Abstract
BACKGROUND
Patients with chronic non-cancer pain (CNCP) have high rates of substance use disorders (SUD). SUD complicates pain treatment and may lead to worse outcomes. However, little information is available describing adherence to opioid treatment guidelines for CNCP generally, or guideline adherence for patients with comorbid SUD.
OBJECTIVE
Examine adherence to clinical guidelines for opioid therapy over 12 months, comparing patients with SUD diagnoses made during the prior year to patients without SUD.
DESIGN
Cohort study.
PARTICIPANTS
Administrative data were collected from veterans with CNCP receiving treatment within a Veterans Affairs regional healthcare network who were prescribed chronic opioid therapy in 2008 (n = 5814).
KEY RESULTS
Twenty percent of CNCP patients prescribed chronic opioid therapy had a prior-year diagnosis of SUD. Patients with SUD were more likely to have pain diagnoses and psychiatric comorbidities. In adjusted analyses, patients with SUD were more likely than those without SUD to have had a mental health appointment (29.7% versus 17.2%, OR = 1.49, 95% CI = 1.26–1.77) and a urine drug screen (UDS) (47.0% versus 18.2%, OR = 3.53, 95% CI = 3.06–4.06) over 12 months. There were no significant differences between groups on receiving more intensive treatment in primary care (63.4% versus 61.0%), long-acting opioids (26.9% versus 26.0%), prescriptions for antidepressants (88.2% versus 85.8%, among patients with depression), or participating in physical therapy (30.6% versus 28.6%). Only 35% of patients with SUD received substance abuse treatment.
CONCLUSIONS
CNCP patients with SUD were more likely to have mental health appointments and receive UDS monitoring, but not more likely to participate in other aspects of pain care compared to those without SUD. Given data suggesting patients with comorbid SUD may need more intensive treatment to achieve improvements in pain-related function, SUD patients may be at high risk for poor outcomes.
KEY WORDS: chronic pain, substance use disorder, opioids, treatment guidelines, medical utilization
INTRODUCTION
Chronic non-cancer pain (CNCP) is a debilitating illness that affects up to 35% of primary care patients.1 It is the most frequent reason for medical visits and the direct economic impact of chronic back pain alone has been estimated to be as high as $86 billion annually, with indirect costs exceeding hundreds of billion dollars.2
Providing treatment for CNCP is challenging and many patients continue to experience persistent pain and disability. The use of prescription opioid medications has increased3,4 and chronic opioid therapy is now the most common treatment for CNCP.5 Clinical guidelines for managing patients with CNCP include recommendations for assessment and follow-up, dose changes, supplementary treatment, managing adverse events, and special approaches for high-risk patients.6–8
CNCP patients with comorbid substance use disorder (SUD) who receive chronic opioid therapy are considered a high-risk group. There is a disproportionately high prevalence of SUDs among CNCP patients.9 Although rates vary by setting and type of substance being assessed, approximately 10% of CNCP in primary care10,11 and 10-30% of patients in specialty pain clinics12–14 have a current SUD. CNCP patients with a history of SUD are more likely to receive opioids for CNCP and at higher daily dose than patients without history of SUD.15–17
Guidelines for CNCP state that history of SUD is not a contraindication for treatment.6 However, patients with CNCP and a history of SUD are at increased risk for medication misuse18–20, and treatment guidelines recommend that patients with a history of SUD receive more intensive monitoring and treatment. Guidelines published by the Department of Veterans Affairs (VA) indicate that opioid therapy is contraindicated for patients with an active SUD who are not in treatment.7
Little data have been published on the extent to which providers adhere to opioid treatment recommendations generally, or for patients considered at high risk. The purpose of this study was to examine adherence to opioid therapy guidelines for CNCP patients based on SUD status. Results from a recent systematic review indicate there is little information available to guide clinicians who treat CNCP in patients with comorbid SUD.9 In this study, we compare implementation of guideline suggested clinical care in: 1) veterans with CNCP who are receiving chronic opioid therapy and have a SUD, and 2) veterans with CNCP receiving chronic opioid therapy and do not have a SUD diagnosis.
