Abstract
Background
Psoriasis afflicts 2-3% of the world’s population. Affected patients commonly have risk factors for cardiovascular disease (CVD). In addition, psoriasis is independently associated with CVD and mortality.
Purpose
To determine which CVD risk factors are associated with psoriasis independent of confounders, whether psoriasis is associated with CVD independent of CVD risk factors, and whether there is increased mortality among patients with psoriasis.
Data Sources
MEDLINE, Embase, and Cochrane Collaborations from inception through October 2009. We reviewed bibliographies of retrieved articles for additional references.
Study Selection
Cross-sectional, cohort-based, case-control, and randomized controlled trials which involved patients with psoriasis.
Data Extraction
Two investigators independently reviewed studies and resolved any discrepancies by consensus.
Data Synthesis
Of the 2,303 articles identified by literature search, 90 studies met inclusion criteria for this review; 15 were cohort-based studies, 45 were case-control, and 30 were cross-sectional.
Limitations
The quality of evidence was limited by study heterogeneity and lack of large scale prospective studies with long-term follow-up.
Conclusions
Patients with psoriasis demonstrate a higher prevalence of cardiovascular risk factors and appear to be at increased risk for ischemic heart disease, cerebrovascular disease, and peripheral arterial disease. This increase in vascular disease may be independent of shared risk factors and may contribute to the increase in all-cause mortality. Future research should aim to more confidently distinguish between a true causal relationship or merely an association resulting from multiple shared risk factors. Physicians should screen for and aggressively treat modifiable risk factors for CVD in patients with psoriasis.
KEY WORDS: psoriasis, cardiovascular disease, alcohol, risk factors, cross-sectional studies, cohort-based studies
INTRODUCTION
Psoriasis afflicts nearly 2-3% of the world’s population, including seven million Americans.1 In addition to its cutaneous manifestations, psoriasis has been associated with arthritis, depression, and reduced quality of life.2–4 Recent understanding has shown that as a systemic inflammatory condition, psoriasis is analogous to other immune disorders, such as systemic lupus erythematosus or rheumatoid arthritis.5 Considering that these specific disorders have been associated with cardiovascular (CVD) and other vascular diseases,6,7 investigators have focused their attention on whether CVD risk factors or CVD, per se, are increased in patients with psoriasis.
For many years, the treatment of psoriasis has been directed at managing both skin and joint involvement with a variety of modalities ranging from topical preparations (corticosteroids, coal tar, anthralin, tazarotene, vitamin D analogues) to phototherapy to systemic medications (methotrexate, cyclosporine, retinoids, biologics). Recently, there have been advances in the therapeutic efficacy and targeted therapy of the biologics with anti-tumor necrosis factor (TNF) class and anti-interleukin 12/23 p40 (ustekinumab). Physicians may soon have to look beyond the skin and joints to consider the impact of these systemic therapies on systemic inflammation.
We conducted a systematic review to address three specific questions related to psoriasis and CVD. First, which CVD risk factors are associated with psoriasis, independently of confounders? Second, is psoriasis associated with CVD independently of cardiovascular disease risk factors? Third, is there associated increase in mortality among patients with psoriasis?
METHODS
Data Sources and Searches
We conducted a key word search using MEDLINE, Embase, and the Cochrane Collaborations for publications from database inception through October 2009, which includes the earliest insights as well as the more recent breakthroughs and landmark publications. We limited the search to studies of human subjects and included English publications. We excluded review articles, guidelines, and case reports. Key search terms came from categories such as CVD risk factors (“diabetes mellitus,” “dyslipidemia,” “smoking,” “hypertension,” “tobacco”), systemic treatments (“methotrexate,” “cyclosporine,” “acitretin,” “etanercept,” “adalimumab,” “infliximab,” “efalizumab”), vascular diseases (“cardiovascular disease,” “stroke,” “ischemic heart disease,” “peripheral vascular disease”), mortality (“mortality”) and “psoriasis.” The online appendix describes the search strategy in detail (Appendix). A review of bibliographies of retrieved articles yielded no additional references.
Study Selection
All studies which were classified as cohort-based, cross-sectional, case-controlled, or randomized controlled trials were further evaluated. Eligible studies were original research articles that examined the association of psoriasis with at least one of the following: CVD risk factors, vascular disease, or the effect of CVD risk factors or vascular disease on mortality.
Data Extraction and Quality Assessment
Two reviewers assessed the quality of each eligible study. We used the Newcastle–Ottawa scales to assess each case control and cohort study. This scale uses a star system to judge nonrandomized studies based on three broad perspectives: the selection of the study group, the comparability of the groups, and the ascertainment of either exposure or outcome of interest for case-control or cohort studies, respectively.8 We assessed cross-sectional studies using cross-sectional/prevalence study quality scale which grades nonrandomized studies based on 11 dichotomous (YES/NO) criteria concerning basic elements of the study’s design, analysis, and completeness of data collection. We used the Jadad scale, a 5-question scale critiquing clinical trials for proper method of randomization, appropriate blinding, and accurate evaluation of withdrawals and dropouts, to evaluate randomized controlled trials. Discrepancies between the two reviewers (R.V.P. and M.L.S.) were resolved by consensus. Due to the heterogeneity of the patients, study designs, interventions, and outcomes data pooling, we were unable to perform a meta-analysis.
We considered a study less prone to bias, and consequently gave it more emphasis, if a consecutive or random sample of psoriasis cases were enrolled and if a comparison group consisted of consecutive, random, or appropriately matched controls.
Data Synthesis and Analysis
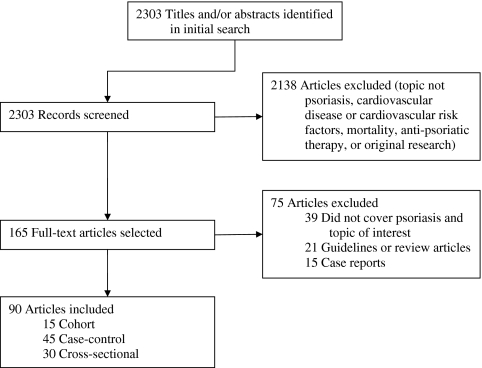
Of 2,303 articles identified by the search, 165 were screened and 90 met inclusion criteria (Fig. 1). Of the 75 excluded articles, 39 did not cover psoriasis and an outcome of interest, 21 were guidelines or reviews, and 15 were case reports. Of the 90 articles included, 15 were cohort-based, 45 were case-control, and 30 were cross-sectional. For each study, we characterized the design, patient data, inclusion/exclusion criteria, major outcomes, and conclusions, along with the established quality assessment.
Figure 1.
Flow of eligible studies of the association of cardiovascular disease and psoriasis.
RESULTS
CVD Risk Factors Associated with Psoriasis
Table 1 details those studies that examined the association of psoriasis with CVD risk factors, including hyperlipidemia, hyperhomocysteinemia/elevated C-reactive protein (CRP), hypertension (HTN), diabetes mellitus type 2 (DM), obesity, metabolic syndrome (MBS), smoking, and alcohol consumption.
Table 1.
