Abstract
We describe a child of Middle Eastern descent by first-cousin mating with idiopathic neurogenic bladder and high grade vesicoureteral reflux at 1 year of age, whose characteristic facial grimace led to the diagnosis of Ochoa (Urofacial) syndrome at age 5 years. We used homozygosity mapping, exome capture and paired end sequencing to identify the disease causing mutation in the proband. We reviewed the literature with respect to the urologic manifestations of Ochoa syndrome. A large region of marker homozygosity was observed at 10q24, consistent with known autosomal recessive inheritance, family consanguinity and previous genetic mapping in other families with Ochoa syndrome. A homozygous mutation was identified in the proband in HPSE2: c.1374_1378delTGTGC, a deletion of 5 nucleotides in exon 10 that is predicted to lead to a frameshift followed by replacement of 132 C-terminal amino acids with 153 novel amino acids (p.Ala458Alafsdel132ins153). This mutation is novel relative to very recently published mutations in HPSE2 in other families. Early intervention and recognition of Ochoa syndrome with control of risk factors and close surveillance will decrease complications and renal failure.
Keywords: Ochoa syndrome, Urofacial syndrome, HPSE2 mutation, Neurogenic bladder
Case report and molecular genetics
The proband is an 11-year-old girl who is the second of three children of parents that are 1st degree cousins. The father and mother had no history of repeated urinary tract infections. A 13 year old brother and 8 year old sister have had no urological abnormalities or urinary tract infections, and each had normal ultrasound, urinalysis and urine culture investigations (data not shown). The siblings also do not demonstrate an inverted facial expression when smiling.
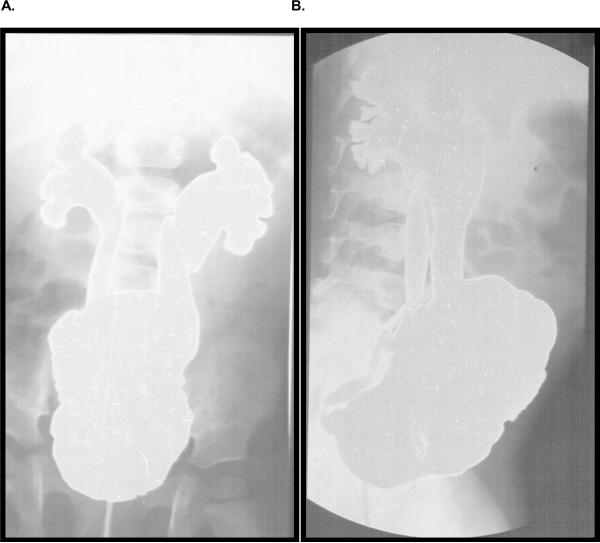
The proband was born at term following a normal vaginal delivery after an uneventful pregnancy. Birth weight was 3200 g and length 49 cm. Development and health were normal until 11 months of age when she developed a febrile E. coli urinary tract infection and was treated with oral antibiotics. Renal ultrasonography showed mildly enlarged kidneys, with ureteral, pelvic and calyceal dilatation, and a large trabeculated bladder. Voiding cystourethrography showed a trabeculated bladder and massive grade V bilateral vesicoureteral reflux into markedly dilated ureters without any sign of anatomical obstruction (Figure 1). The patient had a normal spinal MRI. At that time the diagnosis was idiopathic neurogenic bladder with high grade stage V reflux. This child had repeated pyelonephritis requiring IV antibiotic therapy during the next 6 months. Ureteral reimplantation was performed at age 18 months and she was maintained on prophylactic trimethoprim – sulfamethoxazole and oxybutynin for the next 2 years. Intermittent self catheterization three times per day using clean technique was performed by the parents. The child was stable for the next 2 years and she was free of urinary tract infections but started to exhibit severe constipation and Type II procidentia (rectal prolapse). Complete rectal prolapse signifies the intussusception of the rectal wall in its entirety through the anal sphincter. Type II refers to a complete full-thickness prolapse, and is further divided by severity. First degree is a high or concealed intussusception. Second degree is externally visible with straining, and third degree is externally visible at all times [1]. The patient had Type II second degree rectal prolapse with need for manual reduction after each defecation. With continuous use of lactulose as a stool softener the symptoms declined and rectal prolapse became intermittent with spontaneous reduction. The frequency of intermittent catheterization was increased to 5–6 times per day to reduce the risk of urinary tract infection after urodynamic studies showed a sharp rise in bladder pressure to more than 100mmHg with volumes greater than 85 ml.
Figure 1.
Radiographic documentation of vesicoureteral reflux. Voiding cystourethogram showing neurogenic bladder with a trabeculated, thickened bladder wall and increased bladder capacity. During filling a bilateral grade five reflux into the palvicalceal system was evident.