METHODS
Participants
All data were obtained for veterans receiving medical care at a VA facility in the Pacific Northwest (Washington, Oregon, Idaho, and Alaska) in 2008. Data were extracted from the Veterans Integrated Service Network (VISN)-20 Data Warehouse21 after approval by the Institutional Review Board at the Portland VA Medical Center. The Data Warehouse contains extracts of data from the main clinical software packages of regional facilities and two national VA databases.
Patients were eligible if they received care within VISN-20 during 2008. To identify patients with CNCP, we reviewed electronic medical record pain Numeric Rating Scores (NRS) which are routinely administered during ambulatory visits. Pain intensity is rated on a scale of 0–10, where 0 = no pain and 10 = worst pain imaginable.22 As part of the VA “Pain as the 5th Vital Sign” initiative, NRS are routinely collected at outpatient medical visits; over 95% of veterans with an outpatient visit in VISN-20 in 2008 had a NRS. We defined CNCP as being present when patients had pain intensity scores ≥ 4 on the NRS in three or more different months (for patients whose first pain score occurred in November or December, the first two months of 2009 were considered). This is consistent with definitions that require at least three months duration.23 We chose NRS scores ≥ 4 as indicating moderate to severe pain because it is consistent with prior research and aligns with VA clinical practice and policy regarding indication for further pain assessment.24 To be included, patients must have been prescribed chronic opioid therapy, defined as 90 or more consecutive days.10,25 We identified 28,556 potentially eligible patients. Patients were excluded if they had any cancer diagnosis within the last six years (n = 6,234), surgery within the past six months (n = 1,597), enrollment in a VA opioid substitution program (n = 456), or death in 2008 (n = 592). We retained the 5,814 patients who were prescribed opioids for 90 or more consecutive days. A portion of these data have been reported in a report of factors associated with prescriptions for high doses of opioids.26
We created an index date for all patients to be used as a reference for data collection. The index date was the earliest date in 2008 at the beginning of a 90 consecutive day episode of opioid use.
Demographic, Diagnostic, Pharmacy, and Medical Utilization Data
The independent variable was having a SUD documented within 12 months prior to the index date. All alcohol use disorders, illicit SUDs, and prescription drug use disorders were included. Inpatient and outpatient diagnoses were based on the International Classification of Diseases, Clinical Modification–9th Revision (ICD-9-CM). This may include some patients whose SUD is in remission but whose provider noted it because the patient’s history of SUD may be relevant to current care.
Demographic data included age, gender, race, marital status, and VA service-connected status. We collected ICD-9-CM diagnoses of common psychiatric disorders. Past-year inpatient hospitalization and outpatient diagnoses were used to calculate a Charlson Comorbidity score27, a standardized technique that uses diagnostic codes to provide a measure of illness severity.28,29
Pharmacy data were reviewed for one year after the index date to obtain prescriptions of opioids and non-opioid analgesics. Each opioid prescription was converted to a morphine equivalent.26
We extracted medical utilization data from services considered important in management with opioid therapy for one year after the index date. This included data from primary care, physical therapy, orthopedics, mental health, and substance abuse treatment. Data were also collected on the frequency and results of urine drug screen (UDS) administrations.
Statistical Analyses
CNCP patients with a SUD diagnosis made within one year prior to the index date were compared to patients who did not have a SUD diagnosis. Chi-square tests were used for categorical variables and analysis of variance for linear variables.
Odds ratios (OR) and 95% confidence intervals (95% CI) were derived using generalized estimating equations (GEE) to identify whether a SUD diagnosis was associated with outcome variables. This methodology accounted for within-site correlations among the eight different VA facilities. We specified a model-based estimator covariance matrix because we were accounting for less than 20 facilities. We controlled for the effects of variables that may be associated with treatment utilization. Categorical variables (gender, major depressive disorder diagnosis, SUD) were entered as predictors, while linear variables (age, average NRS score) were entered as covariates. A separate GEE model was constructed for each outcome variable.