Studies of Cardiovascular Disease Risk Factors Associated with Psoriasis
| Hyperlipidemia | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Akhyani et al.22 | 2007 | Patients with psoriasis had elevated triglyceride, total cholesterol, and LDL cholesterol | 4 of 9 |
| Cohen et al.56 | 2007 | Multivariate models adjusting for age and gender found patients with psoriasis had an increased risk for ischemic heart disease, diabetes mellitus, hypertension, obesity, and dyslipidemia | 6 of 9 |
| Jones et al.23 | 2000 | High-density lipoprotein (HDL) cholesterol and its subfraction, HDL3, were reduced and most dense subfraction of low-density lipoprotein (LDL), LDL3, was increased in patients with psoriatic arthritis | 7 of 9 |
| Kaye et al.16 | 2008 | HRs were increased among patients with psoriasis vs. the comparison cohort for incident diabetes , hypertension, obesity , and hyperlipidaemia . They also had higher risks of incident MI, angina , atherosclerosis , PVD, and stroke | 8 of 9 |
| Mallbris et al.17 | 2006 | Patients with psoriasis had higher VLDL as compared to controls, after adjusting for age, gender, smoking, physical exercise, alcohol consumption, body mass index, and systolic BP | 8 of 9 |
| Piskin et al.18 | 2003 | In the patient group, serum total cholesterol and LDL were higher than those of controls. Triglyceride, HDL, and VLDL levels did not show any significant difference between patients and controls | 8 of 9 |
| Rocha-Pereira et al.24 | 2001 | Patients with psoriasis had elevations in cholesterol , triglycerides, LDL, VLDL, apolipoprotein B, and Lp(a), as well as a reduction in HDL and total antioxidant capacity | 5 of 9 |
| Takahashi et al.97 | 2001 | There was positive correlation between BMI, body fat, and visceral adiposity level and the severity of psoriasis. The correlation of body fat or visceral adiposity with PASI was higher than. Plasma adiponectin negatively correlated with BMI , %body fat, and visceral adiposity. In contrast, plasma levels of TNF-a and leptin were positively correlated with BMI, %body fat, and visceral adiposity | 5 of 9 |
| Wu et al.39 | 2008 | Patients with psoriasis were more likely to have the following cardiovascular comorbidities: hypertension, hypercholesterolemia, and diabetes | 7 of 9 |
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Bajaj et al.98 | 2009 | Serum cholesterol, triglycerides and LDL were higher than in the control group. There was no statistical difference in serum levels of VLDL and HDL between the two groups | 7 of 11 |
| Dreiher et al.20 | 2008 | Psoriasis patients had higher prevalence of dyslipidemia after controlling for confounders. In multivariate analysis, triglycerides were higher in psoriasis patients and HDL levels were lower | 7 of 11 |
| Kimball et al.26 | 2008 | Two distinct databases were used to verify these relationships. In IMS Health database, patients with psoriasis had higher OR of CVD, CHF, DM , hyperlipidemia, HTN, ischemic heart disease , acute MI, PVD), and atherosclerosis. Based upon disease severity (presence of systemic therapy), patients with “severe” disease had higher OR for multiple clinical endpoints than “mild” disease | 5 of 11 |
| Naldi et al.85 | 2008 | The OR of psoriasis was 0.8 (95% CI 0.5–1.3) in hypertensive subjects, 1.1 (95% CI 0.6–2.0) in diabetics and 1.1 (95% CI 0.7–1.7) in hyperlipidemic subjects. Since these chronic conditions were not significant at first diagnosis of psoriasis, the reported associations with these factors may be a late effect of the psoriatic disease process | 6 of 11 |
| Neimann et al.27 | 2006 | Patients with mild psoriasis had a higher adjusted OR of DM, HTN, hyperlipidemia, obesity, and smoking than controls. Patients with severe psoriasis had a higher adjusted OR of DM, obesity, and smoking than controls. Additionally, DM and obesity were more prevalent in those with severe vs mild psoriasis | 5 of 11 |
| Pietrzak et al.21 | 2009 | Patients with psoriasis had higher Lp(a) and lower HDL than controls. Lp(a) levels were found to be positively correlated with psoriasis severity as measured by the PASI score | 6 of 11 |
| Reynoso-von Drateln19 | 2003 | Patients with psoriasis had lower levels of HDL compared to controls , but no difference was found in fasting insulin, triglycerides, cholesterol, LDL or VLDL | 6 of 11 |
| Tam et al.86 | 2008 | After adjusting for BMI, PsA patients had a higher prevalence of DM and HTN, but a lower prevalence of low HDL. They have significantly increased systolic and diastolic BP, insulin resistance and inflammatory markers (hs-CRP) compared to controls | 5 of 11 |
| Also see: Prodanovich (Effects on Mortality, 83) | |||
| Hyperhomocysteinemia/Elevated C reactive protein | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Grzybowski et al.87 | 2001 | Psoriatics’ serum levels of glucose, insulin, C-peptide and uric acid were monitored before and after treatment. All measured parameters significantly increased and dependent on the BMI. Compared with pretreatment, values of glucose and uric acid were significantly lower during therapy. The increase in the mean C-peptide and insulin levels after treatment was constant and independent from clinical disease | 5 of 9 |
| Karabudak et al.88 | 2008 | Patients with psoriasis had higher levels of total homocysteine, CRP, and ceruloplasmin, and lower levels of antithrombin-III and folic acid | 6 of 9 |
| Vanizor Kural et al.28 | 2003 | Psoriasis patients had higher levels of total homocysteine, auto-antibody to oxidized LDL, fibrinogen, fibronectin, and lower levels of tissue plasminogen activator, vitamin B12 and folate | 7 of 9 |
| Malerba et al.29 | 2006 | Plasma homocysteine levels were higher in patients with psoriasis as compared with controls. Plasma homocysteine levels correlate directly with PASI scores. In contrast, plasma folic acid levels were lower in cases than controls and levels are inversely correlated with plasma homocysteine levels and PASI scores | 8 of 9 |
| Ohtsuka32 | 2008 | Occurrence rate of elevated maximum BMI in high–highest hs-CRP group was higher than those with low–moderate hs-CRP. Occurrence rates of elevated TG and elevated conventional CRP in high–highest hs-CRP were higher than in low–moderate hs-CRP | 6 of 9 |
| Rocha-Pereira et al.89 | 2004 | Patients with psoriasis had higher levels of inflammatory markers including fibrinogen, ESR, and CRP. Patients with severe psoriasis had higher levels than those with mild disease | 3 of 9 |
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Cakmak et al.30 | 2009 | Psoriasis patients had no significant differences in total homocysteine, folate, or vitamin B12 | 7 of 11 |
| Coimbra et al.96 | 2009 | Patients with psoriasis had higher cholesterol, TG, Lp(a), oxLDL and CRP | 7 of 11 |
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Biyik et al.33 | 2006 | Patients with psoriasis had higher systolic BP and diastolic BP, as well as left ventricular hypertrophy, wall motion abnormalities, and diastolic dysfunction | 7 of 9 |
| Also see: Cohen (Hyperlipidemia, 60), Kay (Hyperlipidemia, 16), Wu (Hyperlipidemia, 42) | |||
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Qureshi et al.34 | 2009 | Women with psoriasis were at increased relative risk for development of diabetes and hypertension even after multivariate analysis controlling for other factors | 8 of 9 |
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Cohen et al.55 | 2008 | Multivariate models adjusting for age, gender, and smoking status of the patients demonstrated that psoriasis was associated with the metabolic syndrome, ischemic heart disease, DM, hypertension, and obesity | 6 of 11 |
| Gerdes et al.35 | 2008 | Patients with psoriasis had higher intake of multiple medications based on National Health survey, including ARBs, diuretics, ACE inhibitors, antidiabetic agents, lipid-reducing agents, and calcium channel blockers. However, there was no increase in the intake of beta-blockers and other heart medications | 5 of 11 |
| Gladman et al.73 | 2009 | In patients with psoriatic arthritis compared to controls from the Canadian Community Health Survey, the standardized prevalence ratios for MI, angina, and hypertension were increased, whereas the SPRs for CHF and CVA were not | 6 of 11 |
| Sommer et al.54 | 2006 | Psoriatics had higher prevalence of metabolic syndrome, DM, HTN, hyperlipoproteinemia, coronary heart disease, moderate, regular, and heavy alcohol consumption, and smoking | 6 of 11 |
| Also see: Han (Heart Disease, 25), Naldi (Hyperlipidemia, 90), Neimann (Hyperlipidemia, 27), Prodanovich (Effects on Mortality, 83), Tam (Hyperlipidemia, 91), Xiao (Heart Disease, 80), Henseler (Obesity, 108) | |||
| Diabetes Mellitus | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Brauchli et al.38 | 2008 | Patients with psoriasis had an increase in HTN, hyperlipidemia, and obesity compared with controls. Multivariate models adjusting for obesity, smoking, HTN, and hyperlipidemia demonstrated increased risk for developing DM in patients with psoriasis | 8 of 9 |
| Also see: Cohen (Hyperlipidemia, 60), Kay (Hyperlipidemia, 16), Wu (Hyperlipidemia, 42) | |||
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Boehncke et al.90 | 2007 | The intima–media thickness of carotid artery was measured by ultrasound, and an oral glucose tolerance test (OGTT) was performed to calculate the homeostasis model assessment of insulin resistance (HOMA). Correlations were confirmed between BMI and HOMA as well as BMI and vessel wall thickness. Also, there was a correlation between PASI score and insulin secretion in the OGTT | 4 of 9 |
| Also see: Cohen (Hyperlipidemia, 60), Kay (Hyperlipidemia, 16), Wu (Hyperlipidemia, 42) | |||
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Cohen et al.91 | 2008 | Diabetes was diagnosed in 13.8% of cases compared with 5.4% of controls . The age-adjusted OR of DM amongst psoriatic patients >20 years was similar between men and women. A multivariate model demonstrated that psoriasis was associated with diabetes, even after controlling for age and gender | 5 of 11 |
| Reeds et al.37 | 1964 | Approximately 25% of psoriatic patients studied had diabetes, with chances of this occurring by chance less than 1 in 2000. Many patients had a family history of diabetes and psoriasis | 7 of 11 |
| Also see: Han (Heart Disease, 25), Kimball (Hyperlipidemia, 26), Naldi (Hyperlipidemia, 90), Neimann (Hyperlipidemia, 27), Prodanovich (Effects on Mortality, 83), Reynoso-von Drateln (Hyperlipidemia, 19), Shapiro (Atherosclerosis, 9), Sommer (HTN, 58), Henseler (Obesity, 108) | |||
| Obesity | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Cerman et al.43 | 2008 | Serum leptin levels, tissue leptin and leptin receptor expression were higher in patients with severe psoriasis than patients with mild-moderate psoriasis and controls . Serum leptin levels showed a positive correlation with PASI. In addition, serum leptin levels, tissue leptin and leptin receptor expression showed a positive correlation with disease duration | 7 of 9 |
| Chen et al.44 | 2008 | Obesity and HTN were observed more often in cases than controls. Psoriasis was independent risk factor for hyperleptinemia after multivariate analysis | 7 of 9 |
| Jin et al.45 | 2008 | Compared to those with waist-hip ratio (WHR) ≤ 0.80, those with WHR 0.80-0.85 and >0.85 have greater associations with psoriasis | 7 of 9 |
| Johnston et al.42 | 2008 | Serum leptin and soluble leptin receptor levels were not elevated. However, resistin, IL-1β, IL-6, CCL2, CXCL8 and CXCL9 were elevated in patients and serum resistin correlated with disease severity. Improvement after UVB treatment was accompanied by decreased serum CXCL8. In vitro, leptin and resistin could induce CXCL8 and TNF-α production by blood monocytes, and leptin also induced IL-1β and IL-1ra production | 8 of 9 |
| Kaur et al.46 | 2008 | Adiponectin level in normal-weight psoriasis patients was more than twice the level in healthy normal-weight controls. Overweight psoriasis patients were characterized by an increased IL-6 level, which correlated negatively with adiponectin. Glutathione redox status, inversely correlated with adiponectin and had increased oxidative stress in the overweight cases | 3 of 9 |