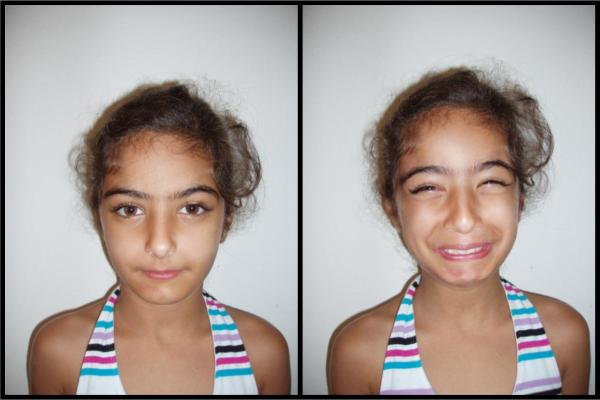
At age 5 ½ years the diagnosis of Ochoa was recognized when she showed the characteristic, inverted, facial expression at a routine examination (Figure 2, age 9). At the age of 7 she was also noted to have slow growth, with height and weight falling below the 25th centile. Psychomotor development has been normal. The child presently performs intermittent self catheterization 7–8 times per day and has an active life. She takes oxybutynin, nitrofurantoin and lactulose daily, remains free of upper urinary tract infections and is able to hold her urine for a reasonable period of time. Routine biochemistry, including urea and creatinine as well as urine microalbumin remain within the normal ranges. She has had four episodes of orthostatic hypotension and presyncope but no syncopal episodes. Workup included a normal EKG and a normal Holter monitor except for sinus tachycardia during a presyncopal attack that was documented during the Holter monitor. She had an exaggerated heart rate increase in response to postural change which is diagnostic of postural tachycardia syndrome POTS [2]. Autonomic reflexes have been otherwise preserved.
Figure 2.
Photograph of proband at 9 years old. Note characteristic facial grimace, which occurs when laughing or smiling.
Family sample collection and molecular studies were approved by the University of Michigan Institutional Review Board and the family described provided informed consent. To exclude a gene dosage abnormality within the 10q24 genomic region, we performed a comparative genomic hybridization experiment using a 244K Agilent chromosomal microarray (data not shown) according to previously described methods in the Michigan Medical Genetics Laboratories [3]; results for the proband were normal. Homozygosity mapping was performed based on genotype data collected using the Affymetrix Human SNP Array 6.0, followed by calculation of nonparametric likelihood ratio-based z-scores as described previously [4]. We next performed variant discovery in the exomes by using the Agilent Sure Select target enrichment system, followed by paired-end sequencing on the Illumina Genome Analyzer II to generate 36-nt sequence reads across ~160,000 coding exons in the human genome. We processed the sequence data in two pipelines, one involving read alignment with BWA [5] and variant calling with the Genome Analysis Toolkit (GATK) from the Broad institute, the other by using the CLC Genomics Workbench™ software (CLC-bio, Aarhus, Denmark). We focused our analysis on reads mapping to the 10q24 region.
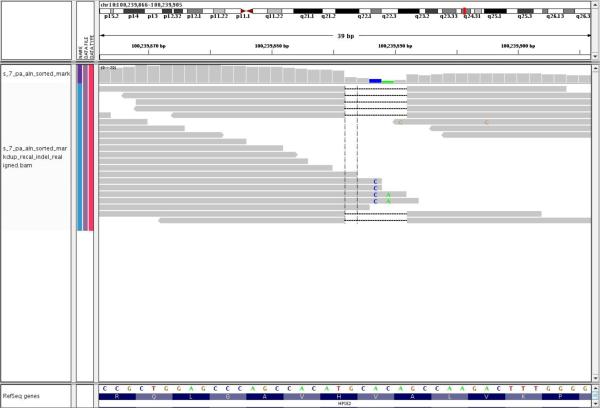
Molecular genetic evaluation disclosed long runs of homozygosity for a number of chromosomal regions including a ~18 Mb region in 10q23–q24 (data not shown) mapped previously by Wang et al. [6] to harbor an Ochoa syndrome gene. We obtained 58,829,100 36-nt Illumina GA-II reads (in 29,414,550 read pairs); 28.8 million reads were correctly mapped pairs and there were 4% duplicate read pairs. Examination of the reads within the 10q23q24 region revealed 7 of 9 reads in exon 10 of HPSE2 demonstrating a 5 base pair deletion (Figure 3). PCR amplification and Sanger sequencing confirmed a homozygous 5 bp deletion in the proband, and both parents are heterozygous mutation carriers (Figure 4). The 5-bp deletion is located in exon 10 of the HPSE gene at c.1374_1378delTGTGC, which causes a frameshift beginning at codon 458 and predicts the replacement of the C-terminal 132 amino acids with a novel 153 amino acid peptide. The mutated sequence has been submitted to GenBank, Accession number: BankIt1402480 Ochoa HQ438075.