For the GEE models, outcome variables were identified a priori that were considered most critical for receiving guideline concordant care. For each outcome variable described below, we provide a grade for the strength of the recommendation (strong, weak) and for quality of the evidence (high, moderate, poor), which are based on published guidelines for chronic opioid therapy.6 Treatment recommendations for CNCP with comorbid SUD suggest “more intensive” follow-up and monitoring by primary care (strong recommendation, low-quality evidence).6 We therefore examined whether patients met with their primary care provider four or more times in one year; four appointments was chosen because a frequency analysis indicated this was the median number of appointments. Whether patients were prescribed a long-acting opioid was included, as this is a common recommendation (strong recommendation, low-quality evidence); however, there are not high quality empirical studies available to support the use of long-acting versus short-acting opioids.30 Physical therapy was measured because it is helpful for improving function and a common treatment recommendation is to participate in multidisciplinary treatment, including physical therapy (strong recommendation, moderate-quality evidence).31 We evaluated whether patients provided a UDS in the year after the index date as it is recommended that patients on chronic opioid therapy provide annual UDS (weak recommendation, low-quality evidence); more intensive UDS monitoring is recommended in patients with SUD (strong recommendation, low-quality evidence).6 We assessed whether patients attended a mental health visit (for patients with a current axis I psychiatric disorder), or received a prescription for antidepressant medication (for patients with major depression or dysthymia). These outcome variables were utilized because mental health interventions are associated with improvements in pain-related function (strong recommendation, moderate-quality evidence).32,33
RESULTS
Of the 5,814 participants, 19.5% (n = 1,136) had a SUD diagnosis, which included abuse or dependence of alcohol (n = 829, 73.0%), cannabis (n = 184, 16.2%), cocaine (n = 128, 11.3%), opioids (n = 172, 15.1%), amphetamines (n = 86, 7.6%), polysubstance (n = 81, 7.1%), or other substance (n = 257, 22.6%). There were statistically significant differences between groups on demographic characteristics (Table 1). Patients with SUD were younger (52.9 versus 55.5 years), more often male (92.6% versus 89.8%), and less likely to be married (33.4% versus 49.7%) than patients without SUD. Patients with SUD had greater pain NRS (6.7 versus 6.6) than patients without SUD, though the clinical difference between groups was small.
Table 1.
Comparison of Demographic Characteristics by Substance Use Disorder (SUD) Status
| Pain and SUD | Pain only | p-value | |
|---|---|---|---|
| (n = 1,136) | (n = 4,678) | ||
| Age, Mean (SD) | 52.9 (9.2) | 55.5 (12.4) | < 0.001 |
| Male Gender, N (%) | 1052 (92.6%) | 4199 (89.8%) | < 0.004 |
| Marital Status | < 0.001 | ||
| Single/Never Married, N (%) | 177 (15.6%) | 439 (9.4%) | |
| Married, N (%) | 379 (33.4%) | 2327 (49.7%) | |
| Separated/Divorced, N (%) | 507 (44.6%) | 1544 (33.0%) | |
| Widow, N (%) | 37 (3.3%) | 241 (5.2%) | |
| Unknown, N (%) | 36 (3.2%) | 127 (2.7%) | |
| Race | 0.004 | ||
| Caucasian, N (%) | 819 (72.1%) | 3285 (70.2%) | |
| Black, N (%) | 94 (8.3%) | 289 (6.2%) | |
| Other, N (%) | 24 (2.1%) | 138 (2.9%) | |
| Unknown or Declined, N (%) | 199 (17.5%) | 966 (20.6%) | |
| VA Service-Connected, N (%) | 71 (68.3%) | 240 (64.2%) | 0.44 |
| Average Pain Score, Mean (SD) | 6.7 (1.3) | 6.6 (1.3) | < 0.001 |
| Average Daily Dose Morphine Equivalent (SD) | 57.2 (86.1) | 56.6 (122.4) | 0.87 |
| Range, Median | 5 – 897, 32 | 5 - 5496, 30 | |
| Charlson Comorbidity Score, Mean (SD) | 1.17 (1.5) | 1.11 (1.5) | 0.23 |
Table 2 displays differences between groups on pain and psychiatric diagnoses. Patients with SUD were more likely to be diagnosed with low back pain, migraine headache, and neck or joint pain than those without SUD. The proportion of patients with three or more documented pain diagnoses significantly differed between groups (31.0% versus 26.7%, p = 0.004). Patients with SUD were more likely to be diagnosed with every psychiatric disorder assessed, with the exception of sleep disorders than those without SUD.