| McGowan et al.47 | 2008 | Using two healthcare databases, there was a trend toward higher BMI in patients with psoriasis | 6 of 9 |
| Murray et al.48 | 2008 | A positive association between psoriasis severity and BMI was found. Physician’s global assessment score increased with BMI. There was also a positive correlation between BMI and BSA% | 5 of 9 |
| Naldi et al.49 | 2005 | The risk of psoriasis was higher in ex- and current smokers than in never-smokers. Frequency of psoriasis varied in relation to a family history of psoriasis in first degree relatives, BMI 26–29, and BMI ≥ 30, and stressful life event score | 6 of 9 |
| Wang et al.50 | 2008 | The serum leptin levels in male and female psoriatic patients were higher than those in male and female controls . The serum leptin levels in patients with psoriasis showed positive correlation with BMI, but not with age | 5 of 9 |
| Wolk et al.41 | 2009 | Obesity (BMI ≥ 30 kg/m2) was found to have increased OR for onset of plaque psoriasis. Further, one unit increment in BMI was associated with a 9% higher risk of disease). Disease activity of plaque psoriasis as measured by PASI was positively associated with increased body weight. Current (regular or occasional) smokers had an increased risk of psoriasis compared with never smokers | 6 of 9 |
| Wolkenstein et al.62 | 2009 | In multivariate analysis, higher BMI, current and former smoking habits and beta-blocker intake were independently associated with a higher risk of psoriasis; intake of statins was associated with a decreased risk | 5 of 9 |
| Also see: Kaye (Hyperlipidemia, 16) | |||
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Henseler et al.101 | 1995 | Patients with psoriasis had increased observed incidence rate of systemic disorders as compared with expected rates, including obesity, hypertension, heart failure, and DM) | 6 of 11 |
| Herron et al.40 | 2008 | Prevalence of obesity in patients within Utah Psoriasis Initiative was higher than controls. Prevalence of smoking was higher than general Utah population and the non-psoriatic population. Specifically, the prevalence for obesity and smoking were higher than controls; the prevalence of obese smokers was also found to be higher in these psoriasis patients | 8 of 11 |
| Setty et al.52 | 2007 | Multivariate relative risk of psoriasis was 1.40 for a BMI of 25.0-29.9, 1.48 a BMI of 30.0-34.9, and 2.69 for a BMI ≥ 35.0. For BMI at age of 18 years, the multivariate RR for top BMI category (≥30.0) was 1.73 and that for a lower BMI category (<21.0) was 0.76. Weight gain from the age of 18 years, higher waist circumference, hip circumference, and waist-hip ratio were all associated with a higher risk of incident psoriasis | 6 of 11 |
| Also see: Neimann (Hyperlipidemia, 27) | |||
| Metabolic Syndrome | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Gisondi et al.51 | 2007 | Metabolic syndrome was more common in psoriatics than in controls after the age of 40 years. They also had a higher prevalence of hypertriglyceridemia and abdominal obesity, whereas hyperglycemia, HTN, and HDL levels were similar. Although psoriasis patients were more frequently smokers, the association of psoriasis with metabolic syndrome was independent from smoking | 7 of 9 |
| Also see: Cohen (Hyperlipidemia, 60) | |||
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Bongiorno et al.57 | 2009 | Psoriatic patients had higher average BMI than controls. Overweight and obesity occurred more often in psoriatics. Metabolic syndrome was more common in patients with psoriasis compared with controls. The associations with DM, HTN and dyslipidemia were also confirmed | 6 of 11 |
| Also see: Cohen (HTN, 59), Sommer (HTN, 58) | |||
| Smoking | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Huerta et al.58 | 2007 | Smoking was found to be an independent risk factor for psoriasis. There was no association between risk of psoriasis and antecedents of stress, DM, HTN, dyslipidemia, heart disease, or RA | 8 of 9 |
| Mills et al.60 | 1992 | There was an association between psoriasis, current smoking status and smoking habits prior to onset of disease. There was marked dose-response relationship; the risk of psoriasis in those smoking >20 cigarettes/day was markedly elevated | 7 of 9 |
| Naldi et al.61 | 1992 | Family history was a risk factor for psoriasis with an adjusted OR of 18.8 for a history in parents and 3.2 for a history in siblings. Risk of psoriasis was higher for current smokers than for those who had never smoked. The adjusted OR was 2.1 for people smoking ≥ 15 cigarettes/day. Also, there was a trend, though not significant, for increased risk of psoriasis in alcohol drinkers | 7 of 9 |
| Miot et al.95 | 2009 | Prevalence of ever smoking was higher those with palmoplantar pustulosis (PPP) than psoriasis vulgaris and other dermatologic patients (NPD). Adjusted OR of PPP ever smoking compared to PV and NPD was 9.5 and 36.2, respectively | 5 of 9 |
| Poikolainen et al.100 | 1994 | Before onset of the skin disease, the recalled mean number of cigarettes smoked daily was higher in psoriatics than for controls. In logistic regression analysis, psoriasis was associated with smoking, but not with alcohol intake, marital status, or social group. The OR for development of psoriasis was 3.3 for patients smoking ≥ 20 cigarettes/day compared to nonsmokers. After onset of disease, psoriasis was associated significantly with alcohol intake, smoking, and the occurrence of negative life events | 7 of 9 |
| Also see: Naldi (Obesity, 53), Naldi (Smoking, 65), Wolk (Obesity, 44), Jankovic (Alcohol Consumption, 72), Wolkenstein (Obesity, 66) | |||
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Setty et al.64 | 2007 | Compared with never smokers, multivariate RR of psoriasis was 1.78 for current smokers and 1.37 for past smokers. Compared with nonsmokers, multivariate RR of psoriasis was 1.60 for those who had smoked 11–20 pack-years for ≥ 21 pack-years. Compared with never smokers, multivariate RR of psoriasis was 1.61 for those who quit <10 years ago, 1.31 for those who quit for 10–19 years ago, and similar in those that quit ≥ 20 years ago | 4 of 9 |
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Davidsson et al.63 | 2005 | Patients admitted to dermatologic wards smoked more cigarettes and drank less wine than the other two groups. The number of cigarettes and the use of tranquilizers, sleeping medications, and antidepressants showed small, but significant, positive correlations with psoriasis quality of life measures | 7 of 11 |
| Fortes et al.65 | 2005 | High intensity of smoking (>20 cigarettes/day) vs. a lower level of consumption (≤10 cigarettes/day) was associated with a more than 2-fold increased risk of more clinically severe psoriasis. Cigarette-years, measured as the product of the intensity and duration (years) of smoking, increased the risk of clinically more severe psoriasis after adjustment for confounding factors | 5 of 11 |
| Jin et al.66 | 2009 | Risk of psoriasis was related to smoking with adjusted OR of 2.07 and a graded positive association between risk of psoriasis and intensity/duration of smoking. Individuals harboring HLA-Cw6 gene and HLA-57 had increased risk of psoriasis with OR 9.93 and 2.41, respectively. A striking finding is that the risk of psoriasis for smokers with HLA-Cw6 increased about 11-fold than non-smokers without HLA-Cw6 | |
| Zhang et al.67 | 2002 | Proportion of male psoriasis patients using tobacco and alcohol was much higher than that of controls, whereas no difference was found in the females. Heavy smokers were more likely to have severe psoriasis. | 5 of 11 |
| Also see: Herron (Obesity, 43), Neimann (Hyperlipidemia, 27), Sommer (HTN, 58) | |||
| Alcohol Consumption | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Jankovic et al.68 | 2009 | Risk of psoriasis is higher in those who were divorced or exposed to environmental tobacco smoke at home. Alcohol consumption and family history of psoriasis are also risk factors for psoriasis. The effect of environmental tobacco smoke at home was found only in women | 6 of 9 |
| Naldi et al.59 | 1999 | Risk for psoriasis was higher in ex-smokers and in current smokers than those who never smoked. Smoking was associated with pustular lesions with adjusted OR 10.5 for those smoking more than 15 cigarettes per day. No significant overall association with alcohol consumption was noted after controlling for smoking habits | 8 of 9 |
| Poikolainen et al.99 | 1990 | Recalled mean alcohol intake before onset of skin diseases was higher among patients with psoriasis than among controls. In logistic regression analysis, psoriasis was associated with alcohol intake but not with coffee consumption, smoking, age, marital state, or social group. For an alcohol intake of 100 g/day compared with no intake, the OR for psoriasis was 2.2. During 12 months before examination, mean daily alcohol intake of patients with psoriasis was higher than in controls | 6 of 9 |
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Poikolainen et al.70 | 1999 | The all-cause standardized mortality ratio (SMR) was 1.62 for men and 1.54 for women. Among men, highest SMRs were found for alcohol psychosis and liver disease. Among women, highest SMR was for liver disease. Excess mortality was high in men and women for all causes of death directly related to alcohol | 5 of 9 |
| Cross-sectional studies | CSQ | ||
| Also see: Sommer (HTN, 58), Zhang (Smoking, 71), Poikolainen (Smoking, 107) | |||
| Abbreviations: CSQ, Cross-sectional Study Quality; NOS, Newcastle–Ottawa Score | |||
Hyperlipidemia Nineteen of 19 studies (8 case-control, 11 cross-sectional) found an association between hyperlipidemia and psoriasis (Table 1). High quality data indicate that psoriasis is associated with an abnormal lipid profile and imbalance of oxidants and antioxidants.15 Patients with a recent diagnosis of psoriasis had higher rates of new onset hyperlipidemia than controls.16 Study subjects had higher levels of very low density lipoproteins,17 total cholesterol and LDL,18 and lower levels of cardioprotective high density lipoproteins (HDL).19 Psoriatic patients also had elevated levels of lipoprotein (a) after controlling for hyperlipidemia.21 Overall, the preponderance of data supports this association with dyslipidemia.22–27
Hyperhomocysteinemia/Elevated C-Reactive Protein (CRP) Six of seven studies evaluating homocysteine or CRP (6 case-control, 1 cohort) linked elevated levels with psoriasis (Table 1). Mean plasma homocysteine levels were significantly higher than sex and age matched controls.29 While Cakmak et al.30 did not observe significant differences in homocysteine, they found that homocysteine levels correlated with Psoriasis Areas and Severity Index (PASI) in the psoriasis group. It is important to note that homocysteine may be a surrogate marker of CVD rather than an etiologic factor, as recent studies of pharmacotherapy to lower homocysteine levels have not impacted vascular outcomes.High-sensitivity CRP (hs-CRP) has proven to be a useful adjunct for global risk assessment in primary prevention of CVD.31 In one report, nearly 30% of patients with psoriasis had high levels of hs-CRP (above 0.20 mg/dL), whereas 95% of controls had low levels of hs-CRP (less than 0.069 mg/dL). 32 Those psoriatics with high levels have rates of myocardial infarction (MI) that are more than twice that of patients with psoriasis with low hs-CRP, suggesting that CRP has prognostic potential for acute MI in those with psoriasis.31,103
Hypertension Fourteen of 16 studies (5 case-control, 11 cross-sectional) found higher rates of HTN among affected cases than among controls (Table 1). Disease duration of psoriasis was associated with increased systolic and diastolic pressures, as well as left ventricular dysfunction.33 Other studies, many of lesser quality, have substantiated this relationship. In a study of 1,200 hospitalized patients with psoriasis, authors demonstrated an independent association with therapeutically-treated HTN and DM.35 They also found an increase in age and sex-adjusted risk for DM, HTN, and dyslipidemia compared to a control population. Importantly, patients may have HTN related to treatment with cyclosporine (CsA),36 but no studies attempted to clarify this issue.