Figure 3.
Paired-end sequencing identifies HPSE2 deletion. Genome Analysis Toolkit screen shot of a portion of HPSE2 exon 10 showing 36 base pair reads with a 5 base pair deletion (gap between the grey bars) encountered in 7/9 reads.
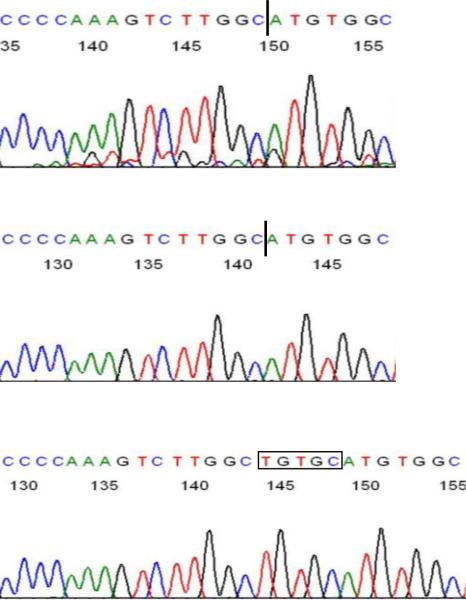
Figure 4.
Confirmation of paired-end sequencing result using Sanger sequencing. PCR amplification and sequencing of genomic DNA from the affected proband and her parents revealed that she is homozygous for the 5 base pair deletion and confirms the high-throughput sequencing data.
TOP: Sequence confirming homozygous deletion in proband. Dark vertical bar represents position of homozygous deletion of TGTGC. MIDDLE: Sequence of paternal PCR-amplified, cloned deleted allele. BOTTOM: Sequence of paternal PCR-amplified, cloned non-deleted allele; TGTGC is deleted in chromatograms shown in TOP and MIDDLE. Maternal PCR-amplified products revealed both deleted and non-deleted products identical to the paternal products shown. The mutated sequence demonstrating the deletion has been submitted to GenBank, submission number 1402171.
Discussion
Elejalde [7] described a syndrome of neuropathic bladder and facial distortion as the Ochoa syndrome (aka: Urofacial syndrome) and thanked Dr. Bernardo Ochoa for referring the patients for study. Ochoa and Gorlin [8] described 36 children with this condition between 1965 and 1986. These children had enuresis and urinary tract infection in association with `inversion' of facial expression when laughing. About two-thirds of the patients had moderate to severe constipation. During the last 40 years, 146 patients have been reported in Colombia, Kuwait, USA, Europe and Brazil. A review of all cases (Supplemental Table I, includes references) suggests a worse outcome compared to idiopathic neurogenic bladder, suggesting that late recognition or lack of recognition of the syndrome maybe a contributing factor.
Micturition is fundamentally a spinal reflex, facilitated and inhibited by higher brain centers, and also subject to voluntary facilitation and inhibition. Interruption of the pathways from the brain stem to the spine results in uncoordinated voiding (detrusor/sphincter dyssynergia) [9]. Complete rectal prolapse signifies the intussusception of the rectal wall in its entirety through the anal sphincter. Feng and Churchill [10] suggested that “dysfunctions in these two systems represent a broad spectrum of functional disturbances best described as elimination dysfunction (ED)”. Urinary and gastrointestinal tracts share the same embryologic origin (endoderm), the same anatomic space (pelvis), and the same innervations (sacral pelvis plexus). Conditions that affect one may significantly affect the other. The evaluation and management of dysfunctional elimination syndromes should be an integral part of the treatment of every child with vesicoureteral reflux [11]. It is unclear whether POTS occurs more frequently in patients with Urofacial syndrome compared to the general population.
We have identified a novel mutation in HPSE2, indicating likely loss of function mutations in this gene as causative of the disorder. Recently, loss of function mutations in the HPSE2 gene were identified in other individuals with Ochoa syndrome [12, 13]. For some of these patients, mutations could not be identified within HPSE2, suggesting genetic heterogeneity. HPSE2 encodes a putative endoglycosidase whose precise function is unknown, yet by homology with HPSE1, is hypothesized to enzymatically cleave heparan sulfate glycosaminoglycans. It will be interesting to see how HPSE2 loss of function leads to facial, genitourinary and gastrointestinal neurologic dysfunction and to investigate individuals with subclinical neurological bladder, occult neuropathic bladder, non-neurogenic neurogenic bladder or Hinman syndrome, dysfunctional voiding, or dysfunctional elimination for mutations in gene(s) in the pathway involving HPSE2.