Table 2.
Comparison of Current Pain and Psychiatric Diagnoses by Substance Use Disorder (SUD) Status
| Pain and SUD | Pain only | p-value | |
|---|---|---|---|
| (n = 1136) | (n = 4678) | ||
| Pain Diagnoses | |||
| Fibromyalgia | 60 (5.3%) | 259 (5.5%) | 0.74 |
| Inflammatory Bowel Disease | 28 (2.5%) | 79 (1.7%) | 0.08 |
| Low Back Pain | 696 (61.3%) | 2632 (56.3%) | 0.002 |
| Migraine Headache | 124 (10.9%) | 407 (8.7%) | 0.02 |
| Neck or Joint Pain | 738 (65.0%) | 2666 (57.0%) | < 0.001 |
| Neuropathy | 71 (6.3%) | 338 (7.2%) | 0.25 |
| Rheumatism/ Arthritis | 500 (44.0%) | 2024 (43.3%) | 0.65 |
| Psychiatric Diagnoses | |||
| Major Depressive Disorder | 606 (53.3%) | 1743 (37.3%) | < 0.001 |
| Dysthymic Disorder | 93 (8.2%) | 240 (5.1%) | < 0.001 |
| Bipolar Disorder | 138 (12.1%) | 202 (4.3%) | < 0.001 |
| Panic Disorder | 50 (4.4%) | 136 (2.9%) | 0.01 |
| Posttraumatic Stress Disorder | 457 (40.2%) | 1181 (25.2%) | < 0.001 |
| Other Anxiety Disorder | 211 (18.6%) | 550 (11.8%) | < 0.001 |
| Schizophrenia | 63 (5.5%) | 108 (2.3%) | < 0.001 |
| Any Sleep Disorder | 128 (11.3%) | 452 (9.7%) | 0.11 |
| Nicotine Disorder | 630 (55.5%) | 1257 (26.9%) | < 0.001 |
There was no significant difference between groups in the average daily dose of opioids in morphine equivalent (57.2 versus 56.6 mg). Figure 1 displays comparisons on types of opioid medications that were prescribed. Patients with SUD were more likely to receive oxycodone (35.7% versus 30.2%), and less likely to be prescribed hydrocodone (53.9% versus 59.3%) and fentanyl (1.0% versus 1.9%) than those without SUD. The groups did not differ in likelihood of being prescribed short-acting (85.7% versus 87.8%) or long acting (26.9% versus 26.0%) opioids. There was no difference in the rates of prescriptions for high doses of opioids (defined as ≥ 180 mg morphine equivalent; 9.2% versus 8.0%). Patients with SUD were more likely to receive concurrent prescriptions for antidepressants (73.0% versus 61.7%), benzodiazepines (27.2% versus 22.5%), and NSAID/acetaminophen (68.6% versus 61.8%) than patients without SUD (Fig. 2).
Figure 1.
Unadjusted comparison of prescriptions for opioid medications based on Substance Use Disorder (SUD) status. Note. * = p < 0.05. ** = p ≤ 0.001.
Figure 2.