Insulin Resistance and Diabetes Mellitus, Type 2 Fourteen of 17 studies (4 case-control, 1 cohort, 12 cross-sectional) found a relationship between insulin resistance, DM, or both, and psoriasis (Table 1). An early study identified a higher prevalence of DM among patients with psoriasis and found higher family history of both diseases together than expected from the general population.37 Using a database of nearly 40,000 psoriasis patients, there was an increased incidence rate of DM after controlling for age, sex, smoking, hypertension, and dyslipidemia.38 Similarly, this increased risk for DM persisted after controlling for age, BMI and smoking in another study.34Of the remaining lesser quality studies, some used control groups which did not meet proper standards of comparison and had poorly matched controls. Others failed to assess the independent role of DM in relation to psoriasis. No studies considered the potential for impact of local or systemic corticosteroid treatment on rates of insulin resistance and DM.
Obesity Seventeen of 19 studies (14 case-control, 5 cross-sectional) identified a relationship between psoriasis and obesity (Table 1). For example, in a cohort of 550 psoriasis patients, BMI was significantly higher than that for age and sex-matched controls from the same area.40 The median body image score of normal weight at disease onset transitioned to a perception of overweight at time of enrollment into the study, suggesting obesity follows onset of disease. Interestingly, obesity was not only a risk factor for onset of psoriasis, but also increased PASI of plaque psoriasis at onset of disease. 41 Thus, the relationship between psoriasis and obesity seems to be quite complicated. Patients with the HLA-Cw6 haplotype have an increased risk for the development of psoriasis, and this risk of psoriasis is enhanced by modifiable factors such as increased BMI and elevated waist-to-hip ratios, which illustrates the complex interplay between genetic predisposition and behavior.45 This relationship with obesity is well-described.46–52
Metabolic Syndrome Five of five studies (2 case-control, 3 cross-sectional) found a relationship between MBS and psoriasis (Table 1). Each publication established cohort and comparison groups with minimal levels of evident bias. MBS represents the clustering of central obesity, dyslipidemia, HTN, a prothrombotic state, and glucose intolerance, and is a strong predictor of CVD, DM, and stroke.53 MBS may confer cardiovascular risk higher than the sum of its components, but this remains controversial. Two well-designed studies of hospital-based adults with plaque-type psoriasis found higher rates of MBS beginning at middle age (40 to 49 years) and persisting throughout life.51,54 In a study evaluating nearly 17,000 patients with psoriasis, the vast majority had components of MBS, and they had significantly higher prevalence of DM, HTN, and obesity as compared with controls.55 Moreover, MBS was much more common in patients with psoriasis as compared to controls.57
Smoking Twenty of 21 studies (11 case-control, 1 cohort, 8 cross-sectional) identified a role of smoking in development and/or severity of psoriasis (Table 1), making this the best studied behavioral-driven CVD risk factor associated with psoriasis.High quality publications found smoking to be an independent risk factor for psoriasis, with risk of psoriasis higher in ex-smokers and current smokers than never smokers.58,59 A marked dose-response relationship exists; the risk for development of psoriasis is highest for people smoking more than 20 cigarettes/day.60 A similar dose-response relationship was confirmed for those smoking more than 15 cigarettes/day.61 Lesser quality studies provide support for the hypothesis that tobacco use is associated with psoriasis, but fail to include appropriately matched comparison groups.63–68
Alcohol Consumption Four of seven studies (4 case-control, 1 cohort, 2 cross-sectional) that investigated the role of alcohol consumption identified a significant relationship with psoriasis (Table 1). In general, there is a J-shaped relationship between alcohol intake and deleterious health outcomes such as HTN, stroke, and CAD, wherein small amounts may be protective and large amounts dangerous.69 In one lesser quality review of over 6,000 hospitalized patients with psoriasis, the majority of deaths were alcohol-related.70 The study was limited by lack of adequate comparison groups and exclusion of patients with other co-morbidities associated with increased alcohol intake. Patients with psoriasis had higher rates of alcohol consumption than controls.68 None of these studies were high quality, making this behavior-driven risk factor an area of interest for additional research.
CVD Types Associated with Psoriasis
Table 2 outlines studies of atherosclerosis (subclinical CVD) and CVD types (heart disease, peripheral vascular disease (PVD), and cerebrovascular disease) in patients with psoriasis.
Table 2.
Studies of Vascular Disease Types Associated with Psoriasis
| Atherosclerosis | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Balci et al.12 | 2009 | Mean intimal medial thickness of right, left, and averaged common carotid artery of psoriasis patients were higher than controls | 6 of 9 |
| Gonzalez-Juanatey et al.10 | 2007 | Flow-mediated endothelial dependent vasodilatation by brachial ultrasonography was impaired in patients compared with controls. A significant correlation between CRP and ESR at time of disease diagnosis and FMD% was found | 8 of 9 |
| Gonzalez-Juanatey et al.11 | 2007 | Patients with PsA exhibited greater carotid artery IMT than matched controls. Adjusted for age, carotid IMT was correlated with age at time of PsA diagnosis, disease duration, total cholesterol, and low-density lipoprotein cholesterol | 8 of 9 |
| Kimhi et al.13 | 2007 | Average IMT for PsA patients was higher compared with controls for the whole group and after adjustment for age, gender, BMI, hypertension, and hyperlipidemia. Average IMT correlated with age, BMI, duration of skin and joint disease, spine involvement, ESR, and fibrinogen | 5 of 9 |
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Shapiro et al.9 | 2006 | The age-adjusted proportion of diabetes was higher in psoriasis patients as compared with the control group. The age-adjusted proportion of atherosclerosis was higher in psoriasis patients as compared with the control group | 7 of 11 |
| Yalcin et al.14 | 2009 | In the myocardial perfusion SPECT, normal coronary perfusion pattern was detected in all patients with psoriasis. All patients reached target heart rate without ST changes | 6 of 11 |
| Also see: Prodanovich (Effects on Mortality, 83) | |||
| Heart Disease | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| McDonald et al.71 | 1978 | In each age/sex group of patients with psoriasis, the rates of occurrence of occlusive vascular disease were higher in predisposed psoriatic patients than in predisposed non-psoriatic patients | 6 of 9 |
| Gonzalez-Juanatey et al.78 | 2006 | In PsA patients, frequency of aortic and tricuspid and mitral regurgitation was not different than matched controls. Also, pulmonary artery systolic pressure was normal in PsA patients. Prevalence of diastolic dysfunction was similar in PsA patients and controls | 8 of 9 |
| Ludwig et al.77 | 2007 | Patients with psoriasis had an increased prevalence and severity of coronary artery calcification (CAC) as compared with controls. Multiple linear regression analysis identified psoriasis as likely independent risk factor for CAC | 8 of 9 |
| Also see: Biyik (HTN, 36), Cohen (Hyperlipidemia, 60) | |||
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Brauchli et al.74 | 2009 | Overall, crude incidence rates of MI, stroke, and TIA were similar among patients with or without psoriasis. However, adjusted OR of developing MI for patients with psoriasis aged < 60 years was elevated compared with patients without psoriasis, while OR for patients aged > or = 60 years was not | 8 of 9 |
| Gelfand et al.72 | 2006 | The incidence of myocardial infarction per 1000 person-years was increased in patients with mild and severe psoriasis, both in young and older populations, though the risk in older populations was less | 8 of 9 |
| Lindegard et al.102 | 1986 | Psoriasis cases are associated with a spectrum of diseases: male as well as female psoriatics seem to show excess rates of viral infections, alcoholism, hypertension, pneumonia, liver cirrhosis, urticaria, and rheumatoid arthritis. Psoriasis in males only seem to be associated with iritis and ankylosing spondylitis, whereas psoriasis in females only is associated with lung cancer, diabetes, obesity, myocardial infarction and asthma | 5 of 9 |
| Markuszeski et al.92 | 2006 | Heart rate was significantly higher both during the day and at night in patients with psoriasis vulgaris than in the control group. There was a positive correlation between the increased heat rate, both during the day and at night, in psoriatic patients and severity of the disease expressed as PASI. Single supraventricular beats were significantly more frequently observed in psoriatic patients vs. the control group | 5 of 9 |
| Wakkee et al.75 | 2009 | Psoriasis patients and controls had similar rates of ischemic heart disease per 100,000 person-years. The age- and gender-adjusted risk of IHD was comparable between both cohorts. Before cohort entry, psoriasis patients used more antihypertensive, antidiabetic, and lipid-lowering drugs and were more often hospitalized. Adjusting for these confounders decreased HR for IHD, but it remained comparable | 7 of 9 |
| Also see: Kaye (Hyperlipidemia, 16) | |||
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Han et al.25 | 2006 | PsA patients had higher prevalence ratio of IHD, atherosclerosis, PVD, CHF, cerebrovascular disease, DM, hyperlipidemia, and HTN | 5 of 11 |
| Xiao et al.76 | 2009 | Patients with mild psoriasis had increased rates of cardiovascular risk factors, including obesity), DM, HTN, hyperlipidemia and smoking. Patients with severe psoriasis had higher adjusted OR of obesity, DM, HTN, hyperlipidemia, and smoking than mild psoriasis and controls. After adjusting for systemic therapies, cardiovascular risk factors, age, and sex, for patients with mild and severe psoriasis, the OR of having an MI was 1.72 and 2.01 (95%, respectively | 8 of 11 |
| Also see: Kimball (Hyperlipidemia, 26), Prodanovich (Effects on Mortality, 83), Sommer (HTN, 58), Cohen (HTN, 59), Gladman (HTN, 77) | |||
| Peripheral Vascular Disease | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Also see: Kaye (Hyperlipidemia, 16) | |||
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Also see: Han (Heart Disease, 25), Kimball (Hyperlipidemia, 26), Prodanovich (Effects on Mortality, 83) | |||
| Cerebrovascular Disease | |||
| Case-control Studies | Year | Clinical variables/outcomes | NOS |
| Also see: Kaye (Hyperlipidemia, 16), Brauchli (Heart Disease, 78) | |||
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Gelfand et al.80 | 2009 | When adjusting for major risk factors for stroke, both mild and severe psoriasis were independent risk factors for stroke. | 8 of 9 |
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Also see: Han (Heart Disease, 25), Prodanovich (Effects on Mortality, 83) | |||
| Abbreviations: CSQ, Cross-sectional Study Quality; NOS, Newcastle–Ottawa Score | |||
Atherosclerosis Six of seven studies (4 case-control, 3 cross-sectional) reported an association between atherosclerosis and psoriasis (Table 2).