Establishing an early diagnosis is relevant since the morphologic and functional damage in the urinary tract is progressive in this syndrome. Severely affected patients can become hypertensive and progress to end-stage renal disease. Because the characteristic grimacing is diagnostic, UFS can easily be queried in patients with an idiopathic neurogenic bladder by eliciting a smile.
Supplementary Material
Acknowledgements
The authors wish to thank the family for their participation and patience in the evaluation of the molecular genetics. This study was supported by funding from NIH ARRA Challenge Grant NIH/RC1-DK086542-01 to F.H. and J.W.I. and the Center for Genetics in Health and Medicine of the Department of Human Genetics to J.W.I. and to J.L. There was no role for the study sponsors in the design of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None of the authors have any conflicts to disclose.
REFERENCES
- [1].Stein EA, Stein DE. Rectal procidentia: diagnosis and management. Gastrointest Endosc Clin N Am. 2006;16:189–201. doi: 10.1016/j.giec.2006.01.014. [DOI] [PubMed] [Google Scholar]
- [2].Mack KJ, Johnson JN, Rowe PC. Orthostatic intolerance and the headache patient. Semin Ped Neurol. 2010;17:109–116. doi: 10.1016/j.spen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- [3].Bedoyan JK, Kumar RA, Sudi J, Silverstein F, Ackley T, Iyer RK, Christian SL, Martin DM. Duplication 16p11.2 in a child with infantile seizure disorder. Am J Med Genet A. 2010;152:1567–1574. doi: 10.1002/ajmg.a.33415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hildebrandt F, Heeringa SF, Rüschendorf F, Attanasio M, Nürnberg G, Becker C, Seelow D, Huebner N, Chernin G, Vlangos CN, Zhou W, O'Toole JF, Hoskins BE, Wolf MT, Hinkes BG, Chaib H, Ashraf S, Schoeb DS, Ovunc B, Allen SJ, Vega-Warner V, Wise E, Harville HM, Lyons RH, Washburn J, Macdonald J, Nürnberg P, Otto EA. A systematic approach to mapping recessive disease genes in individuals from outbred populations. PlosGenetics. 2009;5(1):e1000353. doi: 10.1371/journal.pgen.1000353. PMID: 19165332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang CY, Hawkins-Lee B, Ochoa B, Walker RD, She JX. Homozygosity and linkage- disequilibrium mapping of the urofacial (Ochoa) syndrome gene to a 1-cM interval on chromosome 10q23–q24. Am J Hum Genet. 1997;60:1461–1467. doi: 10.1086/515469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elejalde BR. Genetic and diagnostic considerations in three families with abnormalities of facial expression and congenital urinary obstruction: “The Ochoa syndrome”. Am J Med Genet. 1979;3:97–108. doi: 10.1002/ajmg.1320030114. [DOI] [PubMed] [Google Scholar]
- [8].Ochoa B, Gorlin RJ. Urofacial (Ochoa) syndrome. Am J Med Genet. 1987;27:661–667. doi: 10.1002/ajmg.1320270320. [DOI] [PubMed] [Google Scholar]
- [9].Fernandes ET, Reinberg Y, Vernier R, et al. Neurogenic bladder dysfunction in children: review of pathophysiology and current management. J Pediatr. 1994;124:1–7. doi: 10.1016/s0022-3476(94)70245-4. [DOI] [PubMed] [Google Scholar]
- [10].Feng WC, Churchill BM. Dysfunctional elimination syndrome in children without obvious spinal cord diseases. Pediatr Clin North Am. 2001;48:1489–1504. doi: 10.1016/s0031-3955(05)70387-4. [DOI] [PubMed] [Google Scholar]
- [11].Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160:1019–1022. doi: 10.1097/00005392-199809020-00014. [DOI] [PubMed] [Google Scholar]
- [12].Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JAG, Voelckel M-A, Gu W, Xiong W-C, Mei L, She J-X, Wang C-Y. Loss-of-function mutations in HPSE2 cause the autosomal recessive Urofacial syndrome. Amer J Hum Gen. 2010;86:957–962. doi: 10.1016/j.ajhg.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daly SB, Urquhart JE, Hilton E, McKenzie EA, Kammerer RA, Lewis M, Kerr B, Stuart H, Donnai D, Long DA, Burgu B, Aydogdu O, Derbent M, Garcia-Minaur S, Reardon W, Gener B, Shalev S, Smith R, Woolf AS, Black GC, Newman WG. Mutations in HPSE2 cause Urofacial syndrome. Amer J Hum Gen. 2010;86:963–969. doi: 10.1016/j.ajhg.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.