Unadjusted comparison of prescriptions for other medications based on Substance Use Disorder (SUD) status. Note. This evaluates prescriptions for certain medications at the time of each patient’s index date. * = p ≤ 0.001.
Patients with SUD were more likely to be administered at least one UDS (47.0% versus 18.4%, p < 0.001) and to have a positive UDS for an illicit substance (14.4% versus 4.6%, p < 0.001). Of the patients that had at least one UDS, patients with SUD averaged more total administrations (4.2 versus 2.4, p < 0.001) than patients without SUD. The most common positive UDS results were for cannabis and cocaine, both of which occurred at higher rates among patients with SUD diagnoses (12.1% versus 4.5% and 3.2% versus 0.3%, respectively).
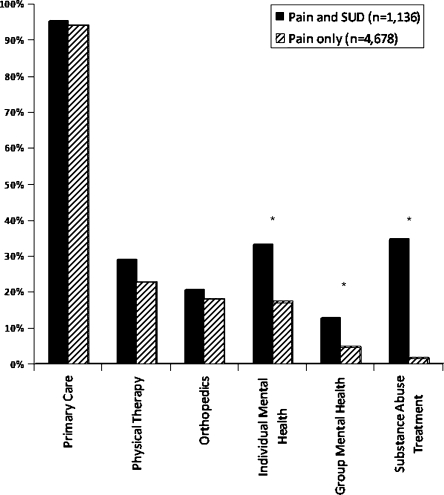
Figure 3 displays unadjusted comparisons between groups analyzing whether or not they received specific medical services. Patients with SUD were more likely to have had mental health and substance abuse appointments (all p-values ≤ 0.001) than those without SUD. There was no difference between groups in visits with primary care, physical therapy, or orthopedics.
Figure 3.
Unadjusted differences in medical utilization based on Substance Use Disorder (SUD) status. Note. This provides dichotomous data comparing whether patient’s received any of these services in the 12 months after each patient’s index date. * = p ≤ 0.001.
Logistic regression models were built to evaluate whether patients with SUD were more likely to receive certain aspects of treatment consistent with clinical guidelines for chronic opioid therapy (Table 3). After controlling for VA facility, age, gender, major depression diagnosis, and pain intensity, patients with SUD were more likely to have had a mental health appointment (OR = 1.49, 95% CI = 1.26–1.77) and to have been administered a UDS (OR = 3.53, 95% CI = 3.06–4.06) than patients without SUD. There was no difference in the likelihood of having more intensive primary care treatment, receiving a long-acting opioid, attending physical therapy, or receiving an antidepressant.
Table 3.
Likelihood of Receiving Guideline-Concordant Care Based on Substance Use Disorder (SUD) Status (n = 5,814)
| Pain and SUD | Pain Only | Odds Ratio (95% confidence interval) | |
|---|---|---|---|
| (n = 1136) | (n = 4678) | ||
| More Intensive Treatment in Primary Care | 1.08 (0.94 – 1.23) | ||
| Number of patients with ≥ 4 visits/year (%) | 720 (63.4%) | 2853 (61.0%) | |
| Average number of appointments (SD), range | 5.7 (4.6), 0-45 | 5.4 (4.7), 0-66 | |
| Long-acting Opioid | 0.94 (0.81 – 1.09) | ||
| Number of patients who received any (%) | 306 (26.9%) | 1216 (26.0%) | |
| Physical Therapy | 1.08 (0.94 – 1.25) | ||
| Number of patients who received any (%) | 348 (30.6%) | 1336 (28.6%) | |
| Average number of appointments (SD), range | 0.9 (2.1), 0-23 | 0.8 (2.4), 0-43 | |
| Urine Drug Screen | 3.53 (3.06 – 4.06) | ||
| Number of patients who received any (%) | 534 (47.0%) | 859 (18.4%) | |
| Average number of administrations (SD), range | 2.2 (4.7), 0-54 | 0.6 (1.8), 0-34 | |
| Mental Health Visit* | 1.49 (1.26 – 1.77) | ||
| Number of patients who received any (%) | 337 (29.7%) | 803 (17.2%) | |
| Average number of appointments (SD), range | 2.5 (6.2), 0-60 | 1.7 (5.4), 0-118 | |
| Antidepressant Medication+ | 1.26 (0.96 – 1.67) | ||
| Number of patients who received any (%) | 554 (88.2%) | 1583 (85.8%) |
Note. The Odds Ratios tested whether patients with a SUD were more likely to receive certain medical services. Analyses controlled for the effects of VA facility, age, gender, average pain score, and major depressive disorder diagnosis. * = For the variable mental health visit, this included only patients who were diagnosed with an axis I psychiatric disorder (n = 3,374). + = For antidepressant use, this included only patients who were diagnosed with major depressive disorder or dysthymia (n = 2,472)