Overall, the quality of studies linking psoriasis and atherosclerosis was high. An Israeli retrospective study reported the age-adjusted proportion of atherosclerosis, as well as DM, to be significantly higher in patients with psoriasis.9 Two studies assessed sub-clinical markers of CVD in patients with psoriasis and psoriatic arthritis.10,11 These studies showed an increase in early atherosclerotic disease and endothelial dysfunction as measured by sonographic markers (carotid artery intima-media thickness and flow-mediated vascular dilation) as compared to healthy age, sex, ethnicity, and BMI-matched controls .
Studies with lower quality designs propose that psoriasis is an independent risk factor for subclinical atherosclerosis. 12-
Heart Disease Twelve of 16 studies (5 case-control, 6 cohort, 5 cross-sectional) demonstrated a relationship between psoriasis and heart disease (Table 2), making this the best-studied vascular disease type. Initial work suggested increased rates of occlusive vascular disease in patients with psoriasis, particularly those with predisposing risk factors.71 Recently, Gelfand et al.72 reported that psoriasis patients had an increased adjusted relative risk for MI that is greatest in young patients with severe psoriasis, attenuates with age, and remains elevated after controlling for age, sex, DM, HTN, hyperlipidemia and smoking. High quality studies validate the prevalence of CVD morbidities in this population, establishing increased standard prevalence ratios for MI, angina, and HTN.73 A recent inception cohort study with nested case-control analysis identified an increased risk of incident MI in patients less than 60 years old with severe disease, but failed to detect increases in strokes or TIA.74 Similarly, in the Netherlands, patients with psoriasis did not have increased hospitalizations from ischemic heart disease.75 To the contrary, in China, there was a higher odds ratio for the development of MI for mild and severe psoriasis, even after adjusting for age, sex, DM, HTN, hyperlipidemia, smoking and systemic therapies.76 While it appears there is an increased risk of incident MI and heart disease in certain subsets of patients, there is a significant gap in research evidence which future prospective study may bridge.
Peripheral Vascular Disease and Cerebrovascular Disease Three of four studies (1 case-control, 3 cross-sectional) evaluating PVD and psoriasis and three of five (2 case-control, 1 cohort, 2 cross-sectional) for cerebrovascular disease and psoriasis reported significant relationships (Table 2). Of these studies, only two were considered high quality, representing a critical need for research. In a study of over 3,000 psoriasis patients from the Veteran’s Administration Hospital database, investigators found higher prevalence rates of ischemic heart disease, stroke and PVD, after controlling for age, sex, DM, HTN, dyslipidemia, and smoking.79 Recent high quality work revealed an increased risk of stroke for mild and severe psoriasis.80 In particular, severe psoriasis confers an increased risk of stroke not explained by major risk factors alone.80 This increased risk of PVD and CVD was confirmed by another study, but analysis did not control for other risk factors.25 While these studies suggest an increased risk for vascular disease, further prospective trials should aim to delineate the possibility of a causal relationship.
Impact of Psoriasis on CVD and Total Mortality
While psoriasis is highly associated with CVD risk factors, CVD, and other vascular diseases, only a few high quality studies have examined the effect on mortality. The majority of studies which aim to investigate mortality fail to adequately select for control groups, or solely investigate the hospitalized patient which limits generalizability. Table 3 highlights data regarding co-morbidities associated with CVD risk factor and the increase in mortality.
Table 3.
Studies of The Effect of Cardiovascular Disease Outcomes on Mortality
| Mortality | |||
|---|---|---|---|
| Cohort Studies | Year | Clinical variables/outcomes | NOS |
| Gelfand et al.82 | 2007 | There was no overall effect of mild psoriasis on mortality, whereas patients with severe psoriasis demonstrated increased overall mortality risk. The association of severe psoriasis with mortality persisted after adjustment for risk factors for mortality and after exclusion of patients with inflammatory arthropathy. Male and female patients with severe psoriasis died 3.5 and 4.4 years younger than patients without psoriasis | 7 of 9 |
| Mallbris et al.83 | 2004 | Using the Swedish Inpatient Registry, there appeared to be no increased cardiovascular mortality among outpatients with psoriasis. Overall risk among inpatients admitted at least once was increased by 50%. Cardiovascular mortality was higher among those admitted at younger ages, specifically for patients aged 20 to 39 years at first admission | 7 of 9 |
| Shbeeb et al.93 | 2000 | Based upon a cohort of patients drawn from community database with psoriatic arthritis, survival was not significantly different from the general population. However, their analysis may exclude patients with severe arthritis who were seen in the hospital setting | 5 of 9 |
| Stern et al.81 | 1988 | Using the Psoralen and UV-A follow-up study, the incidence of death and causes of death were comparable to those expected in the general population. There was no increase in cardiovascular mortality observed, but they found that cirrhosis caused more deaths among their cohort than in the general population) | 7 of 9 |
| Wong et al.84 | 1997 | The 4 leading causes of death observed in patients with PsA were diseases of the circulatory or respiratory systems, malignant neoplasms, , and injuries/poisoning. Specifically, 27.6% of patients died from MIs, 4.3% from CVAs, and 4.3% from congestive heart failure and arteriosclerosis. The SMR for the female cohort was 1.59 and the men 1.65. However, deaths from circulatory diseases did not appear to differ significantly from those expected in the general population | 7 of 9 |
| Also see: Poikolainen (Alcohol Consumption, 74) | |||
| Cross-sectional Studies | Year | Clinical variables/outcomes | CSQ |
| Gladman et al. 94 | 1998 | Patients with psoriatic arthritis had an increased risk of death, after adjustment for age and sex, compared with the general population of Ontario. The combined standardized mortality ratio (SMR) for both men and women was estimated to be 1.62, with an associated confidence interval of 1.21-2.12. For women, the SMR was 1.59, and for men, it was 1.65, indicating a 59% and 66% increase in the death rate among female and male patients with PsA, respectively. | 5 of 11 |
| Prodanovich et al.79 | 2009 | After controlling for risk factors, patients with psoriasis had a higher prevalence of ischemic heart disease, cerebrovascular, and peripheral vascular disease . Psoriasis was also found to be an independent risk factor for mortality | 9 of 11 |
| Abbreviations: CSQ, Cross-sectional Study Quality; NOS, Newcastle–Ottawa Score | |||
Eight studies (6 cohort, 2 cross-sectional) investigated the effects of psoriasis on mortality (Table 3). Early evidence stimulated research interest in this apparent CVD risk and possible increase in mortality.71 Using the PUVA (Psoralen + UV-A) Follow-Up Study, the incidence of and causes of death were comparable to the general population. 81 They found no increase in CVD mortality. Severe psoriasis, defined by use of systemic medications,82 hospitalization at an early age, or repeated hospitalization,83 has been shown by two high quality cohort studies to increase the risk of death. These patients with severe disease have increased mortality, which persisted after adjustment for DM, HTN, BMI, and smoking and after exclusion of patients with inflammatory arthropathy.82 In contrast, mild psoriasis had no effect. In a multivariate analysis, psoriasis was an independent risk factor for all-cause mortality, even after controlling for CVD risk factors and vascular disease.79 Moreover, there are increased standardized mortality ratios for men and women with PsA.84
Comment
The preponderance of available evidence confirms that CVD risk factors such as hyperlipidemia, hyperhomocysteinemia, elevated CRP, HTN, DM, obesity, MBS, and smoking are more prevalent among patients with psoriasis than in the general population. Emerging data indicate increased rates of heart disease independent of traditional CVD risk factors; however, data are limited for cerebrovascular and peripheral vascular disease. It remains a challenge to properly adjust for confounding factors and independently assess the role of a particular CVD risk factor or disease type, as many risk factors are common to both patients with psoriasis and with CVD. Specifically, smoking, alcohol, and obesity are relevant risk factors that are associated with increased risk of both psoriasis and CVD. Residual confounding is likely, whereby a true causal link between psoriasis and vascular disease cannot be clearly defined. High quality studies perform multivariate analysis to adjust for these factors, but the overall data quality is limited by heterogeneity. Retrospective studies based upon expansive healthcare databases have large sample sizes, but the majority of prospective investigations are limited by smaller numbers of patients. This again highlights the great need for larger prospective collection of clinical data.
