham QP, Spicer PP, Hudson JL, Wilson LJ, et al. In vitro cytotoxicity of single-walled carbon nanotube/biodegradable polymer nanocomposites. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31671. [DOI] [PubMed] [Google Scholar]
- 91.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 92.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 93.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Badylak SF, Taylor D, Uygun K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annual review of biomedical engineering. 2010 doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaheri A, Alitalo K, Hedman K, Keski-Oja J, Kurkinen M, Wartiovaara J. Fibronectin and the pericellular matrix of normal and transformed adherent cells. Ann N Y Acad Sci. 1978;312:343–353. doi: 10.1111/j.1749-6632.1978.tb16812.x. [DOI] [PubMed] [Google Scholar]
- 96.Pena SD, Hughes RC. Fibroblast to substratum contacts mediated by the different forms of fibronectin. Cell Biol Int Rep. 1978;2:339–344. doi: 10.1016/0309-1651(78)90019-x. [DOI] [PubMed] [Google Scholar]
- 97.Liu G, Eskin SG, Mikos AG. Integrin alpha(v)beta(3) is involved in stimulated migration of vascular adventitial fibroblasts by basic fibroblast growth factor but not platelet-derived growth factor. J Cell Biochem. 2001;83:129–135. doi: 10.1002/jcb.1208. [DOI] [PubMed] [Google Scholar]
- 98.Tanahashi K, Mikos AG. Effect of hydrophilicity and agmatine modification on degradation of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res A. 2003;67:1148–1154. doi: 10.1002/jbm.a.10147. [DOI] [PubMed] [Google Scholar]
- 99.Tanahashi K, Mikos AG. Protein adsorption and smooth muscle cell adhesion on biodegradable agmatine-modified poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res A. 2003;67:448–457. doi: 10.1002/jbm.a.10077. [DOI] [PubMed] [Google Scholar]
- 100.Schmoekel HG, Weber FE, Schense JC, Gratz KW, Schawalder P, Hubbell JA. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89:253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- 101.Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem. 2007;101:1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 102.Serban MA, Prestwich GD. Modular extracellular matrices: solutions for the puzzle. Methods. 2008;45:93–98. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prestwich GD. Engineering a clinically-useful matrix for cell therapy. Organogenesis. 2008;4:42–47. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9:549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 107.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 109.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pham QP, Kasper FK, Mistry AS, Sharma U, Yasko AW, Jansen JA, et al. Analysis of the osteoinductive capacity and angiogenicity of an in vitro generated extracellular matrix. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31875. [DOI] [PubMed] [Google Scholar]
- 111.Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29:2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 112.Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MAK, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33:63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 113.Sikavitsas VI, van den Dolder J, Bancroft GN, Jansen JA, Mikos AG. Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue-engineered constructs using a rat cranial critical size defect model. J Biomed Mater Res A. 2003;67:944–951. doi: 10.1002/jbm.a.10126. [DOI] [PubMed] [Google Scholar]
- 114.Hollister SJ. Scaffold engineering: a bridge to where? Biofabrication. 2009;1:012001. doi: 10.1088/1758-5082/1/1/012001. [DOI] [PubMed] [Google Scholar]
- 115.Mason C, Manzotti E. Regenerative medicine cell therapies: numbers of units manufactured and patients treated between 1988 and 2010. Regenerative Med. 2010;5:307–313. doi: 10.2217/rme.10.37. [DOI] [PubMed] [Google Scholar]
- 116.Hellman KB. Biomedical applications of tissue engineering technology: regulatory issues. Tissue Eng. 1995;1:203–210. doi: 10.1089/ten.1995.1.203. [DOI] [PubMed] [Google Scholar]
- 117.Lee MH, Arcidiacono JA, Bilek AM, Wille JJ, Hamill CA, Wonnacott KM, et al. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B Rev. 2010;16:41–54. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- 118.Carpenter MK, Couture LA. Regulatory considerations for the development of autologous induced pluripotent stem cell therapies. Regenerative Med. 2010;5:569–579. doi: 10.2217/rme.10.55. [DOI] [PubMed] [Google Scholar]
- 119.Baylis F. Betwixt and between human stem cell guidelines and legislation. Health Law Rev. 2002;11:44–50. [PubMed] [Google Scholar]
- 120.Liras A. Future research and therapeutic applications of human stem cells: general, regulatory, and bioethical aspects. Journal of Transl Med. 2010;8:131. doi: 10.1186/1479-5876-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ginty PJ, Singh PB, Smith D, Hourd P, Williams DJ. Achieving reimbursement for regenerative medicine products in the USA. Regenerative Med. 2010;5:463–469. doi: 10.2217/rme.10.13. [DOI] [PubMed] [Google Scholar]