DISCUSSION
This study provides information on overall adherence to clinical recommendations for managing patients on chronic opioid therapy, with a focus on patients with SUD. Overall, we found mixed results between guideline recommendations and clinical practice. In the total sample of patients prescribed chronic opioid therapy, in the 12 months after the index date, 26% received a long-acting opioid, 24% were administered a UDS, and 29% received physical therapy. Conversely, two-thirds of patients were seen by their primary care provider four or more times in the 12 month period and 86% with a depressive disorder received an antidepressant medication. Whether adherence to clinical guidelines is associated with improvements in pain severity and function remains to be tested.
Twenty percent of CNCP patients who were prescribed chronic opioid therapy had a SUD diagnosis, which may be an underestimate as SUDs are often under-recognized in clinical settings. Results indicate that providers should consistently screen CNCP patients for SUD comorbidity. Two guideline concordant recommendations occurred at higher rates among patients with SUD. Patients with SUD were 1.5 times more likely to participate in a mental health session and 3.5 times more likely to be given a UDS. These data are important, as psychological interventions have consistently strong outcome data in treating pain and improving function33, including studies of patients with pain and SUD34, and regular UDS monitoring is central for the provision of safe treatment with opioids and improves the detection of aberrant medication use.35 However, only half of CNCP patients with SUD received at least one UDS and 35% received substance abuse treatment, suggesting that additional steps are needed to increase guideline adherence. In addition, despite patients with SUD being at higher risk, there was no significant difference in the likelihood of receiving more intensive treatment in primary care, receiving a long-acting opioid, participating in physical therapy, or receiving a prescription for an antidepressant medication. Patients with SUD were also more likely to have a concurrent prescription for benzodiazepine medications, which may increase risk of overdose and death. A number of approaches may need to be used to increase adherence to treatment guidelines, including better system support to assist providers.
The best methods for treating CNCP among patients with comorbid SUD are unclear. Unfortunately, SUD patients are often excluded from clinical trials, resulting in little empirical data available regarding treatment options.9 Recent uncontrolled treatment studies indicate that more intensive, and integrated, treatment options may help to reduce pain and improve functioning among CNCP patients with comorbid SUD.36,37 These resources are often beyond the scope of usual practice in primary care, but could be an option for referral.
The results from this study have additional implications for clinical practice. Recent treatment guidelines suggest the presence of SUD is not a contraindication to opioid therapy, though it is recommended that patients with a SUD receive more intensive treatment and monitoring6 and participate in substance abuse treatment.7 One recent study showed that CNCP patients with a history of SUD are 70% less likely to have clinically significant improvements in pain-related function when receiving usual pain care.38 However, in this prior study, patients with a SUD history had improvements in pain-related function similar to patients without SUD when receiving more intensive and multidisciplinary treatment.38 Given this information as well as current findings showing that patients with SUD did not obtain more intensive care, it is likely that CNCP patients with comorbid SUD are at risk for sub-optimal pain treatment outcomes.