Along with this increase in vascular disease, patients with psoriasis appear to have an increase in mortality. It seems plausible that these vascular diseases may be a significant contributor to this increase, but data are insufficient data to confirm this. The majority of studies that report mortality rates are biased by restriction to hospitalized patients, thus limiting generalizability. Several high quality studies did report higher mortality in younger patients with more severe psoriasis. However, the determination of disease severity relied upon systemic treatments and/or hospitalizations rather than PASI or PGA scores. There does appear to be an overall trend towards an increase in mortality.
It is possible that systemic medications used in the treatment of psoriasis may worsen some cardiovascular risk factors, and could be one mechanism for the observed increase in CVD. Of course, it is also plausible that these same systemic medications may reduce systemic inflammation in and improve comorbidities. The available data do not allow a determination of the impact of systemic therapy on CVD rates.
Based on the available data, it appears that patients with psoriasis have an increased risk for the development of CVD risk factors. At the next level, that of development of vascular disease, there remains much controversy. Psoriasis has significant associations with coronary artery, peripheral vascular and cerebrovascular disease in large cross-sectional studies. Yet, these epidemiologic associations do not yet confirm causal relationship.
We suggest a paradigm shift to focus on psoriasis as a systemic inflammatory disease. Much like rheumatoid arthritis104 and systemic lupus erythematosus105, patients with psoriasis and psoriatic arthritis have increased levels of interleukins and proinflammatory cytokines that may be a pathophysiologic link to atherosclerosis and vascular disease.106 While this inflammatory milieu is a plausible basis for these associations, it remains possible that other, yet undiscovered, explanations may exist. At a minimum, these observations suggest that clinicians should be vigilant about identifying and treating CVD risk factors in this population.
This observational research provides important insights into caring for patients with psoriasis. Clinical screening and treatment guidelines are needed to advise the dermatologist, cardiologist, internist, and patient on the optimal identification and management of CVD risk factors in patients with psoriasis. Future research should study the extent to which systemic treatments may improve CVD outcomes and mortality in this population.
Acknowledgments
Author Contributions Drs Patel, Shelling, Prodanovich, Federman, and Kirsner had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest None disclosed.
Abbreviations
- AuAb-oxLDL
Autoantibodies against oxidized LDL
- BMI
Body Mass Index
- BP
Blood Pressure
- CAD
Coronary Artery Disease
- CCA
Common Carotid Arteries
- CCB
Calcium Channel Blockers
- CHF
Congestive Heart Failure
- CI
Confidence Interval
- CRP
C-Reactive Protein
- CSQ
Cross-sectional Study Quality
- CVD
Cardiovascular Disease
- DM
Diabetes Mellitus
- ESR
Erythrocyte Sedimentation Rate
- EtOH
Alcohol
- FMD
Flow Mediated Dilation
- GERD
Gastroesophageal Reflux Disease
- HDL
High Density Lipoprotein
- HOMA
Homeostasis Model Assessment of Insulin Resistance
- HR
Hazard Ratio
- Ht
Height
- HTN
Hypertension
- IHD
Ischemic Heart Disease
- IMT
Intima Media Thickness
- LDL
Low Density Lipoprotein
- Lp(a)
Lipoprotein A
- LV
Left Ventricle
- LVH
Left Ventricular Hypertrophy
- MBS
Metabolic Syndrome
- MI
Myocardial Infarction
- MTX
Methotrexate
- NBUVB
Narrow Band Ultraviolet B Therapy
- N-O
Newcastle–Ottawa Score
- NPF
National Psoriasis Foundation
- NWHS
National Health and Wellness Survey
- OGTT
Oral Glucose Tolerance Test
- OR
Odds Ratio
- Outpt
Outpatient
- PAI
Plasminogen activator inhibitor-1
- PASI
Psoriasis Area and Severity Index
- PCOS
Polycystic Ovary Syndrome
- PGA
Physicians’ Global Assessment
- PDI
Psoriasis Disability Index
- PLSI
Psoriasis Life Stress Inventory
- PPP
Palmoplantar Pustulosis
- PR
Prevalence Ratio
- PsA
Psoriatic Arthritis
- Pt
Patient
- PUVA
Psoralen and Ultraviolet A Therapy
- PV
Psoriasis Vulgaris
- PVD
Peripheral Vascular Disease
- RF
Risk Factor
- SMR
Standard Mortality Ratio
- SPR
Standard Prevalence Ratio
- TBSA
Total body surface area
- tcHy
Serum Total Homocysteine
- TG
Triglycerides
- TNF
Tumor Necrosis Factor
- tPA
Tissue plasminogen activator
- Tx
Treatment
- Wt
Weight
- XS
Excess
References
- 1.National Psoriasis Foundation. Psoriasis statistics. Available at www.psoriasis.org/about/stats. Accessed March 11, 2011
- 2.Esposito M, Saraceno R, Guinta A, Maccarone M, Chimenti S. An Italian study on psoriasis and depression. Dermatology. 2006;212(2):123–7. doi: 10.1159/000090652. [DOI] [PubMed] [Google Scholar]
- 3.Russo PA, Ilchef R, Cooper AJ. Psychiatric morbidity in psoriasis: a review. Australas J Dermatol. 2004;45(3):155–9. doi: 10.1111/j.1440-0960.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 4.Kimball AB, Jacobson C, Weiss C, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6(6):383–92. doi: 10.2165/00128071-200506060-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kremers H, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. J Am Acad Dermatol. 2007;57(2):347–54. doi: 10.1016/j.jaad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Svenungsson E, Jensen-Urstad K, Heimbürger M, Silveira A, Hamsten A, Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104(16):1887–93. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 7.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. 1997;24(3):445–51. [PubMed] [Google Scholar]
- 8.Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed March 11, 2011
- 9.Shapiro J, Cohen AD, David M, Hodak E, Chodik G, Viner A, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: A case-control study. J Am Acad Dermatol. 2007;56(4):629–34. doi: 10.1016/j.jaad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Juanatey C, Lorca J, Mirando-Filloy JA, Amigo-Diaz E, Testa A, Garcia-Porrua C, et al. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic arthrosclerosis risk factors. Arthritis Rheum. 2007;57(2):287–93. doi: 10.1002/art.22530. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Juanatey C, Lorca J, Amigo-Diaz E, Dierssen T, Javier M, Gonzalez-Gay M. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinical evident cardiovascular disease or classic arthrosclerosis risk factors. Arthritis Rheum. 2007;57(6):1074–80. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- 12.Balci D, Balci A, Karazincir S, Ucar E, Iyigun U, Yalcin F, et al. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol. 2009;23(1):1–6. doi: 10.1111/j.1468-3083.2008.02936.x. [DOI] [PubMed] [Google Scholar]
- 13.Kimhi O, Caspi D, Bornstein NM, Maharshak N, Gur A, Arbel Y, et al. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum. 2007;36(4):203–9. doi: 10.1016/j.semarthrit.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Yalcin H, Balci DD, Ucar E, Ozcelik N, Tasci C, Seyfeli E, et al. Myocardial perfusion is preserved in patients with psoriasis without clinically evident cardiovascular disease. J Eur Acad Dermatol Venereol. 2009;23(7):798–802. doi: 10.1111/j.1468-3083.2009.03178.x. [DOI] [PubMed] [Google Scholar]
- 15.Uyanik BS, Ari Z, Onur E, Gündüz K, Tanülkü S, Durkan K. Serum lipids and apolipoproteins in patients with psoriasis. Chin Chem Lab Med. 2002;40(1):65–8. doi: 10.1515/CCLM.2002.013. [DOI] [PubMed] [Google Scholar]
- 16.Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895–902. doi: 10.1111/j.1365-2133.2008.08707.x. [DOI] [PubMed] [Google Scholar]
- 17.Mallbris L, Granath F, Hamsten A, Ståhle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J Am Acad Dermatol. 2006;54(4):614–21. doi: 10.1016/j.jaad.2005.11.1079. [DOI] [PubMed] [Google Scholar]
- 18.Piskin S, Gurkok F, Ekuklu G, Senol M. Serum lipid levels in psoriasis. Yonsei Med J. 2003;44(1):24–6. doi: 10.3349/ymj.2003.44.1.24. [DOI] [PubMed] [Google Scholar]
- 19.Reynoso-von Drateln C, Martínez-Abundis E, Balcázar-Muñoz BR, Bustos-Saldaña R, González-Ortiz M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. J Am Acad Dermatol. 2003;48(6):822–5. doi: 10.1067/mjd.2003.446. [DOI] [PubMed] [Google Scholar]
- 20.Dreiher J, Weitzman D, Davidovici B, Shapiro J, Cohen AD. Psoriasis and dyslipidaemia: a population-based study. Acta Derm Venereol. 2008;88(6):561–5. doi: 10.2340/00015555-0510. [DOI] [PubMed] [Google Scholar]