Consistent with prior research, CNCP patients with a SUD diagnosis were more likely to have comorbid psychiatric diagnoses38,39, highlighting the need to integrate pain care and mental health services.40 In contrast to previous findings, patients with SUD were not prescribed higher opioid doses17, despite statistically significant differences in pain scores. Patients with SUD may have decreased pain thresholds, suggesting the need for higher doses to achieve a similar response.41,42
Analyses for this study were based on treatment recommendations, which are often based on expert panels and not empirical data. There are other limitations from this study. All data were obtained from electronic medical records as part of standard care. Diagnoses were not confirmed with clinical interviews. The primary study variable was a past-year SUD diagnosis. This is not necessarily consistent with having a current SUD, as a diagnosis may have been given, but the actual substance use may be in remission. Furthermore, 9% of patients in the pain only group subsequently received a SUD diagnosis in the 12 months after the index date. We chose to leave these participants in the pain only group because we made an a priori decision to measure SUD up to the time of the index date. The overall findings from this study, and resulting implications, would not have been substantially influenced if we were to include these patients in the SUD group.
Given our reliance on electronic medical record data, we could not compare differences in pain-related function between the groups. Some patients may have had pain for years prior to this study, and potentially received some interventions in the past (e.g., physical therapy, mental health), but this would not be included in the current analyses. Although we controlled for differences in practice variation based on facility, medical care for CNCP may differ elsewhere. Other aspects of research methodology may limit generalizability. All patients were veterans, the majority of whom were male, and results may not generalize to non-veterans or women. Additionally, patients with mild pain were excluded, and the results may be limited to those with moderate to severe pain.
Results from this study provide data on overall adherence to clinical guidelines for opioid therapy and compare adherence based on SUD status. Prior research has documented the increased rates of opioid use for the treatment of CNCP among patients with SUD and psychiatric disorders.17,43 We have extended this research by detailing the use of medical services to treat pain among patients with CNCP and a past-year SUD diagnosis. These findings indicate that while several elements of care may be different (referral for mental health services, UDS), overall patients with CNCP and SUD receive pain care in a manner that does not differ from patients without a SUD. Further research is needed to assess the impact of improved adherence on clinical outcomes.
Acknowledgments
This study was supported in part by award K23DA023467 from the National Institute on Drug Abuse to Dr. Morasco. Jonathan Duckart, MPS, was supported by grant REA 06–174 from the VA Health Services Research and Development service. The authors appreciate support provided from the Oregon Clinical and Translational Research Institute, grant number Ul1RR024140 from the National Center for Research Resources, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or the Department of Veterans Affairs.
Conflict of Interest None disclosed.
References
- 1.Gureje O, Korff M, Simon GE, Gater R. Persistent pain and wellbeing: a World Health Organization Study in Primary Care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 2.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 3.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn BM. Opioid prescriptions soar. JAMA. 2007;297:249–251. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 5.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18:355–365. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Veterans Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. 2010. http://www.healthquality.va.gov/Chronic_Opioid_Therapy_COT.asp. Accessed 4/1/11.
- 8.American Academy of Pain Medicine and American Pain Society. The use of opioids for the treatment of chronic pain: A consensus statement from the American Academy of Pain Medicine and the American Pain Society. 1997. Glenview, IL. [PubMed]
- 9.Morasco BJ, Gritzner S, Lewis L, Oldham R, Turk DC, Dobscha SK. Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. Pain. 2011;152:488–497. doi: 10.1016/j.pain.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007;8:573–582. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishbain DA, Cutler R, Rosomoff H, Rosomoff R. Validity of self-reported drug use in chronic pain patients. Clinical Journal of Pain. 1999;15:184–191. doi: 10.1097/00002508-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hooten WM, Townsend CO, Bruce BK, Warner DO. The effects of smoking status on opioid tapering among patients with chronic pain. Anesth Analg. 2009;108:308–315. doi: 10.1213/ane.0b013e31818c7b99. [DOI] [PubMed] [Google Scholar]
- 14.Manchikanti L, Damron KS, McManus CD, Barnhill RC. Patterns of illicit drug use and opioid abuse in patients with chronic pain at initial evaluation: a prospective, observational study. Pain Physician. 2004;7:431–437. [PubMed] [Google Scholar]
- 15.Breckenridge J, Clark J. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–350. doi: 10.1016/S1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 16.Massey GM, Dodds HN, Roberts CS, Servoss TJ, Blondell RD. Toxicology screening in orthopedic trauma patients predicting duration of prescription opioid use. J Addict Dis. 2005;24:31–41. doi: 10.1300/J069v24n04_03. [DOI] [PubMed] [Google Scholar]
- 17.Weisner CM, Campbell CI, Ray GT, et al. Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain. 2009;145:287–293. doi: 10.1016/j.pain.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morasco BJ, Dobscha SK. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen Hosp Psychiatry. 2008;30:93–99. doi: 10.1016/j.genhosppsych.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Reid M, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schieffer BM, Pham Q, Labus J, et al. Pain medication beliefs and medication misuse in chronic pain. J Pain. 2005;6:620–629. doi: 10.1016/j.jpain.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Veterans Integrated Service Network-20 Data Warehouse. http://moss.v20.med.va.gov/v20dw/default.aspx. Accessed 4/1/10.
- 22.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 23.Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. NEJM. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Schall M, Nolan K, et al. Rapid improvement in pain management: The Veterans Health Administration and the Institute for Healthcare Improvement Collaborative. Clin J Pain. 2003;19:298–305. doi: 10.1097/00002508-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Korff M, Saunders K, Ray GT, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151:625–632. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CM administrative data. Medical Care. 2002;40:675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Medical Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 30.Kalso E, Allan L, Dobrogowski J, et al. Do strong opioids have a role in the early management of back pain? Recommendations from a European expert panel. Current Med Res Opinion. 2005;21:1819–28. doi: 10.1185/030079905X65303. [DOI] [PubMed] [Google Scholar]
- 31.Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary rehabilitation for chronic low back pain: Systematic review. BMJ. 2001;322:1511–1516. doi: 10.1136/bmj.322.7301.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: A meta-analytic review. Pain. 1992;49:221–230. doi: 10.1016/0304-3959(92)90145-2. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655–662. doi: 10.1037/0022-006X.69.4.655. [DOI] [PubMed] [Google Scholar]
- 34.Currie SR, Hodgins DC, Crabtree A, Jacobi J, Armstrong S. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4:91–100. doi: 10.1054/jpai.2003.17. [DOI] [PubMed] [Google Scholar]
- 35.Katz NP, Sherburne S, Beach M, Rose RJ, Vielguth J, Bradley J, Fanciullo GJ. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097–1102. doi: 10.1213/01.ANE.0000080159.83342.B5. [DOI] [PubMed] [Google Scholar]
- 36.Chelminski PR, Ives TJ, Felix KM, et al. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5:3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodin A, Gronbladh L, Nilsson LH, Gordh T. Methadone treatment of chronic non-malignant pain and opioid dependence – A Long-term follow-up. Eur J Pain. 2006;10:271–278. doi: 10.1016/j.ejpain.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Morasco BJ, Corson K, Turk DC, Dobscha SK. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12:352–359. doi: 10.1016/j.jpain.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haller DL, Acosta MC. Characteristics of pain patients with opioid-use disorder. Psychosomatics. 2010;51:257–266. doi: 10.1176/appi.psy.51.3.257. [DOI] [PubMed] [Google Scholar]
- 40.Savage SR. Management of opioid medications inpatients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377–384. doi: 10.1007/s11920-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 41.Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/S0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 42.Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alc Depend. 2006;82:218–223. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Edlund MJ, Martin BC, Devries A, Fan M, Braden JB, Sullivan MD. Trends in use of opoids for chronic noncancer pain among individuals with mental health and substance use disorders: The TROUP study. Clin J Pain. 2010;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]