- 21.Pietrzak A, Kadzielewski J, Janowski K, Roliński J, Krasowska D, Chodorowska G, et al. Lipoprotein (a) in patients with psoriasis: associations with lipid profiles and disease severity. Int J Dermatol. 2009;48(4):379–87. doi: 10.1111/j.1365-4632.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 22.Akhyani M, Ehsani AH, Robati RM, Robati AM. The lipid profile in psoriasis: a controlled study. J Eur Acad Dermatol Venereol. 2007;21(10):1330–2. doi: 10.1111/j.1468-3083.2007.02260.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones SM, Harris PD, Lloyd J, Stirling CA, Reckless JP, McHugh NJ. Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheum Dis. 2000;59(11):904–9. doi: 10.1136/ard.59.11.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dyslipidemia and oxidative stress in mild and severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. 2001;303(1–2):33–9. doi: 10.1016/s0009-8981(00)00358-2. [DOI] [PubMed] [Google Scholar]
- 25.Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–72. [PubMed] [Google Scholar]
- 26.Kimball AB, Robinson D, Wu Y, Guzzo C, Yeilding N, Paramore C, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001–2002. Dermatology. 2008;217(1):27–37. doi: 10.1159/000121333. [DOI] [PubMed] [Google Scholar]
- 27.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Vanizor Kural B, Orem A, Cimsit G, Uydu HA, Yanid YE, Alver A. Plasma homocysteine and its relationships with atherothrombotic markers in psoriatic patients. Clin Chim Acta. 2003;332(1–2):23–30. doi: 10.1016/s0009-8981(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 29.Malerba M, Gisondi P, Radaeli A, Sala R, Calzavara Pinton PG, Girlomoni G. Plasma homocysteine and folate levels in patients with chronic plaque type psoriasis. Br J Dermatol. 2006;155(6):1165–9. doi: 10.1111/j.1365-2133.2006.07503.x. [DOI] [PubMed] [Google Scholar]
- 30.Cakmak SK, Gül U, Kiliç C, Gönül M, Soylu S, Kiliç A. Homocysteine, vitamin B12 and folic acid levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2009;23(3):300–3. doi: 10.1111/j.1468-3083.2008.03024.x. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 32.Ohtsuka T. The relation between high-sensitivity C-reactive protein and maximum body mass index in patients with psoriasis. Br J Dermatol. 2008;158(5):1141–3. doi: 10.1111/j.1365-2133.2008.08467.x. [DOI] [PubMed] [Google Scholar]
- 33.Biyik I, Narin A, Bozok MA, Ergene O. Echocardiographic and clinical abnormalities in patients with psoriasis. J Intl Med Res. 2006;34(6):632–9. doi: 10.1177/147323000603400608. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension. Arch Dermatol. 2009;145(4):379–82. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerdes A, Zahl VA, Knopf H, Weichenthal M, Mrowietz U. Comedication related to comorbidities: a study in 1203 hospitalized patients with severe psoriasis. Br J Dermatol. 2008;159(5):1116–23. doi: 10.1111/j.1365-2133.2008.08786.x. [DOI] [PubMed] [Google Scholar]
- 36.Ho VC. The use of cyclosporine in psoriasis: a clinical review. Br J Dermatol. 2004;150(suppl 67):1–10. doi: 10.1111/j.0366-077X.2004.05950.x. [DOI] [PubMed] [Google Scholar]
- 37.Reeds RE, Jr, Fusaro RM, Fisher I. Psoriasis vulgaris I: a clinical survey of the association with diabetes mellitus. Arch Dermatol. 1964;89:205–8. doi: 10.1001/archderm.1964.01590260043007. [DOI] [PubMed] [Google Scholar]
- 38.Brauchli YB, Jick SS, Meier CR. Psoriasis and the risk of incident diabetes mellitus: a population-based study. Br J Dermatol. 2008;159(6):1331–7. doi: 10.1111/j.1365-2133.2008.08814.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Mills D, Bala M. Psoriasis: cardiovascular risk factors and other disease comorbidities. J Drug Dermatol. 2008;7(4):373–7. [PubMed] [Google Scholar]
- 40.Herron MD, Hinckley M, Hoffman MS, Papenfuss J, Hansen CB, Callis KP, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141(12):1527–34. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 41.Wolk K, Mallbris L, Larsson P, Rosenblad A, Vingård E, Ståhle M. Excessive body weight and smoking associates with a high risk of onset of plaque psoriasis. Acta Derm Venereol. 2009;89(5):492–7. doi: 10.2340/00015555-0711. [DOI] [PubMed] [Google Scholar]
- 42.Johnston A, Arnadottir S, Gudjonnson JE, Aphale A, Sigmarsdottir AA, Gunnarsson SI, et al. Obesity in psoriasis: leptin and resistin as mediators of cutaneous inflammation. Br J Dermatol. 2008;159(2):342–50. doi: 10.1111/j.1365-2133.2008.08655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerman AA, Bozkurt S, Sav A, Tulunay A, Elbaşi MO, Ergun T. Serum leptin levels, skin leptin, and leptin receptor expression in psoriasis. Br J Dermatol. 2008;159(4):820–6. doi: 10.1111/j.1365-2133.2008.08742.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen YJ, Wu CY, Shen JL, Chu SY, Chen CK, Chang YT, et al. Psoriasis independently associated with hyperleptinemia contributing to metabolic syndrome. Arch Dermatol. 2008;144(12):1571–5. doi: 10.1001/archderm.144.12.1571. [DOI] [PubMed] [Google Scholar]
- 45.Jin Y, Zhang F, Yang S, Kong Y, Xiao F, Hou Y, et al. Combined effects of HLA-Cw6, body mass index, and waist-hip ratio on psoriasis vulgaris in Chinese Han population. J Dermatol Sci. 2008;52(2):123–9. doi: 10.1016/j.jdermsci.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Kaur S, Zilmer K, Kairane C, Kals M, Zilmer M. Clear differences in adiponectin and glutathione redox status revealed in obese and normal-weight patients with psoriasis. Br J Dermatol. 2008;159(6):1364–7. doi: 10.1111/j.1365-2133.2008.08759.x. [DOI] [PubMed] [Google Scholar]
- 47.McGowan JW, Pearce DJ, Chen J, Richmond D, Balkrishnan R, Feldman SR. The skinny of psoriasis and obesity. Arch Dermatol. 2005;141(12):1601–2. doi: 10.1001/archderm.141.12.1601. [DOI] [PubMed] [Google Scholar]
- 48.Murray ML, Bergstresser PR, Adams-Huet B, Cohen JB. Relationship of psoriasis severity to obesity using same-gender siblings as controls for obesity. Clin Exp Dermatol. 2008;34(2):140–4. doi: 10.1111/j.1365-2230.2008.02791.x. [DOI] [PubMed] [Google Scholar]
- 49.Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125(1):61–7. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Chen J, Zhao Y, Geng L, Song F, Chen HD. Psoriasis is associated with increased levels of serum leptin. Br J Dermatol. 2008;158(2):1134–5. doi: 10.1111/j.1365-2133.2008.08456.x. [DOI] [PubMed] [Google Scholar]
- 51.Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital based case-control study. Br J Dermatol. 2007;157(1):68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- 52.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and risk of psoriasis in women. Arch Intern Med. 2007;167(15):1670–5. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 53.Eckel RH, Grundy SM, Zimmer PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 54.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol. 2006;298(7):321–8. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 55.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216(2):152–5. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 56.Cohen AD, Gilutz H, Henkin Y, Zahger D, Shapiro J, Bonneh DY, et al. Psoriasis and the metabolic syndrome. Acta Dermatol Venereol. 2007;87(6):506–9. doi: 10.2340/00015555-0297. [DOI] [PubMed] [Google Scholar]
- 57.Bongiorno MR, Doukaki S, Rizzo D, Aricò M. The prevalence of the obesity in patients with moderate to severe psoriasis in Sicily populations. JEADV 2009; [Epub ahead of print] [DOI] [PubMed]
- 58.Huerta C, Rivero E, Garcia Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143(12):1559–65. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 59.Naldi L, Peli L, Parazzini F. Association of early-stage psoriasis with smoking and male alcohol consumption: evidence from an Italian case-control study. Arch Dermatol. 1999;135(12):1479–84. doi: 10.1001/archderm.135.12.1479. [DOI] [PubMed] [Google Scholar]
- 60.Mills CM, Srivastava ED, Harvey IM, Swift GL, Newcombe RG, Holt PJ, et al. Smoking habits in psoriasis: a case control study. Br J Dermatol. 1992;127(1):18–21. doi: 10.1111/j.1365-2133.1992.tb14818.x. [DOI] [PubMed] [Google Scholar]
- 61.Naldi L, Parazzini F, Brevi A, Peserico A, Veller Fornasa C, Grosso G, et al. Family history, smoking habits, alcohol consumption and risk of psoriasis. Br J Dermatol. 1992;127(3):212–7. doi: 10.1111/j.1365-2133.1992.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 62.Wolkenstein P, Revuz J, Roujeau JC, Bonnelye G, Grob JJ, Bastuji-Garin S. Psoriasis in France and associated risk factors: results of a case-control study based on a large community survey. Dermatology. 2009;218(2):103–9. doi: 10.1159/000182258. [DOI] [PubMed] [Google Scholar]
- 63.Davidsson S, Blomqvist K, Molin L, et al. Lifestyle of Nordic people with psoriasis. Intl J Dermatol. 2005;44(5):378–83. doi: 10.1111/j.1365-4632.2005.01925.x. [DOI] [PubMed] [Google Scholar]
- 64.Setty AR, Curhan G, Choi HK. Smoking and the Risk of Psoriasis in Women: Nurses’ Health Study II. Am J Med. 2007;120(11):953–9. doi: 10.1016/j.amjmed.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fortes C, Mastroeni S, Leffondre K, et al. Relationship between smoking and clinical severity of psoriasis. Arch Dermatol. 2005;141(12):1580–4. doi: 10.1001/archderm.141.12.1580. [DOI] [PubMed] [Google Scholar]
- 66.Jin Y, Yang S, Zhang F, Kong Y, Xiao F, Hou Y, et al. Combined effects of HLA-Cw6 and cigarette smoking in psoriasis vulgaris: A hospital-based case-control study in China. J Eur Acad Dermatol Venereol. 2009;23(2):132–7. doi: 10.1111/j.1468-3083.2008.02951.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, Wang H, Te-shao H, Yang S, Wang F. Frequent use of tobacco and alcohol in Chinese psoriasis patients. Intl J Dermatol. 2002;41(10):659–62. doi: 10.1046/j.1365-4362.2002.01595.x. [DOI] [PubMed] [Google Scholar]
- 68.Jankovic S, Raznatovic M, Marinkovic J, Jankovic J, Maksimovic N. Risk factors for psoriasis: a case-control study. J Dermatol. 2009;36(6):328–34. doi: 10.1111/j.1346-8138.2009.00648.x. [DOI] [PubMed] [Google Scholar]
- 69.O’Keefe JAH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double edged sword. J Am Coll Cardiol. 2007;50(11):1019–24. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 70.Poikolainen K, Karvonen J, Pukkala E. Excess mortality related to alcohol and smoking among hospital-treated patient with psoriasis. Arch Dermatol. 1999;135(12):1490–3. doi: 10.1001/archderm.135.12.1490. [DOI] [PubMed] [Google Scholar]
- 71.McDonald CJ, Calabresi P. Psoriasis and occlusive vascular disease. Br J Dermatol. 1978;99:469–75. doi: 10.1111/j.1365-2133.1978.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 72.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 73.Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis (PsA) Ann Rheum Dis. 2009;68(7):1131–5. doi: 10.1136/ard.2008.094839. [DOI] [PubMed] [Google Scholar]
- 74.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol. 2009;160(5):1048–56. doi: 10.1111/j.1365-2133.2008.09020.x. [DOI] [PubMed] [Google Scholar]
- 75.Wakkee M, Herings RM, Nijsten T. Psoriasis may not be an independent risk factor for acute ischemic heart disease hospitalizations: results of a large population-based Dutch cohort. J Invest Dermatol. 2009;[Epub ahead of print] [DOI] [PubMed]
- 76.Xiao J, Chen LH, Tu YT, Deng XH, Tao J. Prevalence of myocardial infarction in patients with psoriasis in central China. J Eur Acad Dermatol Venereol. 2009;23(11):1311–5. doi: 10.1111/j.1468-3083.2009.03318.x. [DOI] [PubMed] [Google Scholar]
- 77.Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156(2):271–6. doi: 10.1111/j.1365-2133.2006.07562.x. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Juanetey C, Amigo-Diaz E, Miranda-Filloy JA, Testa A, Revuelta J, Garcia Purrua C, et al. Lack of echocardiographic and Doppler abnormalities in psoriatics arthritis patients without clinically evident cardiovascular disease or classic arthrosclerosis risk factors. Semin Arthritis Rheum. 2006;35(5):333–9. doi: 10.1016/j.semarthrit.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145(6):700–3. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 80.Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–8. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stern RS, Lange R. Cardiovascular disease, cancer, and cause of death in patients with psoriasis: 10 years prospective experience in a cohort of 1,380 patients. J Invest Dermatol. 1988;91(3):197–201. doi: 10.1111/1523-1747.ep12464847. [DOI] [PubMed] [Google Scholar]
- 82.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis. Arch Dermatol. 2007;143(12):1493–9. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 83.Mallbris L, Akre O, Granath F, Yin L, Lindelof B, Ekbom A, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19(3):225–30. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- 84.Wong K, Gladman DD, Husted J, Long JA. Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997;40(10):1868–72. doi: 10.1002/art.1780401021. [DOI] [PubMed] [Google Scholar]
- 85.Naldi L, Chatenoud L, Belloni A, Peserico A, Balato N, Virgili AR, et al. Medical history, drug exposure and the risk of psoriasis: Evidence from an Italian case-control study. Dermatology. 2008;216(2):125–30. doi: 10.1159/000111509. [DOI] [PubMed] [Google Scholar]
- 86.Tam LA, Tomlinson B, Chu TT, Li M, Leung YY, Kwok LW, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls- the role of inflammation. Rheumatol. 2008;47(5):718–23. doi: 10.1093/rheumatology/ken090. [DOI] [PubMed] [Google Scholar]
- 87.Grzybowski A, Grzybowski G, Druzdz A, Zaba R. Evaluation of glucose, insulin, C-peptide uric acid serum levels in patients with psoriasis. Pol Merkur Lekarski. 2001;11(66):495–8. [PubMed] [Google Scholar]
- 88.Karabudak O, Ulusoy RE, Erikci AA, Solmazgul E, Dogan B, Harmanyeri Y. Inflammation and hypercoaguable state in adult psoriatic men. Acta Derm Venereol. 2008;88(4):337–40. doi: 10.2340/00015555-0456. [DOI] [PubMed] [Google Scholar]
- 89.Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. The inflammatory response in mild and severe psoriasis. Br J Dermatol. 2004;150(5):917–28. doi: 10.1111/j.1365-2133.2004.05984.x. [DOI] [PubMed] [Google Scholar]
- 90.Boehncke S, Thaci D, Beschmann H, Ludwig RJ, Ackermann H, Badenhoop K, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157(6):1249–51. doi: 10.1111/j.1365-2133.2007.08190.x. [DOI] [PubMed] [Google Scholar]
- 91.Cohen AD, Dreiher J, Shapiro Y, Vidavsky L, Vardy DA, Davidovici B, et al. Psoriasis and diabetes: a population-based cross-sectional study. J Eur Acad Dermatol Venereol. 2008;22(5):585–9. doi: 10.1111/j.1468-3083.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 92.Markuszeski L, Bissinger A, Janusz I, Narbutt J, Jedrzejowska AS, Zalewska A. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38(1):64–9. doi: 10.1016/j.arcmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Shbeeb M, Uramoto KM, Gibson LE, O'Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000;27(5):1247–50. [PubMed] [Google Scholar]
- 94.Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arth Rheum. 1998;41(6):1103–10. doi: 10.1002/1529-0131(199806)41:6<1103::AID-ART18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 95.Miot HA, Miot LD, Lopes PS, Haddad GR, Marques SA. Association between palmoplantar pustulosis and cigarette smoking in Brazil: a case-control study. J Eur Acad Dermatol Venereol. 2009;23(10):1173–7. doi: 10.1111/j.1468-3083.2009.03282.x. [DOI] [PubMed] [Google Scholar]
- 96.Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Circulating levels of adiponectin, oxidized LDL and C-reactive protein in Portuguese patients with psoriasis vulgaris, according to body mass index, severity and duration of the disease. J Dermatol Sci. 2009;55(3):202–4. doi: 10.1016/j.jdermsci.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi H, Tsuji H, Takahashi I, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Prevalence of obesity/adiposity in Japanese psoriasis patients: adiposity is correlated with the severity of psoriasis. J Dermatol Sci. 2009;55(1):74–6. doi: 10.1016/j.jdermsci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Bajaj DR, Mahesar SM, Devrajani BR, Iqbal MP. Lipid profile in patients with psoriasis presenting at Liaquat University Hospital Hyderabad. J Pak Med Assoc. 2009;59(8):512–5. [PubMed] [Google Scholar]
- 99.Poikolainen K, Reunala T, Karvonen J, Lauharanta J, Kärkkäinen P. Alcohol intake: a risk factor for psoriasis in young and middle aged men? BMJ. 1990;300(6727):780–3. doi: 10.1136/bmj.300.6727.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poikolainen K, Reunala T, Karvonen J. Smoking, alcohol and life events related to psoriasis among women. Br J Dermatol. 1994;130(4):473–7. doi: 10.1111/j.1365-2133.1994.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 101.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32(6):982–6. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 102.Lindegard B. Diseases associated with psoriasis in a general population of 159,200 middle-aged, urban, native Swedes. Dermatologica. 1986;172:298–304. doi: 10.1159/000249365. [DOI] [PubMed] [Google Scholar]
- 103.Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: Rationale for screening and primary prevention. Am J Cardiol. 2003;92(suppl):17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- 104.Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol. 2003;30(1):36–40. [PubMed] [Google Scholar]
- 105.Rhew EY, Ramsey-Goldman R. Premature atherosclerotic disease in systemic lupus erythematosus–role of inflammatory mechanisms. Autoimmun Rev. 2006;5(2):101–5. doi: 10.1016/j.autrev.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Prodanovich S, Shelling ML, Federman DG, Kirsner RS. Cytokine milieu in psoriasis and cardiovascular disease may explain the epidemiological findings relating these 2 diseases. Arch Dermatol. 2008;144(11):1518–9. doi: 10.1001/archderm.144.11.1518. [DOI] [PubMed] [Google Scholar]